Abstract
Energy, freshwater, and the environment are interrelated features that infuse all human activities. Addressing this nexus in an integrated energy conversion system is a big challenge for the research community. Adsorption desalination system, which is a good alternative to traditional desalination systems, could solve this problem because it uses eco-friendly working fluids and can be powered by renewable energy. Many experimental prototypes for the adsorption desalination cycle were built and tested in the last decades. Also, different adsorbent materials were developed and characterized. Therefore, this paper reviews adsorbent materials with water vapor utilized in experimental adsorption desalination studies, which is considered the first step in constructing an efficient system. After that, the paper comprehensively reviews all previous experimental adsorption desalination studies. It focuses on the design of the experimental test rig, the mass of adsorbent material, and system performance, such as the specific daily water production, coefficient of performance, and specific cooling power. This work also discusses the properties of heat exchangers (i.e., adsorbent beds) employed in adsorption desalination systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy, freshwater, and the environment are interrelated features that infuse all our activities on the earth. Furthermore, they are becoming the most significant and common areas in recent research fields [1]. World energy consumption is projected to increase by 2.6% annually to 2030 [2]. The electrical energy utilization growth rate in Egypt is about 7% annually. It would need to increase its current generation capacity by a higher rate (more than 7%). The energy rate utilized by refrigeration air conditioning systems represents 30% of the total worldwide consumed energy and 32% in Egypt [3].
Due to population growth, desalination is a practical solution to the water shortage problem [4]. Distillation, membrane, and crystallization are examples of traditional desalination methods. Membrane-based reverse osmosis (RO), multi-stage flashing (MSF), and multi-effect distillation (MED) are examples of commercial desalination technologies [5]. Table 1 expresses comparing analysis for almost all common desalination technologies [6]. On the other hand, traditional desalination technologies have a significant initial investment and running cost [7, 8]. The energy costs of producing unit water by MSF or RO are higher than producing potable water from surface and subterranean water resources. Desalination costs vary depending on the location. The cheapest seawater reverse osmosis cost was 0.5 US$/m3 in 2016 [9]. Traditional energy-based desalination plants consume a lot of natural resources. As a result, solar, geothermal, wind, and other pollutant-free renewable energy sources are becoming increasingly popular for desalination. However, more research is needed to identify the most suitable technology for desalination applications [10].
Adsorption desalination system (ADS) is becoming a promising future technology for saving the required freshwater [11, 12]. It is based on using porous material that could be regenerated via low-grade thermal energy [13, 14]. The ADS has the advantage of efficiently utilizing low heat sources such as waste heat and/or solar energy [15,16,17]. It has some advantages over the commercial desalination methods, such as (i) the employment of the low-temperature excess or waste heat, (ii) lesser corrosion and fouling, (iii) and low maintenance cost. In addition, the ADS has two significant outcomes over the current desalination technologies, namely, (i) removing any “bio-contamination” and (ii) decreasing global warming due to the employment of excess waste heat [2, 18, 19]. The adsorption system can also be driven by renewable energy, reducing global warming resulting from carbon dioxide emissions of electricity generation [20]. The adsorption systems can be utilized for cooling purposes [21, 22]. The idea for utilizing these systems in desalination was firstly presented by Zejli et al. [23] in 2004, in which the earliest ADS simulation was performed. Till now, mathematical results [4,5,6, 24,25,26,27,28] achieved high values of specific daily water production (SDWP) and coefficient of performance (COP) of 98 m3/ton.day and 2.1, respectively [4]. However, this performance was not proven experimentally on either lab-scale, prototype, or pilot scale. Experimental measurements are still low as SDWP did not increase more than 18 m3/ton.day [29], and COP did not increase than 0.77 [30]. This trend shows a research gap between the theoretical and experimental studies in this field because of the lack of understanding of the effect of heat and mass transfer mechanisms on cycle performance. Also, many adsorbent materials have been developed in the last years, and however, their performance was tested theoretically without considerable investigation about their thermal effect on the system performance [14, 31].
Therefore, the present review presents an innovative review focusing on experimental studies of ADS. It also discusses the effect of the employed adsorbents' heat and mass transfer characteristics on the system efficiency for the first time in reviewing ADS. Thus, the paper identifies the huge difference in performance between the experimental and numerical studies in adsorption desalination. It also discusses this technology's future perspective, challenges, and outlook to fill the world water demand and supply gap. The present review is divided into two main sections besides the introduction. The first section explores research that expresses experimental adsorbent materials with water vapor, which is the first step in constructing an ADS. The second section explores experimental investigations for ADS with and without evaporator condenser heat recovery. This work emphasizes the experimental test rig design, the mass of adsorbent material adsorption, and desalination system performance as SDWP, COP, and specific cooling power (SCP) for each experimental device. This review states the properties of utilized heat exchangers of adsorbent beds in ADS.
Adsorbent materials used in ADS
Many researchers focused on developing new adsorbent material or improving its adsorption uptake to enhance ADS effectiveness. Therefore, this section presents adsorption materials tested with water vapor as adsorbate.
Silica gel
Silica gel is the common material utilized in the ADS. Silica gel is a category of amorphous synthetic silica that consists of a rigid and continuous net of colloidal silica associated with SiO4 particles. The main advantages of silica gel are that it can regenerate with temperatures as low as 100 °C and thermal stability. Still, they have low adsorption capacity compared to new adsorbents such as MOF [15]. White [32] theoretically illustrated the effect of silica gel granular diameter (1, 2, 3 mm) on the water adsorption rate. The study showed that reducing granule size raises the adsorption rate. Table 2 summarizes different types of silica gel and their adsorption uptakes.
Zeolite
Zeolite is a crystalline alumina silicate composed of alkali/alkali soil, namely molecular sieve, and alumina silicate skeletal has 0.2–0.5 cm3/g of porosity. The adsorption capability of zeolite is related to the proportion between aluminum and silicon. The main advantages of zeolite are non-toxic, non-flammable, and environmentally friendly. It needs high regeneration temperatures and low adsorption capacity compared to new adsorbents such as MOF [36]. About 40 types of natural zeolite and around 150 types of artificial zeolite regarding a synthesis method [37]. Table 3 summarizes different types of zeolite and their adsorption uptakes.
Metal–organic frameworks (MOFs)
Heat transformation technologies require the creation of adsorbent materials. In this regard, new materials appropriate to adsorption–desorption working fluid must yet be discovered for this technology to be remarkable [41, 42]. Metal–organic frameworks (MOFs), also known as porous coordination polymers (PCPs), have shown outstanding adsorbent properties and were investigated for heat transformation uses. MOFs also comprise hydrophilic and hydrophobic moieties, each with adsorption characteristics. Because of their high adsorption capacity for guest molecules such as water, MOF materials offer significant potential for heat transformation compared to a large range of natural and manufactured adsorbents. However, their stability and long-time synthesis process are the main challenges facing this family of nanoporous materials [42, 43]. Compared to silica gel, MOFs with hydrophilic characteristics have the preference since they have an unlimited water uptake capacity at high pressures. At first, MOFs were demonstrated as adsorbent materials by looking at their ability to use solid–gas adsorption for energy transformations. MOF materials offer a wide range of energy storage and heat transformation (cooling/heating) uses. Because water is commonly utilized as a working fluid, the examined adsorbent materials were evaluated using water adsorption–desorption properties. MOFs have also been examined for water adsorption studies to investigate structural characteristics and adsorption performance. The metal clusters must first coordinate water molecules before the poetical condensation procedure in the solid adsorbent's pores (MOFs) occurs in water adsorption [42, 44]. Therefore, metal groups categorize MOF materials for water adsorption and heat/energy transformation applications. In addition, several frameworks showed geometric plasticity and reversible structural change in guest adsorption. Thus, water adsorption on MOF materials was previously used to estimate their heat transformation performance.
