Abstract
The use of silver nanoparticles (AgNPs) in many consumer products is expected to increase in the future due to their multifaceted properties. With the increasing demand for AgNPs, manufacturers are focusing more on exploring various methods for synthesizing AgNPs that are safer, cost-effective, and scalable. Synthesis of AgNPs using biological processes is advantageous over physical and chemical methods, primarily due to low cost and being free of hazardous chemical reagents. Among the biological processes, various groups of algae have been explored for the synthesis of AgNPs. Different biogenic substances present in the algae serve as excellent reducing and capping agents for the synthesis of NPs. Moreover, in comparison to plants and other microorganisms, algae are easy to maintain and show mass multiplication. In this review, state-of-the-art information has been collected regarding the usage of different groups of algae for the synthesis of AgNPs, their physico-chemical properties, mechanism of synthesis, and bioactivity in detail. One of the applications of AgNPs i.e., antimicrobial activity, is discussed in detail. Further, a conceptual framework regarding various techniques used for characterization of nanoparticles is described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is a growing scientific field with many beneficial roles for humans and the environment [1]. In principle, NPs are described as materials having sizes 1–100 nm at least in one dimension. The emergence of nanoparticles (NPs) with unique functions and size-dependent physio-chemical properties differing from the bulk form make them useful for various applications [2]. Smaller-sized NPs display a higher surface versus volume ratio, an essential characteristic for catalytic reactivity, chemical stability, thermal conductivity, nonlinear optical performance, and antimicrobial activity. These features make NPs play an important role in the medical field diagnostics, drug delivery systems, gene therapy, cancer therapy, tissue engineering, forensic, agriculture, wastewater treatment, hygiene products, development of biosensors, printing of holograms, and a variety of coating areas including energy contact actions, optoelectronics, electronics, automobiles, etc. [3, 4].
Nanoparticles (NPs) are mainly classified into different categories. (1) One type is organic NPs (which have carbon NPs). Examples of carbon-based NPs are fullerenes, carbon nanotubes, carbon nanofibers, and lampblack graphene. Organic NPs also include dendrimers, liposomes, micelles, polymer NPs, etc. (2) Another type is inorganic NPs that include noble metal NPs, magnetic NPs, and semiconductor NPs. Inorganic-based NPs include metals such as Ag, Au, and metal oxides such as iron oxide and titanium dioxide. [5]. (3) A third type is composite NPs which include a combination of carbon-based, metal-based, or organic-based NPs with any sort of metal, ceramic, or polymer bulk materials [6].
NPs are synthesized via physical, chemical, and biological ways. Laser ablation, thermoreduction, ultrasonication, and electrochemical and microwave-assisted synthesis methods are used in physical processes. While synthesizing NPs via physical methods needs high-energy inputs in the form of pressure and temperature, chemical methods mainly use various reagents for reduction/oxidation through the sol–gel processes, atomic or molecular condensation, chemical etching, sputtering, and spray pyrolysis. At an industrial scale, NPs are synthesized by chemical and physical methods. However, in the chemical process, many toxic reagents are considered unsafe, wherein the physical form uses high-energy inputs and incurs high manufacturing costs [1, 7].
In recent years, efforts have been concentrated on developing methods to fabricate safe and low-cost NPs. The biogenic synthesis of NPs has been proposed as an environmental and cost-effective alternative to the physical and chemical methods [8]. In this way, the biological process, known as bio-nanofabrication, is attempted to synthesize safe, biocompatible, and cost-effective NPs [9, 10]. Biologically synthesized NPs of metals such as gold, silver, copper, iron, zinc, and titanium are used in various biomedical applications including magnetically reactive drug delivery vehicles, photothermal therapy, contrast enhancer for molecular imaging, etc.