MOFs have significantly more promise for this use than current adsorbents for heat transformation applications, like alumina phosphates or zeolites, owing to their composition, pore structure, also topology. Furthermore, additional improvement of the porosity structure of the MOFs, allowing for tailoring of their adsorption capabilities, modification or functionalization of metal clusters/ions, and biological linkers are still achievable [42, 45]. This opens up exciting possibilities for MOF production with specified properties optimized for specific working situations, such as heat transformations [42, 43]. Interestingly, development in MOF chemistry has progressed. Numerous techniques to synthesize and develop water-stable MOFs have paved the path for water-sorbent candidates with improved water adsorption and associated applications, see Table 4 [46,47,48,49,50]. From where water uptake capacity and corresponding relative pressure at which the pore filling occurs, the adsorption capabilities of MOFs are highly variable from a qualitative standpoint. Hydrolytically stable porous materials with large pore volumes, on the other hand, are likely to have large water adsorption capabilities. Hunt for hydrolytically stable and recyclable MOFs with higher total water uptake is a major focus of MOF chemistry research [45, 47, 51,52,53].
Comparison among MOF materials and conventional materials
The authors showed and compared various MOF materials that outperform existing porous materials like silica gel and zeolites in water adsorption [69, 70], see Fig. 1. The water adsorption isotherms of MOF materials are shown in Fig. 2. When comparing Figs. 1 and 2, it is clear that the MIL-101(Cr) outperformed the typical greatest adsorption capacity. For the adsorption (desalination and cooling) process, desalinated water and cooling effects are influenced by the value of the adsorption capacity of the adsorbent material. The available adsorbed amount in water vapor adsorption uptake (∆w) at adsorption and adsorption pressures are mainly affected by the available adsorbed amount. The ∆w represents the difference between expected material concentrations in the adsorption and desorption model (cycle sorption quantity). The adsorbent material with a step increase in water vapor uptake before (P/Ps = 0.25) is suitable for cooling applications. Some adsorbent materials take their most adsorption capacity after P/Ps = 50%, which indicates that it is more suitable for desalination than cooling applications. In cooling applications, the evaporator pressure could be considered 1 kPa to get a cooling effect at around 7 °C, while in the desalination application, the evaporator pressure could be higher, around 2.25 kPa to take benefit most adsorption capacity of material as ∆w [36]. Based on this Figure, MIL-101(Cr), MIL-100(Fe), and aluminum fumarate are suitable for applications requiring high P/Ps, like desalination [68] or energy storage, whereas CPO-27(Ni) is better suited for applications requiring low P/Ps like energy storage [71], cooling [72] or dual effect desalination [73, 74]. For more illustration of whether an adsorbent is suitable more in desalination or cooling applications, or both. This is calculated based on ∆w at evaporation and condensation pressures and temperatures using the Gibbs energy change (− RT ln(P/Ps)) relationship as illustrated in Fig. 3. The figure expresses that the MIL-101(Cr)-UoB has the best performance in desalination mode. It is achieved ∆w (1.22 kg/kg) in desalination application. But it has not a good performance in cooling mode as it achieves ∆w (0.06 kg/kg) due to its isotherm performance, which has a jump in adsorbed vapor uptake after P/Ps = 0.4. The figure also expresses that Aluminum fumarate and MIL-100(Fe)-UoB have the best performance in cooling mode as they achieve around ∆w (0.32 kg/kg) in cooling mode.
Isotherm of water adsorption for some MOF materials like MIL-101(Cr)-UoB, CPO-27(Ni), Aluminum fumarate, and MiL-100(Fe)-UoB [69] at 25 °C
Adsorption characteristic of MOF [69] at 25 °C
Enhancing the properties of MOF materials
Because of its huge pore size and high free volume, MIL-101(Cr) has exceptional features, including exceptionally low thermal conductivity. During both adsorption and desorption stages, low thermal conductivity makes it difficult for heat transfer processes to reach the required operating temperatures fast. To enhance the thermal conductivity of parent MIL-101, a composite of MIL-101(Cr)/GrO was utilized (Cr) [78]. Two approaches were used to create a composite of MIL-101(Cr) and GrO: physically mixing the two components and integrating them into the synthesis process. Owing to the limited porosity of GrO, it was discovered that the composites made via the physical mixing method had a decreased water uptake. In the synthesis process, the 2% GrO synthesis composite demonstrated comparable water uptake at low relative pressures and outperformed the pristine material at high relative pressures. Because of the crystal structural distortion, 5% GrO synthesis composite reduced water uptake [78].
Composite adsorption materials
Many researchers have improved the current adsorbent materials by the composition method, whether by compositing two or more host matrix materials together or by compositing a host matrix with a salt hydrate to improve the performance of systems that use those materials [36]. Table 5 summarizes the adsorption characteristics of composite materials. The table illustrates that composite adsorbent materials achieved higher adsorption water capacity than the base materials, which expresses that composite materials can achieve higher performance as cooling power or desalination effect. But these composite adsorbents need to be examined experimentally in adsorption desalination/cooling devices to realize what can enhance performance.
Experimental adsorption desalination systems
This section explores all presented experimental investigations for ADS separated into two categories. Experimental investigation types are ADS with and without evaporator condenser heat recovery. This section explores all experimental adsorption desalination (AD) studies; the work emphasizes the experimental test rig design, the mass of adsorbent material adsorption, and desalination system performance as SDWP, COP, and specific cooling power (SCP) of each experimental device. This work also reviews the properties of the used heat exchangers of adsorbent beds in ADS.
Experimental adsorption desalination studies
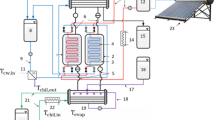
In this sub-section, experimental investigations of ADS are presented. These experimental investigations are for producing both desalination and cooling effects. The first excremental test rig for ADS was presented by Wang et al. [8], as illustrated in Fig. 4. The highest SDWP obtained was 4.7 m3/ton of silica gel at Tdes = 85 °C and Tcw = 30 °C. This study also reported that SDWP yielded from the plant could be further boosted by adopting a higher chilled water temperature supply (Tchi) and lowering adsorption cooling water (Tcwi). It demonstrated that ADS was also more efficient when the heat source temperature was lowered to 65 °C. Thu et al. [118] reported ADS performances with two-bed and four-bed operational modes. Figure 5 shows the used test rig for ADS. The tested results are estimated in terms of (i) SDWP, cycle time, and (ii) performance ratio (PR) for several driving temperatures (Tdrv). It was found that the maximum SDWP is about 10 m3 /tone.day with PR 0.61. The study also provided a valuable guideline for the operational approach of ADS. The study employed four adsorption units with 36 kg of silica gel per adsorption unit. Wu et al. [119] presented ADS as an alternative to traditional desalination systems that could be utilized by waste heat or solar energy to generate potable. The study investigated a practical implementation of theoretical ADS cycles and their validity experimentally. As shown in Fig. 6, the study employed one adsorption unit with 2.124 kg of silica gel.
Schematic diagram of the ADS used test facility [8]
Schematic diagram of used ADS experimental test rig stated in [118]
One adsorption unit with 2.124 kg of silica gel [119]
Ng et al. [120] analyzed the performance of ADS utilized by waste heat for producing desalinated water and cooling effect. A theoretical simulation for ADS was preceded. The cycle was explored using key performance parameters like (i) SCP, (ii) SDWP, (iii) COP, and (iv) overall conversion ratio (OCR). The mathematical results were certified by experimental data. Figure 7 expresses the advanced ADS cycle with 4 adsorption units with 36 kg silica gel per unit. At Twhi = 85 °C, the cycle produced 3.6 m3 of desalinated water and 23 Raton at Tcho = 10 °C.
Advanced ADS cycle with 4 adsorption units [120]
Mitra et al. [121] evaluated two-stage ADS for both cooling-cum-desalination. Figure 8 shows a schematic of the experimental facility. The study showed that a single-stage ADS system could not be used with an air-cooled condenser under tropical conditions, and this was realized by operating the system in a 2-stage model. Also, the study expressed a developed simulation model that was closer to experimental results than the previous one.
Schematic of 2-bed two-stage ADS [121]
Gao et al. [122] investigated an innovative single-stage vacuum evaporator to extract saltwater. The system was settled to utilize an ultra-low-grade heat source of 50 °C. Figure 9 illustrates the investigated system. The adsorbent bed comprised 5 arrays of U-shaped aluminum finned tube heat-exchanger with 0.8 kg of silica gel (Type A). It was conducted that utilizing lower Tcwi enhanced the desorption process, which boosted the performance of the developed system.
Schematic of the used experimental facility [122]
Alsaman et al. [15] proposed and designed a new solar ADS for cooling and desalination. The proposed ADS was built and tested under Egypt's climate conditions. Figure 10 expresses the designed ADS 13.5 kg with silica gel. The Adsorption characteristics were also presented for the selected material. The theoretical model was close to experimental results. The results showed that SCP was 112 W/kg and SDWP was 4 m3/day.ton with COP of 0.45. Dakkama et al. [74] investigated MOF development for producing ice and freshwater. Figure 11 expresses a schematic diagram of the investigated system. The adsorber bed contained 670 g of CPO-27 (Ni). Results indicated that the optimal operating salinity concentration was 35,000 ppm to produce ice (8.3 m3/day/ton) with COP 0.9. The SDWP was 1.8 m3/day/ton.
Schematic diagram of the solar ADC test rig [15]
Schematic diagram of ADS for ice making and freshwater [74]
Youssef et al. [73] explored experimentally using CPO-27 (Ni) as adsorbent material for ADS applications. Experimental and numerical investigation for utilizing 0.67 kg of CPO-27(Ni) with a one-bed ADS system was obtained, as expressed in Fig. 12. Results demonstrated that by increasing Teva and reducing Tcond, SCP was improved. The ADS created 65 Rton/ton at (Tevap = 20 °C). SDWP was improved to 22.8 m3/ton.day at (Tevap = 40 °C, Tcon = 5 °C and Tdes = 95 °C). Olkis et al. [123,124,125,126] presented three papers illustrating the design of an experimental small-scale ADS desalinator for producing freshwater. The study introduced the world’s smallest ADS with 0.2 kg silica gel, as shown in Fig. 12. The system achieved an SDWP of 7.7 kg/kgsg.day. The ADS demonstrated the profits of heat combination between the adsorbent beds to reduce the consumed energy by 25% and raise the PR to 0.6.
Elsayed et al. [127] reported that MOF materials were recommended to substitute the traditional adsorbents. The study presented an experimental test of 0.375 kg aluminum fumarate in ADS. The performance of aluminum fumarate was higher than that of ADS utilizing silica gel and CPO-27(Ni) for desalination effect only at high P/Ps. Zhang et al. [128] presented a pilot-scale ADS with freshwater production of 100 kg/h, as illustrated in Fig. 13. The system was constructed based on small-scale system optimization and enhancement. The results exhibited that the desalinated water was less than 100 kg/h at Thwi = 55 °C. At higher Thwi, the desalinated water rate was improved to 191.3 kg/h at Thwi = 80 °C.
Schematic diagram of pilot-scale ADS [76]
Advanced experimental desalination investigations
In this section, the advanced adsorption desalination experimental investigations will be expressed. These experimental investigations are for only the desalination effect. In these systems, a heat recovery between evaporator and condenser was utilized. Thu et al. [25]expressed the results of an investigation advanced AD cycle with internal heat recovery between condenser and evaporator. Figure 14 expresses the advanced AD cycle with 4 adsorption units with 36 kg silica gel per unit. A mathematically advanced AD cycle was developed and validated with experimental results. The advanced ADS could yield an SDWP of 9.24 m3/ton daily at 70 °C with PR = 0.77. The proposed system could be operated at 50 °C Thwi with SDWP 4.3. The advanced cycle SDWP was two times that of the traditional AD cycle.
Advanced ADS cycle with 4 adsorption units [25]
Ma et al. [30] investigated an experimental heat recovery between adsorber and desorber beds for ADS with 29.17 kg silica gel per adsorption bed, as illustrated in Fig. 15. The results showed that the SDWP and PR were 4.69 and 0.766, respectively.
Schematic diagram for heat recovery between adsorber and desorber beds [30]
This heat recovery employment could not rise SDWP, but it could save consuming energy. Kim et al. [129] investigated the water quality measurements of AD plants. Feedwater was taken from the Red Sea. Figure 16 expresses the schematic AD cycle with 4 adsorption units. Water quality was assessed by complying with the Environmental Protection Agency (EPA) principles with major primary and minor inorganic drinking water contaminants and other usually tested water quality considerations. Desalinated water testing ensured the good quality of generated freshwater. Test results showed that ADS effectively removes all forms of salts to less than 10 ppm. Bai et al. [29] investigated the mass recovery between adsorber and desorber beds for ADS and the feedwater quality effect on ADS performance. The results showed that the SDWP and SCP were 18.08 and 490, respectively.
schematic ADS cycle with 4 adsorption units [129]
Hybrid adsorption desalination with MED system
There are many studies on hybrid ADS with other systems such as RO-AD, AD-MVC, and AD-HDH. Still, many of these studies are theoretically investigated. The only experimental hybrid investigation systems were expressed between AD and MED. Shahzad et al. [130] presented an experimentally new hybrid “MEDAD” system, a coupling of the conventional MED and ADS, as expressed in Fig. 17. The main advantage of the MEDAD cycle is that it allowed some MED stages to work below ambient temperature, indifferent to the conventional MED. The hybrid system significantly increases desalinated water to 2.5–3 folds of conventional MED.
MEDAD schematic diagram for experimental rig installed in NUS [130]
Son et al. [131] explored experimentally hybrid “MEDAD” desalination, applying synergetic impact for utilizing energy to improve the MEDAD performance, as expressed in Fig. 18. The MEDAD system significantly increased desalinated water up to 2–5 folds of conventional MED of the same rating.
Pictorial view of MED-AD system [131]
Table 6 summarizes the results of previous experimental ADS studies. The SDWP for ADS that utilized silica gel varied from 3.6 to 14.2 m3/day.ton. This wide range illustrates the significant effect of ADS system design and operating conditions. Therefore, the next section summarizes the heat exchanger configurations and their effect on ADS performance.
Effect of heat transfer of adsorption bed on ADS performance
In this section, the effect of heat transfers of adsorption bed on ADS performance. The current study expresses all adsorption desalination experimental investigations. The heat transfer parameters of adsorption beds are expressed in Table 7. The following equation illustrates heat transfer parameters.
To simplify the computation, the TMinherent of sorbed refrigerant was ignored. It is simplified in two ways: (1) the computed thermal mass no longer includes the sorbent's equilibrium composition, and (2) the thermal mass may be viewed as constant in sorption and desorption operations. Neglecting the TM of sorbed refrigerant will have a minor influence on TMtotal for many adsorption heat exchangers (HXs).
The thermal mass of the adsorption bed is given by
where HTF represents heating thermal fluid.
The specific thermal mass is given by.
Figures 19 and 20 express the effect of bed design representing thermal masses of heat exchangers on ADS performance through the previous experimental studies. In the previous experimental studies, the STM varied from 1.74 to 6.58 kJ/kg. Figure 19 illustrates the COP variation by changing the adsorption bed's specific thermal mass (STM). The COP decreases from 0.766 to 0.36 with STM increasing 1.74–6.05 kJ/kg for silica gel adsorbent duo to increase the adsorption bed's thermal mass, which means more heat losses in the heating adsorption bed. This heat loss is represented in the heating of the heat exchanger. Figure 20 expresses the SDWP variation by changing the adsorption bed's specific thermal mass (STM). STM has a significant effect on SDWP. The SDWP increases from 3.6 to 18 m3/day.ton (about 500% increasing) with STM increasing from 1.74 to 6.58 kJ/kg as a result of increasing the overall heat transfer coefficient due to increasing the thermal mass of the adsorption bed. This means more adsorbent vapor is released in desorption mode, leading to more desalinated water production in the condenser.
Challenges and perspectives
This section expresses the research gap, the required research topics in ADS, and future marketing challenges of marketing these ADS. Despite the many advantages of ADS, as they can be driven by renewable and/or waste energy, they still face difficulties in marketing and dissemination. This is because it suffers from high volume and relatively low efficiency compared to traditional devices, which decreases the productivity of these systems and makes them unattractive. Moreover, the number of experimental researches in this field is still limited compared to its importance. From this standpoint, it was necessary to show the gap and clarify the deficiency in this area. This is what this work is trying to show, as after reviewing the published research, it was found that the number of devices built to study desalination systems does not exceed a dozen. This clearly shows that the field still needs more effort, research, and the development of new methods and materials to raise the efficiency of this system. Therefore, it is recommended to do more experimental research to encourage the industrial sector and investors to build AD plants. The authors recommend these future researches focus on the following:-
-
1
Finding new adsorbent materials with a high adsorption capacity to reduce AD plant volume and increase performance in terms of SDWP and COP.