Biosynthesized AgNPs are more acceptable for medical applications due to their superior biocompatibility than chemically synthesized ones [3]. AgNPs are easy to synthesize, have tunable photophysical attributes, chemical stability, good conductivity, high thermal conductivity, and high catalytic and antimicrobial activity than any other noble metals. AgNPs have various biological applications, including bioimaging, biosensors, gene delivery, drug delivery, catalysis, and antioxidant activity [11]. Further, AgNPs play an important role as biomedicine against drug-resistant bacteria [12]. Ag is generally considered a noble metal used to treat burns, open wounds, and cuts. Applications of AgNPs in nanogels, nano solutions, Ag-based dressings, and coating of medical devices are also in progress [13]. Ag can act as a medicine in its most varied forms in ionic, colloidal, combined form, or the form of NPs. This element has potentially been used for a series of treatments of diseases, including cancer, malaria (vector control), and inflammation, mainly in the uterus [14]. Further, the AgNPs have received attention in the area of wastewater treatment, food packaging, cosmetic products preservation, agriculture, etc. [13, 15, 16].
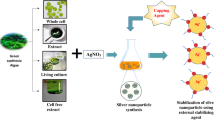
During the green synthesis of AgNPs, different biomolecules present in the extract act as stabilizing and/or capping agents, reducing the Ag+ to Ag0 [17]. Various studies revealed that plant phytochemicals such as ascorbic acid, apiin, cyclic peptide, citric acid, epicatechin gallate, ellagic acid, gallic acid, galangin, pinocembrin, phyllanthin, sorbic acid, retinoic acid, and theaflavin had been recognized as responsible agents for the synthesis of AgNPs [18].
Algae are a rich source of biomolecules, as they contain various carbohydrates, fats, nucleic acids, pigments, proteins, and secondary metabolites such as some aromatic compounds, macrolides, terpenes, and peptides [19]. Synthesis of NPs utilizing algae is advantageous over other methods, including plant extract due to shorter duration and minimum use of solvents/reagents, and accordingly considered as cost-effective and safe [20, 21]. Due to the rich, diverse chemical ecology of algae, significant research efforts are needed to understand the precise mechanism of how algae are involved in the synthesis of metallic NPs in a more effective manner, with advantages over other organisms in the syntheses of NPs [12]. Many studies have been carried out over the years on algae for NP synthesis. However, there is a need to critically analyze the information of various algae groups used to synthesize AgNPs to understand the role and efficacy of synthesis decisively.
Further, more insight is required into how the bioactivity of algae molecules influences the various physico-chemical properties, viz, size, shape, functional groups, etc. It is worth studying AgNPs through algae customized for shape, size, and other valuable properties for better use [19, 22]. This review attempts to compute and analyze the state-of-the-art information of understanding the synthesis mechanism and factors involved in NPs synthesis through various groups of algae. Further, the surface chemistry of AgNPs leading to bioactivity will also be discussed [23].
Synthesis of silver nanoparticles mediated by algae
Many studies have been conducted to evaluate the potential of different groups of algae for the synthesis of AgNPs. The following section discusses the various parameters that influence the size and shape of the AgNPs during biogenic synthesis using algal extract.
Cyanobacteria
Although cyanobacteria are not per se classified under the algae, since they are photosynthetic prokaryotes, we have included the studies of cyanobacteria in this discussion. The aqueous extract of Trichodesmium erythraeum is used for the synthesis of AgNPs. The synthesized AgNPs show cubical and crystalline shapes of 26.5 nm size. The antibacterial activity of AgNPs is also observed against Proteus mirabilis and Staphylococcus aureus (clinical strains), S. pneumoniae (penicillin R), S. aureus (tetracycline R), and E. coli (amikacin R) (drug-resistant bacterial strains). The maximum anti-cancerous effect was observed at 50 mg concentration against HeLa and MCF-7 cell lines, and the IC50 values were 25.0 ± 0.50 mg/ml and 30.0 ± 0.50 mg/ml, respectively, at 24 h [24]. In another study, the cell-free aqueous extract of Microchaete NCCU-342 (cyanobacteria) was found to reduce agents for the synthesis of AgNPs. The AgNPs were synthesized at an optimized 1 mM AgNO3, biomass quantity of 80 μg/ml, duration of 60 min UV light exposure, the temperature of 60 °C, and pH 5.5. The NPs synthesized were found to be spherical in shape and their size ranges from 60 to 80 nm. The study also found that AgNPs have shown appreciable dye discoloration capacity compared to cyanobacterial extract. Hence, AgNPs can mitigate the contamination from the dye [25]. In another report, methanol extract of Oscillatoria sp. was used for the synthesis of AgNPs. In this report, synthesized NPs were spherical and were found to be about 10 nm in size. Further, these NPs showed antibacterial and antibiofilm activity. The antibiofilm activity was reported against S. aureus ATCC 29213, E. coli ATCC 35218, P. aeruginosa ATCC27853, Citrobacter sp., S. typhi ATCC 14028, E. coli ATCC 11775, and Bacillus cereus. Maximum inhibition was observed against Pseudomonas aeruginosa ATCC 27853, and the minimum inhibition was observed against Citrobacter sp. [26]. These studies revealed that several parameters such astemperature, species, pH, the concentration of AgNO3, etc., during synthesis influence the shape and size of the AgNPs, thereby changing the application scenario.