-
2
Finding new composite adsorbent materials for higher adsorption capacity
-
3
How to increase the COP of the AD cycle by increasing the overall heat transfer coefficient of adsorption beds and evaporator and condenser.
-
4
Applying the recent theoretical research of ADS experimentally to close the gap between theoretical and experimental studies. Mathematical results [4,5,6, 24,25,26,27,28] achieved high values of SDWP and COP of 98 m3/ton.day and 2.1, respectively [4]. However, this performance was not proven experimentally on either lab-scale, prototype, or pilot scale. Experimental measurements are still low as SDWP did not increase more than 18 m3/ton.day, and COP did not increase than 0.78.
-
5
Applying the theoretical hybridization between ADS and RO, HDH, salt hydrate, and absorption system experimentally to realize the benefits of these combinations. Also, study new combinations of ADS and other desalination systems.
-
6
Establish more pilot plants and scale up adsorption desalination plants to encourage the industrial sector to invest in these ADS. Finding new adsorbent materials with a high adsorption capacity to reduce AD plant volume and increase performance in terms of SDWP and COP.
Conclusions
This review presents a survey about the constructed and tested experimental water distillation systems that consider adsorption technology. Not so many systems have been found, as less than ten systems were built worldwide to take off freshwater from the salty water by adsorption evaporation technology. One of these few systems had been built in Egypt. The majority of these systems employed silica gel as an adsorbent material; its composites and metal–organic framework were also used. The amounts of the used adsorbents varied from less than 1 kg, reaching 1440 kg. Produced amount of pure water per day per ton of adsorbent has been varied as well, from 1.8 m3/ton/day up to 25 m3/ton/day. The whole presented system used a fin tube-type heat exchanger. It is clear that the technology is still in the cradle, and more experimental test rigs are required to be built and tested at different operating conditions. Also, more adsorbent materials are needed to be employed in such systems.
References
Harby, K., Ali, E.S., Almohammadi, K.M.: A novel combined reverse osmosis and hybrid absorption desalination-cooling system to increase overall water recovery and energy efficiency. J. Clean. Prod. 287, 125014 (2021). https://doi.org/10.1016/j.jclepro.2020.125014
Thu, K., Chakraborty, A., Kim, Y.D., Myat, A., Saha, B.B., Ng, K.C.: Numerical simulation and performance investigation of an advanced adsorption desalination cycle. Desalination 308, 209–218 (2013). https://doi.org/10.1016/j.desal.2012.04.021
Sadri, S., Khoshkhoo, R.H., Ameri, M.: Optimum exergoeconomic modeling of novel hybrid desalination system (MEDAD+RO). Energy 149, 74–83 (2018). https://doi.org/10.1016/j.energy.2018.02.006
Ali, E.S., Mohammed, R.H., Qasem, N.A.A., Zubair, S.M., Askalany, A.: Solar-powered ejector-based adsorption desalination system integrated with a humidification-dehumidification system. Energy Convers. Manag. 238, 114113 (2021). https://doi.org/10.1016/j.enconman.2021.114113
Ali, E.S., Mohammed, R.H., Askalany, A.: A daily freshwater production of 50 m3/ton of silica gel using an adsorption-ejector combination powered by low-grade heat. J. Clean. Prod. 282, 124494 (2021). https://doi.org/10.1016/j.jclepro.2020.124494
Askalany, A.A., Ali, E.S.: A new approach integration of ejector within adsorption desalination cycle reaching COP higher than one. Sustain. Energy Technol. Assess. 41, 100766 (2020). https://doi.org/10.1016/j.seta.2020.100766
Ng, K.C., Shahzad, M.W., Son, H.S., Hamed, O.A.: An exergy approach to efficiency evaluation of desalination. Appl. Phys. Lett. 110, 184101 (2017). https://doi.org/10.1063/1.4982628
Wang, X., Ng, K.C.: Experimental investigation of an adsorption desalination plant using low-temperature waste heat. Appl. Therm. Eng. 25, 2780–2789 (2005). https://doi.org/10.1016/j.applthermaleng.2005.02.011
Shemer, H., Semiat, R.: Sustainable RO desalination – Energy demand and environmental impact. Desalination 424, 10–16 (2017). https://doi.org/10.1016/j.desal.2017.09.021
Ding, D., Huang, J., Deng, X., Fu, K.: Recent advances and perspectives of nanostructured amorphous alloys in electrochemical water electrolysis. Energy Fuels 35, 15472–15488 (2021). https://doi.org/10.1021/acs.energyfuels.1c02706
Kim, Y.D., Woo, S.Y., Lee, H.S., Ji, H.: Adsorption isotherm model for analyzing the adsorption characteristics of water vapor to commercially available silica gel adsorbents for adsorption desalination applications. J. Chem. Eng. Data. 66, 1144–1156 (2021). https://doi.org/10.1021/acs.jced.0c00927
Ali, E.S., Askalany, A.A., Harby, K., Diab, M.R., Hussein, B.R.M., Alsaman, A.S.: Experimental adsorption water desalination system utilizing activated clay for low grade heat source applications. J. Energy Storage 43, 103219 (2021). https://doi.org/10.1016/j.est.2021.103219
Yang, M., Wang, X., Li, J., Zheng, J.N., Jiang, L.: Effects of particle sizes on growth characteristics of propane hydrate in uniform/nonuniform sands for desalination application. Energy Fuels 36, 1003–1014 (2022). https://doi.org/10.1021/acs.energyfuels.1c03709
Ali, E.S., Askalany, A.A., Harby, K., Diab, M.R., Alsaman, A.S.: Adsorption desalination-cooling system employing copper sulfate driven by low grade heat sources. Appl. Therm. Eng. 136, 169–176 (2018). https://doi.org/10.1016/j.applthermaleng.2018.03.014
Alsaman, A.S., Askalany, A.A., Harby, K., Ahmed, M.S.: Performance evaluation of a solar-driven adsorption desalination-cooling system. Energy 128, 196–207 (2017). https://doi.org/10.1016/j.energy.2017.04.010
Amin, Z.M., Hawlader, M.N.A.: Analysis of solar desalination system using heat pump. Renew. Energy 74, 116–123 (2015). https://doi.org/10.1016/j.renene.2014.07.028
Ali, E.S., Mohammed, R.H., Zohir, A.E., Farid, A.M., Elshaer, R.N., El-Ghetany, H.H., Askalany, A.A.: Novel ultrasonic dynamic vapor sorption apparatus for adsorption drying, cooling and desalination applications. Energy Rep. 8, 8798–8804 (2022). https://doi.org/10.1016/j.egyr.2022.06.026
Askalany, A., Habib, K., Ghazy, M., Assadi, M.K.: Adsorption cooling system employing activated carbon/ hfc410a adsorption pair. ARPN J. Eng. Appl. Sci. 11, 12253–12257 (2016)
Ghazy, M., Askalany, A.A., Ibrahim, E.M.M., Mohamed, A.S.A., Ali, E.S., AL-Dadah, R.: Solar powered adsorption desalination system employing CPO-27(Ni). J. Energy Storage 53, 105174 (2022). https://doi.org/10.1016/j.est.2022.105174
Ghazy, M., Ibrahim, E.M.M., Mohamed, A.S.A., Askalany, A.A.: Cooling technologies for enhancing photovoltaic–thermal (PVT) performance: a state of the art. Int. J. Energy Environ. Eng. (2022). https://doi.org/10.1007/s40095-022-00491-8
Ghazy, M., Askalany, A., Kamel, A., Khalil, K.M.S., Mohammed, R.H., Saha, B.B.: Performance enhancement of adsorption cooling cycle by pyrolysis of Maxsorb III activated carbon with ammonium carbonate. Int. J. Refrig. 126, 210–221 (2021). https://doi.org/10.1016/j.ijrefrig.2020.12.036
Askalany, A.A., Saha, B.B.: Towards an accurate estimation of the isosteric heat of adsorption—a correlation with the potential theory. J. Colloid Interface Sci. 490, 59–63 (2017). https://doi.org/10.1016/j.jcis.2016.11.040
Zejli, D., Benchrifa, R., Bennouna, A., Bouhelal, O.K.: A solar adsorption desalination device: first simulation results. Desalination 168, 127–135 (2004). https://doi.org/10.1016/j.desal.2004.06.178
Thu, K., Yanagi, H., Saha, B.B., Ng, K.C.: Performance investigation on a 4-bed adsorption desalination cycle with internal heat recovery scheme. Desalination 402, 88–96 (2017). https://doi.org/10.1016/j.desal.2016.09.027
Thu, K., Saha, B.B., Chakraborty, A., Chun, W.G., Ng, K.C.: Study on an advanced adsorption desalination cycle with evaporator-condenser heat recovery circuit. Int. J. Heat Mass Transf. 54, 43–51 (2011). https://doi.org/10.1016/j.ijheatmasstransfer.2010.09.065
Ali, E.S., Askalany, A.A., Zohir, A.E.: Innovative employing of salt hydration with adsorption to enhance performance of desalination and heat transformation systems. Appl. Therm. Eng. 179, 115614 (2020). https://doi.org/10.1016/j.applthermaleng.2020.115614
Askalany, A., Ali, E.S., Mohammed, R.H.: A novel cycle for adsorption desalination system with two stages-ejector for higher water production and efficiency. Desalination 496, 114753 (2020). https://doi.org/10.1016/j.desal.2020.114753
Ali, E.S., Muhammad Asfahan, H., Sultan, M., Askalany, A.A.: A novel ejectors integration with two-stages adsorption desalination: away to scavenge the ambient energy. Sustain. Energy Technol. Assess. 48, 101658 (2021). https://doi.org/10.1016/j.seta.2021.101658
Bai, S., Ho, T.C., Ha, J., An, A.K., Tso, C.Y.: Study of the salinity effects on the cooling and desalination performance of an adsorption cooling cum desalination system with a novel composite adsorbent. Appl. Therm. Eng. 181, 115879 (2020). https://doi.org/10.1016/j.applthermaleng.2020.115879
Ma, H., Zhang, J., Liu, C., Lin, X., Sun, Y.: Experimental investigation on an adsorption desalination system with heat and mass recovery between adsorber and desorber beds. Desalination 446, 42–50 (2018). https://doi.org/10.1016/j.desal.2018.08.022
Askalany, A.A., Ernst, S.J., Hügenell, P.P.C., Bart, H.J., Henninger, S.K., Alsaman, A.S.: High potential of employing bentonite in adsorption cooling systems driven by low grade heat source temperatures. Energy 141, 782–791 (2017). https://doi.org/10.1016/j.energy.2017.07.171
White, J.: A CFD simulation on how the different sizes of silica gel will affect the adsorption performance of silica gel. Model. Simul. Eng. 2012, 1–12 (2012). https://doi.org/10.1155/2012/651434
Thu, K., Chakraborty, A., Saha, B.B., Ng, K.C.: Thermo-physical properties of silica gel for adsorption desalination cycle. Appl. Therm. Eng. 50, 1596–1602 (2013). https://doi.org/10.1016/j.applthermaleng.2011.09.038
Robens, E., Wang, X.: Investigation on the isotherm of silica gel+water systems. J. Therm. Anal. Calorim. 76, 659–669 (2004). https://doi.org/10.1023/b:jtan.0000028045.96239.7e
Mohammed, R.H., Mesalhy, O., Elsayed, M.L., Su, M., Chow, C.L.: Revisiting the adsorption equilibrium equations of silica-gel/water for adsorption cooling applications. Int. J. Refrig. 86, 40–47 (2018). https://doi.org/10.1016/j.ijrefrig.2017.10.038
Alsaman, A.S., Ibrahim, E.M.M., Ahmed, M.S., Askalany, A.A.: Composite adsorbent materials for desalination and cooling applications: a state of the art. Int. J. Energy Res. 46, 10345–10371 (2022). https://doi.org/10.1002/er.7894
Bahgaat, A.K., Hassan, H.E., Melegy, A.A., Abd-El Kareem, A.M., Mohamed, M.H.: Synthesis and characterization of zeolite-Y from natural clay of Wadi Hagul Egypt. Egypt J Chem 63, 3791–3800 (2020). https://doi.org/10.21608/EJCHEM.2020.23195.2378
Sayilgan, ŞÇ., Mobedi, M., Ülkü, S.: Effect of regeneration temperature on adsorption equilibria and mass diffusivity of zeolite 13x-water pair. Microporous Mesoporous Mater. 224, 9–16 (2016). https://doi.org/10.1016/j.micromeso.2015.10.041
Kayal, S., Baichuan, S., Saha, B.B.: Adsorption characteristics of AQSOA zeolites and water for adsorption chillers. Int. J. Heat Mass Transf. 92, 1120–1127 (2016). https://doi.org/10.1016/j.ijheatmasstransfer.2015.09.060
Teo, H.W.B., Chakraborty, A., Han, B.: Water adsorption on CHA and AFI types zeolites: modelling and investigation of adsorption chiller under static and dynamic conditions. Appl. Therm. Eng. 127, 35–45 (2017). https://doi.org/10.1016/j.applthermaleng.2017.08.014
Henninger, S.K., Schmidt, F.P., Henning, H.M.: Water adsorption characteristics of novel materials for heat transformation applications. Appl. Therm. Eng. 30, 1692–1702 (2010). https://doi.org/10.1016/j.applthermaleng.2010.03.028
Chaemchuen, S., Xiao, X., Klomkliang, N., Yusubov, M., Verpoort, F.: Tunable metal-organic frameworks for heat transformation applications. Nanomaterials 8, 661 (2018). https://doi.org/10.3390/nano8090661
Tatlier, M., Munz, G., Henninger, S.K.: Relation of water adsorption capacities of zeolites with their structural properties. Microporous Mesoporous Mater. 264, 70–75 (2018). https://doi.org/10.1016/j.micromeso.2017.12.031
Furukawa, H., Gándara, F., Zhang, Y.B., Jiang, J., Queen, W.L., Hudson, M.R., Yaghi, O.M.: Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 136, 4369–4381 (2014). https://doi.org/10.1021/ja500330a
Canivet, J., Bonnefoy, J., Daniel, C., Legrand, A., Coasne, B., Farrusseng, D.: Structure-property relationships of water adsorption in metal-organic frameworks. New J. Chem. 38, 3102–3111 (2014). https://doi.org/10.1039/c4nj00076e
Burtch, N.C., Jasuja, H., Walton, K.S.: Water stability and adsorption in metal-organic frameworks. Chem. Rev. 114, 10575–10612 (2014). https://doi.org/10.1021/cr5002589
Administrator, O.J.S.T.: Metal-organic frameworks applied for water purification. Resour Technol (2018). https://doi.org/10.18799/24056537/2018/1/177
Taylor, J.M., Vaidhyanathan, R., Iremonger, S.S., Shimizu, G.K.H.: Enhancing water stability of metal-organic frameworks via phosphonate monoester linkers. J. Am. Chem. Soc. 134, 14338–14340 (2012). https://doi.org/10.1021/ja306812r
Canivet, J., Fateeva, A., Guo, Y., Coasne, B., Farrusseng, D.: Water adsorption in MOFs: fundamentals and applications. Chem. Soc. Rev. 43, 5594–5617 (2014). https://doi.org/10.1039/c4cs00078a
Jasuja, H., Burtch, N.C., Huang, Y.G., Cai, Y., Walton, K.S.: Kinetic water stability of an isostructural family of zinc-based pillared metal-organic frameworks. Langmuir 29, 633–642 (2013). https://doi.org/10.1021/la304204k
Li, S., Chen, Y., Pei, X., Zhang, S., Feng, X., Zhou, J., Wang, B.: Water purification: adsorption over metal-organic frameworks. Chin J. Chem. 34, 175–185 (2016). https://doi.org/10.1002/cjoc.201500761