Green algae
Many studies have been conducted to evaluate the potential of green algae for the synthesis of AgNPs (Table 1). In one study, a fresh extract of Caulerpa racemose (marine green algae) was used to synthesize AgNPs and later analyzed for their antibacterial activity. The SPR shows a peak at 413 nm, and synthesis was monitored by UV–Vis spectroscopy. The FTIR analysis revealed that the functional group (peptides), present in the extract of Caulerpa racemosa, acts as a stabilizing and reducing agent for AgNPs. The characterization techniques unravel that the synthesized NPs were crystalline with FCC geometry with 5–25 nm in size. These AgNPs showed antibacterial activity against human pathogens such as Proteus mirabilis and Staphylococcus aureus. The zeta potential of the particles was −16 mV, indicating moderate stability of the AgNPs in the environment [27]. In another study, Chlorella vulgaris was explored to reduce Ag ion, and the size of the synthesized AgNPs was reported to be in the range of 8–20 nm with a mean size of 12.62 nm [28]. A facile, green method has been demonstrated for biogenic synthesis of highly stable AgNPs using an aqueous extract from Caulerpa racemosa. The AgNPs synthesized are very durable and protected by various phytochemicals of Caulerpa racemosa. The AgNPs synthesized were highly crystalline and mainly oriented in the plane with the FCC geometry, supported by the XRD and SAED models. The average particle size of the synthesized AgNPs is 25 nm with a deformed spherical shape identified from HR-TEM. Besides, AgNPs have significant catalytic activity toward the degradation of methyl bromide. Thus, the synthesized AgNPs can be used as an active catalyst to degrade methyl bromide by NaBH4 [29]. The bio-reduction of aqueous Ag ions into AgNPs using Spirogyra varians has also been demonstrated. The procedure applied is simple, economical, and environmental friendly. The average size of the synthesized NPs was 17.6 nm, and the SEM image confirmed the synthesis of relatively uniform NPs. The antibacterial effect of AgNPs was also tested on several microorganisms by measuring the zone of inhibition, MIC, and MBC. The results confirmed that NPs could act as a powerful antibacterial agent against various pathogenic bacteria [30]. The algal extract of Chlorella vulgaris was found to act as a capping, reducing, and stabilizing agent for the synthesis of AgNPs, and synthesized AgNPs were used for the synthesis of quinolines. The synthesis of AgNPs from C. vulgaris algae and its application is a new synthetic approach for the biocatalytic conversion of 2-aminobenzyl alcohol into important substituted quinolines [31]. In another recently performed study, a green alga Botryococcus braunii was used for the synthesis of AgNPs. As revealed by SEM, the synthesized NPs showed a peak at 490 nm and were about 88 nm in size. FTIR analysis revealed that the polysaccharides, long-chain fatty acids, proteins, and amides acted as reducing, stabilizing, and capping agents. These AgNPs were used as a catalyst for synthesizing 2-arylbenzimidazoles and utilized to reduce nitroaniline [32]. The synthesis of AgNPs from the dried biomass of Chlorella ellipsoidea was confirmed by absorbance at 436 nm on the UV–Vis spectrum. FTIR analysis revealed that the flavonoid/phenolic groups are responsible for the synthesis of AgNPs. The AgNPs synthesized are mainly spherical in shape and crystalline in nature. The average size of the AgNPs was found to be 220.8 ± 31.3 nm with a polydispersity index of 0.408. The antibacterial activity of the synthesized AgNPs showed significant activity against one Gram-positive bacteria, S. aureus, and three Gram-negative bacteria, E. coli, K. pneumoniae, and P. aeruginosa, by the disc diffusion method [33]. Likewise, an extract of Chlorella vulgaris was used for the synthesis of AgNPs. Characterization of synthesized AgNPs was carried out by UV–Vis spectrophotometry, SEM, TGA, DLS, EDX, and XRD. UV–Vis spectrophotometry showed a peak at 300 nm. DLS measurement showed that the synthesized AgNPs were 82.19 and 505.3 nm with a polydispersity index of 0.505. The AgNPs were thermally stable and showed antibiofilm and antibacterial activities [34].