Li, N., Xu, J., Feng, R., Hu, T.L., Bu, X.H.: Governing metal-organic frameworks towards high stability. Chem. Commun. 52, 8501–8513 (2016). https://doi.org/10.1039/c6cc02931k
Towsif Abtab, S.M., Alezi, D., Bhatt, P.M., Shkurenko, A., Belmabkhout, Y., Aggarwal, H., Weseliński, ŁJ., Alsadun, N., Samin, U., Hedhili, M.N., Eddaoudi, M.: Reticular chemistry in action: a hydrolytically stable mof capturing twice its weight in adsorbed water. Chem. 4, 94–105 (2018). https://doi.org/10.1016/j.chempr.2017.11.005
Reinsch, H., Marszalek, B., Wack, J., Senker, J., Gil, B., Stock, N.: A new Al-MOF based on a unique column-shaped inorganic building unit exhibiting strongly hydrophilic sorption behaviour. Chem. Commun. 48, 9486–9488 (2012). https://doi.org/10.1039/c2cc34909d
Reinsch, H., van der Veen, M.A., Gil, B., Marszalek, B., Verbiest, T., de Vos, D., Stock, N.: Structures, sorption characteristics, and nonlinear optical properties of a new series of highly stable aluminum mOFs. Chem. Mater. 25, 17–26 (2013). https://doi.org/10.1021/cm3025445
Akiyama, G., Matsuda, R., Kitagawa, S.: Highly porous and stable coordination polymers as water sorption materials. Chem. Lett. 39, 360–361 (2010). https://doi.org/10.1246/cl.2010.360
Jeremias, F., Khutia, A., Henninger, S.K., Janiak, C.: MIL-100(Al, Fe) as water adsorbents for heat transformation purposes—a promising application. J. Mater. Chem. 22, 10148–10151 (2012). https://doi.org/10.1039/c2jm15615f
Wickenheisser, M., Jeremias, F., Henninger, S.K., Janiak, C.: Grafting of hydrophilic ethylene glycols or ethylenediamine on coordinatively unsaturated metal sites in MIL-100(Cr) for improved water adsorption characteristics. Inorg. Chim. Acta. 407, 145–152 (2013). https://doi.org/10.1016/j.ica.2013.07.024
Ehrenmann, J., Henninger, S.K., Janiak, C.: Water adsorption characteristics of MIL-101 for heat-transformation applications of MOFs. Eur. J. Inorg. Chem. 2011, 471–474 (2011). https://doi.org/10.1002/ejic.201001156
Akiyama, G., Matsuda, R., Sato, H., Hori, A., Takata, M., Kitagawa, S.: Effect of functional groups in MIL-101 on water sorption behavior. Microporous Mesoporous Mater. 157, 89–93 (2012). https://doi.org/10.1016/j.micromeso.2012.01.015
Khutia, A., Rammelberg, H.U., Schmidt, T., Henninger, S., Janiak, C.: Water sorption cycle measurements on functionalized MIL-101Cr for heat transformation application. Chem. Mater. 25, 790–798 (2013). https://doi.org/10.1021/cm304055k
Jeremias, F., Lozan, V., Henninger, S.K., Janiak, C.: Programming MOFs for water sorption: amino-functionalized MIL-125 and UiO-66 for heat transformation and heat storage applications. Dalt. Trans. 42, 15967–15973 (2013). https://doi.org/10.1039/c3dt51471d
Shigematsu, A., Yamada, T., Kitagawa, H.: Wide control of proton conductivity in porous coordination polymers. J. Am. Chem. Soc. 133, 2034–2036 (2011). https://doi.org/10.1021/ja109810w
Wade, C.R., Corrales-Sanchez, T., Narayan, T.C., Dincǎ, M.: Postsynthetic tuning of hydrophilicity in pyrazolate MOFs to modulate water adsorption properties. Energy Environ. Sci. 6, 2172–2177 (2013). https://doi.org/10.1039/c3ee40876k
Liu, J., Wang, Y., Benin, A.I., Jakubczak, P., Willis, R.R., LeVan, M.D.: CO2/H2O adsorption equilibrium and rates on metal-organic frameworks: HKUST-1 and Ni/DOBDC. Langmuir 26, 14301–14307 (2010). https://doi.org/10.1021/la102359q
Schoenecker, P.M., Carson, C.G., Jasuja, H., Flemming, C.J.J., Walton, K.S.: Effect of water adsorption on retention of structure and surface area of metal-organic frameworks. Ind. Eng. Chem. Res. 51, 6513–6519 (2012). https://doi.org/10.1021/ie202325p
Cmarik, G.E., Kim, M., Cohen, S.M., Walton, K.S.: Tuning the adsorption properties of uio-66 via ligand functionalization. Langmuir 28, 15606–15613 (2012). https://doi.org/10.1021/la3035352
Elsayed, E., Al-Dadah, R., Mahmoud, S., Elsayed, A., Anderson, P.A.: Aluminium fumarate and CPO-27(Ni) MOFs: characterization and thermodynamic analysis for adsorption heat pump applications. Appl. Therm. Eng. 99, 802–812 (2016). https://doi.org/10.1016/j.applthermaleng.2016.01.129
Al Dadah, R., Mahmoud, S., Elsayed, E., Youssef, P., Al-Mousawi, F.: Metal-organic framework materials for adsorption heat pumps. Energy 190, 116356 (2020). https://doi.org/10.1016/j.energy.2019.116356
Mohammed, R.H., Rezk, A., Askalany, A., Ali, E.S., Zohir, A.E., Sultan, M., Ghazy, M., Abdelkareem, M.A., Olabi, A.G.: Metal-organic frameworks in cooling and water desalination: synthesis and application. Renew. Sustain. Energy Rev. 149, 111362 (2021). https://doi.org/10.1016/j.rser.2021.111362
Elsayed, A., Elsayed, E., Al-Dadah, R., Mahmoud, S., Elshaer, A., Kaialy, W.: Thermal energy storage using metal–organic framework materials. Appl. Energy. 186, 509–519 (2017). https://doi.org/10.1016/j.apenergy.2016.03.113
Shi, B.: Development of an Mof based adsorption air conditioning system for automotive. http://etheses.bham.ac.uk/id/eprint/6017 (2015)
Youssef, P.G., Dakkama, H., Mahmoud, S.M., Al-Dadah, R.K.: Experimental investigation of adsorption water desalination/cooling system using CPO-27Ni MOF. Desalination 404, 192–199 (2017). https://doi.org/10.1016/j.desal.2016.11.008
Dakkama, H.J., Youssef, P.G., Al-Dadah, R.K., Mahmoud, S.: Adsorption ice making and water desalination system using metal organic frameworks/water pair. Energy Convers. Manag. 142, 53–61 (2017). https://doi.org/10.1016/j.enconman.2017.03.036
Rezk, A., Al-Dadah, R., Mahmoud, S., Elsayed, A.: Experimental investigation of metal organic frameworks characteristics for water adsorption chillers. Proc. Inst. Mech. Eng. Part. C J. Mech. Eng. Sci. 227, 992–1005 (2013). https://doi.org/10.1177/0954406212456469
Kummer, H., Füldner, G., Henninger, S.K.: Versatile siloxane based adsorbent coatings for fast water adsorption processes in thermally driven chillers and heat pumps. Appl. Therm. Eng. 85, 1–8 (2015). https://doi.org/10.1016/j.applthermaleng.2015.03.042
Cheung, O., Hedin, N.: Zeolites and related sorbents with narrow pores for CO2 separation from flue gas. RSC Adv. 4, 14480–14494 (2014). https://doi.org/10.1039/c3ra48052f
Elsayed, E., Wang, H., Anderson, P.A., Al-Dadah, R., Mahmoud, S., Navarro, H., Ding, Y., Bowen, J.: Development of MIL-101(Cr)/GrO composites for adsorption heat pump applications. Microporous Mesoporous Mater. 244, 180–191 (2017). https://doi.org/10.1016/j.micromeso.2017.02.020
Jänchen, J., Ackermann, D., Stach, H., Brösicke, W.: Studies of the water adsorption on zeolites and modified mesoporous materials for seasonal storage of solar heat. Sol. Energy. 76, 339–344 (2004). https://doi.org/10.1016/j.solener.2003.07.036
Casey, S.P., Elvins, J., Riffat, S., Robinson, A.: Salt impregnated desiccant matrices for “open” thermochemical energy storage-selection, synthesis and characterisation of candidate materials. Energy Build. 84, 412–425 (2014). https://doi.org/10.1016/j.enbuild.2014.08.028
Mrowiec-Białoń, J., Jarzȩbski, A.B., Lachowski, A.I., Malinowski, J.J., Aristov, Y.I.: Effective inorganic hybrid adsorbents of water vapor by the sol-gel method. Chem. Mater. 9, 2486–2490 (1997). https://doi.org/10.1021/cm9703280
Wu, H., Wang, S., Zhu, D.: Effects of impregnating variables on dynamic sorption characteristics and storage properties of composite sorbent for solar heat storage. Sol. Energy. 81, 864–871 (2007). https://doi.org/10.1016/j.solener.2006.11.013
Mrowiec-Bialoń, J., Lachowski, A.I., Jarzȩbski, A.B., Gordeeva, L.G., Aristov, Y.I.: SiO2-LiBr nanocomposite sol-gel adsorbents of water vapor: preparation and properties. J. Colloid Interface Sci. 218, 500–503 (1999). https://doi.org/10.1006/jcis.1999.6406
Gordeeva, L.G., Glaznev, I.S., Malakhov, V.V., Aristov, Y.I.: Influence of calcium chloride interaction with silica surface on phase composition and sorption properties of dispersed salt. Russ. J. Phys. Chem. 77, 1843–1847 (2003)
Aristov, Y.I., Tokarev, M.M., Restuccia, G., Cacciola, G.: Selective water sorbents for multiple applications, 2 CaCl2 confined in micropores of silica gel: sorption properties. React. Kinet. Catal. Lett. 59, 335–342 (1996). https://doi.org/10.1007/BF02068131
Simonova, I.A., Freni, A., Restuccia, G., Aristov, Y.I.: Water sorption on composite “silica modified by calcium nitrate.” Microporous Mesoporous Mater. 122, 223–228 (2009). https://doi.org/10.1016/j.micromeso.2009.02.034
Aristov, Y.I., Sapienza, A., Ovoshchnikov, D.S., Freni, A., Restuccia, G.: Reallocation of adsorption and desorption times for optimisation of cooling cycles. Int. J. Refrig. 35, 525–531 (2012). https://doi.org/10.1016/j.ijrefrig.2010.07.019
Tanashev, Y.Y., Krainov, A.V., Aristov, Y.I.: Thermal conductivity of composite sorbents “salt in porous matrix” for heat storage and transformation. Appl. Therm. Eng. 61, 401–407 (2013). https://doi.org/10.1016/j.applthermaleng.2013.08.022
Tokarev, M.M., Aristov, Y.I.: Selective water sorbents for multiple applications, 4 CaCl2 confined in silica gel pores: sorption/desorption kinetics. React. Kinet. Catal. Lett. 62, 143–150 (1997). https://doi.org/10.1007/BF02475725
Gordeeva, L.G., Restuccia, G., Cacciola, G., Aristov, Y.I.: Selective water sorbents for multiple applications, 5 LiBr confined in mesopores of silica gel: sorption properties. React. Kinet. Catal. Lett. 63, 81–88 (1998). https://doi.org/10.1007/BF02475434
Ristić, A., Logar, N.Z.: New composite water sorbents CaCl2-PHTS for low-temperature sorption heat storage: determination of structural properties. Nanomaterials 9, 27 (2019). https://doi.org/10.3390/nano9010027
Ponomarenko, I.V., Glaznev, I.S., Gubar, A.V., Aristov, Y.I., Kirik, S.D.: Synthesis and water sorption properties of a new composite “CaCl2 confined into SBA-15 pores.” Microporous Mesoporous Mater. 129, 243–250 (2010). https://doi.org/10.1016/j.micromeso.2009.09.023
Jabbari-Hichri, A., Bennici, S., Auroux, A.: Effect of aluminum sulfate addition on the thermal storage performance of mesoporous SBA-15 and MCM-41 materials. Sol. Energy Mater. Sol. Cells. 149, 232–241 (2016). https://doi.org/10.1016/j.solmat.2016.01.033
Dong, H., Askalany, A.A., Olkis, C., Zhao, J., Santori, G.: Hydrothermal stability of water sorption ionogels. Energy 189, 116186 (2019). https://doi.org/10.1016/j.energy.2019.116186
Askalany, A., Olkis, C., Bramanti, E., Lapshin, D., Calabrese, L., Proverbio, E., Freni, A., Santori, G.: Silica-supported ionic liquids for heat-powered sorption desalination. ACS Appl. Mater. Interfaces. 11, 36497–36505 (2019). https://doi.org/10.1021/acsami.9b07602
Askalany, A.A., Freni, A., Santori, G.: Supported ionic liquid water sorbent for high throughput desalination and drying. Desalination 452, 258–264 (2019). https://doi.org/10.1016/j.desal.2018.11.002
Gordeeva, L.G., Restuccia, G., Freni, A., Aristov, Y.I.: Water sorption on composites “LiBr in a porous carbon.” Fuel Process. Technol. 79(3), 225–231 (2002)
Yu, Q., Zhao, H., Sun, S., Zhao, H., Li, G., Li, M., Wang, Y.: Characterization of MgCl2/AC composite adsorbent and its water vapor adsorption for solar drying system application. Renew. Energy. 138, 1087–1095 (2019). https://doi.org/10.1016/j.renene.2019.02.024
Tso, C.Y., Chao, C.Y.H.: Activated carbon, silica-gel and calcium chloride composite adsorbents for energy efficient solar adsorption cooling and dehumidification systems. Int. J. Refrig. 35, 1626–1638 (2012). https://doi.org/10.1016/j.ijrefrig.2012.05.007
Huang, H., Oike, T., Watanabe, F., Osaka, Y., Kobayashi, N., Hasatani, M.: Development research on composite adsorbents applied in adsorption heat pump. Appl. Therm. Eng. 30, 1193–1198 (2010). https://doi.org/10.1016/j.applthermaleng.2010.01.036
Grekova, A., Gordeeva, L., Aristov, Y.: Composite sorbents “li/Ca halogenides inside multi-wall carbon nano-tubes” for thermal energy storage. Sol. Energy Mater. Sol. Cells. 155, 176–183 (2016). https://doi.org/10.1016/j.solmat.2016.06.006
Grekova, A.D., Gordeeva, L.G., Lu, Z., Wang, R., Aristov, Y.I.: Composite “LiCl/MWCNT” as advanced water sorbent for thermal energy storage: sorption dynamics. Sol. Energy Mater. Sol. Cells. 176, 273–279 (2018). https://doi.org/10.1016/j.solmat.2017.12.011
Brancato, V., Gordeeva, L.G., Grekova, A.D., Sapienza, A., Vasta, S., Frazzica, A., Aristov, Y.I.: Water adsorption equilibrium and dynamics of LICL/MWCNT/PVA composite for adsorptive heat storage. Sol. Energy Mater. Sol. Cells. 193, 133–140 (2019). https://doi.org/10.1016/j.solmat.2019.01.001
Hongois, S., Kuznik, F., Stevens, P., Roux, J.J.: Development and characterisation of a new MgSO4-zeolite composite for long-term thermal energy storage. Sol. Energy Mater. Sol. Cells. 95, 1831–1837 (2011). https://doi.org/10.1016/j.solmat.2011.01.050
Chan, K.C., Chao, C.Y.H., Sze-To, G.N., Hui, K.S.: Performance predictions for a new zeolite 13X/CaCl2 composite adsorbent for adsorption cooling systems. Int. J. Heat Mass Transf. 55, 3214–3224 (2012). https://doi.org/10.1016/j.ijheatmasstransfer.2012.02.054
Oh, H.T., Lim, S.J., Kim, J.H., Lee, C.H.: Adsorption equilibria of water vapor on an alumina/zeolite 13X composite and silica gel. J. Chem. Eng. Data. 62, 804–811 (2017). https://doi.org/10.1021/acs.jced.6b00850
Teo, H.W.B., Chakraborty, A.: Water adsorption on various metal organic framework. IOP Conf. Ser. Mater. Sci. Eng. 272, 012019 (2017). https://doi.org/10.1088/1757-899X/272/1/012019
Yan, J., Yu, Y., Ma, C., Xiao, J., Xia, Q., Li, Y., Li, Z.: Adsorption isotherms and kinetics of water vapor on novel adsorbents MIL-101(Cr)@GO with super-high capacity. Appl. Therm. Eng. 84, 118–125 (2015). https://doi.org/10.1016/j.applthermaleng.2015.03.040
Elsayed, E., Anderson, P., Al-Dadah, R., Mahmoud, S., Elsayed, A.: MIL-101(Cr)/calcium chloride composites for enhanced adsorption cooling and water desalination. J. Solid State Chem. 277, 123–132 (2019). https://doi.org/10.1016/j.jssc.2019.05.026