Brown algae
Brown algae are primarily marine and have unique biochemistry with regard to cell wall composition (having alginic and fucinic acid), pigment (Chl-a, Chl-c, violaxanthin, fucoxanthin, etc.), and stored food (laminarin, mannitol, etc.). Therefore, the extract of brown algae is expected to have a different profile, and utilizing the potential of that extract might be important. In this direction, Dhas et al. reported the synthesis of AgClNPs using an aqueous extract of the brown alga, Sargassum plagiophyllum, in 1 mM AgNO3 treated for 24 h at RT. The size of the spherical AgClNPs varied from 18 to 42 nm with an λmax at 417 nm. The chlorine content of the extract was attributed to the formation of AgClNPs. In this study, the AgClNPs have been shown to inhibit Gram-negative bacteria, E. coli, in a dose-dependent manner [35].
Similarly, Rajesh Kumar et al. reported that exposure time and pH play an essential role in controlling the synthesis of NPs using an aqueous extract of Sargassum longifolium. The maximum biosynthesis of spherical AgNPs was at pH 8.4 after 64 h, as observed by a color change from yellow to brown and an λmax at 460 nm. In this investigation, the biomedical potential of the particles for their antifungal activity was evaluated against three pathogenic fungi, Candida albicans, Aspergillus fumigatus, and Fusarium sp. [36]. The potential of brown algae is not fully explored. More investigation is required to understand the role of different biochemical profiles of brown algae for the green synthesis of AgNPs.
Red algae
The red algae have a different set of dominant pigments (Chl-a, Chl-d, and r-phycoerythrin), and floridean starch is the reserve food material. Therefore, investigating the potential of red algae for the biogenic synthesis of AgNPs is of considerable interest. To understand this potential, the aqueous extract of Turbinaria conoides (seaweed) was used as an effective reducing and capping agent for the biosynthesis of AgNPs and AuNPs [37]. The AgNPs have been biosynthesized by macro marine algae, such as Ulva fasciata, Gelidium crinale, Corallina elongate, Cystoseira myrica, Laurencia obtuse, and Turbinaria turbinata. The AgNPs, biosynthesized by Turbinaria turbinata, were the most cytotoxic against Ehrlich ascites carcinoma (EAC). The AgNPs biologically formed by Turbinaria turbinata have uniform shape and are very small in size, and these properties indicate these AgNPs as having marked cytotoxic activity [38]. The AgNPs synthesized using an extract of Laurencia aldingensis and Laurenciella sp. were characterized by UV–Vis spectroscopy, XRD, EDX, HR-TEM, and zeta potential measurements. Besides, cell viability was tested to assess the cytotoxicity of these AgNPs on the P4 human foreskin fibroblast cell line and in human uterine sarcoma (MES-SA) and the corresponding doxorubicin-resistant mutant MES-SA/Dx5 cells. High toxicity was observed in the two cancer cell lines tested with IC50 values ranging from 1 to 4 μM; however, there was no toxicity in P4 cells. The biological method allows the use of renewable and disposable algae extracts, which leads to the preparation of AgNPs with remarkable cytotoxicity against sarcoma tumor cells [39]. These different studies revealed the potential of other algal groups for the biogenic synthesis of AgNPs (Table 1). Based on these studies, a consensus reduction mechanism was drawn and is explained in the next section.
Reduction mechanism during the biogenic synthesis of AgNPs
The synthetic/chemical approach involves the synthesis of metal NPs in the solution by employing three significant components: (1) metal precursors, (2) reducing agents, and (3) stabilizing agents or capping agents. The most commonly used reducing agents in these methods are sodium citrate, sodium borohydride, ethylene glycol, and glucose. These reducing agents are responsible for reducing metal ions, followed by nucleation and finally lead to metal NPs. The stabilizing agents are added during the preparation, which aids the growth of NPs and protects the NPs from forming agglomeration.