Liu, Z., Gao, W., Qi, X., Lou, F., Lang, H.: Experimental study on salt–metal organic framework composites for water absorption. Inorg. Chim. Acta. 500, 119214 (2020). https://doi.org/10.1016/j.ica.2019.119214
Sapienza, A., Glaznev, I.S., Santamaria, S., Freni, A., Aristov, Y.I.: Adsorption chilling driven by low temperature heat: new adsorbent and cycle optimization. Appl. Therm. Eng. 32, 141–146 (2012). https://doi.org/10.1016/j.applthermaleng.2011.09.014
Aristov, Y.I., Restuccia, G., Tokarev, M.M., Buerger, H.D.D., Freni, A.: Selective water sorbents for multiple applications. 11 CaCl2 confined to expanded vermiculite. React. Kinet. Catal. Lett. 71, 377–384 (2000). https://doi.org/10.1023/A:1010351815698
Tokarev, M., Gordeeva, L., Romannikov, V., Glaznev, I., Aristov, Y.: New composite sorbent CaCl2 in mesopores for sorption cooling/heating. Int. J. Therm. Sci. 41, 470–474 (2002). https://doi.org/10.1016/S1290-0729(02)01339-X
Liu, H., Nagano, K., Togawa, J.: A composite material made of mesoporous siliceous shale impregnated with lithium chloride for an open sorption thermal energy storage system. Sol. Energy. 111, 186–200 (2015). https://doi.org/10.1016/j.solener.2014.10.044
Liu, H., Nagano, K., Sugiyama, D., Togawa, J., Nakamura, M.: Honeycomb filters made from mesoporous composite material for an open sorption thermal energy storage system to store low-temperature industrial waste heat. Int. J. Heat Mass Transf. 65, 471–480 (2013). https://doi.org/10.1016/j.ijheatmasstransfer.2013.06.021
Nakabayashi, S., Nagano, K., Nakamura, M., Togawa, J., Kurokawa, A.: Improvement of water vapor adsorption ability of natural mesoporous material by impregnating with chloride salts for development of a new desiccant filter. Adsorption 17, 675–686 (2011). https://doi.org/10.1007/s10450-011-9363-1
Alsaman, A.S., Ibrahim, E.M.M., Salem Ahmed, M., Ali, E.S., Farid, A.M., Askalany, A.A.: Experimental investigation of sodium polyacrylate-based innovative adsorbent material for higher desalination and cooling effects. Energy Convers. Manag. 266, 115818 (2022). https://doi.org/10.1016/j.enconman.2022.115818
Thu, K., Ng, K.C., Saha, B.B., Chakraborty, A., Koyama, S.: Operational strategy of adsorption desalination systems. Int. J. Heat Mass Transf. 52, 1811–1816 (2009). https://doi.org/10.1016/j.ijheatmasstransfer.2008.10.012
Wu, J.W., Biggs, M.J., Pendleton, P., Badalyan, A., Hu, E.J.: Experimental implementation and validation of thermodynamic cycles of adsorption-based desalination. Appl. Energy. 98, 190–197 (2012). https://doi.org/10.1016/j.apenergy.2012.03.022
Ng, K.C., Thu, K., Saha, B.B., Chakraborty, A.: Study on a waste heat-driven adsorption cooling cum desalination cycle. Int. J. Refrig. 35, 685–693 (2012). https://doi.org/10.1016/j.ijrefrig.2011.01.008
Mitra, S., Kumar, P., Srinivasan, K., Dutta, P.: Performance evaluation of a two-stage silica gel + water adsorption based cooling-cum-desalination system. Int. J. Refrig. 58, 186–198 (2015). https://doi.org/10.1016/j.ijrefrig.2015.06.018
Gao, W., Li, C., Xu, C., Wang, D., Wu, D.: An experimental investigation of salt-water separation in the vacuum flashing assisted with heat pipes and solid adsorption. Desalination 399, 116–123 (2016). https://doi.org/10.1016/j.desal.2016.08.016
Olkis, C., Brandani, S., Santori, G.: Cycle and performance analysis of a small-scale adsorption heat transformer for desalination and cooling applications. Chem. Eng. J. 378, 122104 (2019). https://doi.org/10.1016/j.cej.2019.122104
Olkis, C., Brandani, S., Santori, G.: Design and experimental study of a small scale adsorption desalinator. Appl. Energy. 253, 113584 (2019). https://doi.org/10.1016/j.apenergy.2019.113584
Olkis, C., Brandani, S., Santori, G.: A small-scale adsorption desalinator. Energy Procedia 158, 1425–1430 (2019). https://doi.org/10.1016/j.egypro.2019.01.345
Olkis, C., Al-Hasni, S., Brandani, S., Vasta, S., Santori, G.: Solar powered adsorption desalination for Northern and Southern Europe. Energy 232, 120942 (2021). https://doi.org/10.1016/j.energy.2021.120942
Elsayed, E., Al-Dadah, R., Mahmoud, S., Anderson, P., Elsayed, A.: Experimental testing of aluminium fumarate MOF for adsorption desalination. Desalination 475, 114170 (2020). https://doi.org/10.1016/j.desal.2019.114170
Zhang, H., Ma, H., Liu, S., Wang, H., Sun, Y., Qi, D.: Investigation on the operating characteristics of a pilot-scale adsorption desalination system. Desalination 473, 114196 (2020). https://doi.org/10.1016/j.desal.2019.114196
Kim, Y.D., Thu, K., Masry, M.E., Ng, K.C.: Water quality assessment of solar-assisted adsorption desalination cycle. Desalination 344, 144–151 (2014). https://doi.org/10.1016/j.desal.2014.03.021
Shahzad, M.W., Thu, K., Kim, Y.D., Ng, K.C.: An experimental investigation on MEDAD hybrid desalination cycle. Appl. Energy. 148, 273–281 (2015). https://doi.org/10.1016/j.apenergy.2015.03.062
Son, H.S., Shahzad, M.W., Ghaffour, N., Ng, K.C.: Pilot studies on synergetic impacts of energy utilization in hybrid desalination system: multi-effect distillation and adsorption cycle (MED-AD). Desalination 477, 114266 (2020). https://doi.org/10.1016/j.desal.2019.114266
Askalany, A., Alsaman, A.S., Ghazy, M., Mohammed, R.H., Al-Dadah, R., Mahmoud, S.: Experimental optimization of the cycle time and switching time of a metal organic framework adsorption desalination cycle. Energy Convers. Manag. 245, 114558 (2021). https://doi.org/10.1016/j.enconman.2021.114558
Ghazy, M., Ibrahim, E.M.M., Mohamed, A.S.A., Askalany, A.A.: Experimental investigation of hybrid photovoltaic solar thermal collector (PV/T)-adsorption desalination system in hot weather conditions. Energy 254, 124370 (2022). https://doi.org/10.1016/j.energy.2022.124370
Albaik, I., Badawy Elsheniti, M., Al-Dadah, R., Mahmoud, S., Solmaz, İ: Numerical and experimental investigation of multiple heat exchanger modules in cooling and desalination adsorption system using metal organic framework. Energy Convers. Manag. 251, 114934 (2022). https://doi.org/10.1016/j.enconman.2021.114934
Saleh, M.M., Elsayed, E., Al-Dadah, R., Mahmoud, S.: Experimental testing of wire finned heat exchanger coated with aluminium fumarate MOF material for adsorption desalination application. Therm. Sci. Eng. Prog. 28, 101050 (2022). https://doi.org/10.1016/j.tsep.2021.101050
Acknowledgements
This research is a part of a research project supported by the Academy of Scientific Research and Technology (ASRT) through call Egypt scientists -2. Call no. 2/2019/ASRT-Nexus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zohir, A.E., Ali, E.S., Farid, A.M. et al. A state-of-the-art of experimentally studied adsorption water desalination systems. Int J Energy Environ Eng 14, 573–599 (2023). https://doi.org/10.1007/s40095-022-00536-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40095-022-00536-y