AgNO3 acts as a precursor in this chemical reaction, and NaBH4 acts as a reducing agent [40]. The chemical method utilizes high-energy and toxic chemicals. In the biological process, natural reducing and capping agents are used to synthesize NPs without any harmful by-products (Fig. 1). Different biomolecules are present in the extract of the other organisms (algae, plant, fungi, bacteria, etc.), which act as a reducing agent to reduce metal salt into metal NPs [41].
Biomolecule serves as a reducing agent for reducing the Ag+ ion into neutral Ag0 during the initial steps of the synthesis of AgNPs. Reducing agents provide electrons to Ag+ and release hydrogen atoms in the reaction medium, which causes the acidification of the media. The neutral Ag0 is then partially covered up by these reducing agents, which now serve as a capping agent. Therefore, during the green synthesis of NPs, a cocktail of biomolecules that initially serves as reducing agents can be used as a capping agent. In contrast, during chemical synthesis, separate reducing agents and capping agents are employed to synthesize NPs. Partially capped Ag particles result in crystal structures after nucleation and these grow in size by adsorption of more Ag particles. Capping agents stabilize these crystal structures of nano-size particles (Fig. 2). Reduction of Ag+ into Ag0can be monitored and confirmed by the color change of the solution using UV–Vis spectra [42]. X-ray photoelectron spectrophotometry can be employed to monitor the reduction reaction of Ag+ into Ag0 [43]. AgNO3 itself is gradually reduced in the water, while in reducing agents the rate of reduction rapidly increases [44]. ICP-AES is mainly used to measure the alteration of Ag+ to AgNPs [45].
During the biogenic synthesis of NPs, many different kinds of natural components act as reducing agents for Ag+, such as amino acids, proteins, enzymes, vitamins, alkaloids, flavones, terpenoid, phenolic, tannins, saponins, polysaccharides, and alcoholic components [42]. In the membrane of algae, polysaccharides, proteins, and lipids are present, which act as reducing agents. Functional group of alkaloids, flavones, and anthracenes, such as –C–O–C–, –C–O–, –C = C–, and –C = O–, act as a reducing agent. Different agents such as enzymes, citric acid, polyphenols, biodegradable polymers, silica, and vitamins (B, C, D, and K) can stabilize and functionalize the NPs without unfavorable effects on the environment and biosynthesis [46]. Metallic NPs, viz., copper, cobalt, silver, gold, platinum, palladium, iron, zirconium, cadmium, and metal oxides such as zinc oxide, titanium oxide, magnetite, etc. have been the particular focus of green biosynthesis [47].
The coat of biomolecule on the surface of NPs makes them biocompatible compared to the NPs synthesized by chemical strategies [47]. The biocompatibility of NPs plays a significant role in biomedicine and related fields [41]. Progress in nanotechnology has enabled us to modify the surface of NPs with various moieties, including chemical and functional groups, radioactive agents, and biological molecules according to the target application [48]. The synthesized NPs have been used to target cancer sites by modifying the surface with various moieties such as proteins, antibodies, and aptamers. Aptamers are short single-stranded oligonucleotides that are less immunogenic. In the cancer cell membrane, highly expressed AS1411 protein-specific aptamer exhibited conjugation and were internalized in cancer cells [49]. Therefore, it is possible to design the surface chemistry of NPs in such a way that they can bind specifically to the cancerous cells and destroy them. Research in this direction of making designer NPs is required in the future.
Antimicrobial activity of AgNPs
The AgNPs have a broad spectrum of antibacterial, antiviral, and antifungal properties. The NPs of silver can penetrate bacterial cell walls, alter the structure of cell membranes, and even cause cell death. Their efficiency is due to their nano-scale size and their large surface area to volume ratio. They can increase the permeability of cell membranes, produce reactive oxygen species, and interrupt deoxyribonucleic replication by releasing silver ions [50]. The interaction between AgNPs and microorganisms begins with the adhesion of AgNPs to the wall and membrane of microbial cells, which is based on the electrostatic attraction between the negatively charged microbial cell membrane and positively or less negatively charged AgNPs [51]. During such attraction and interaction, morphological changes in membrane structure are triggered by the nanoparticle, thus leading to disruption of membrane permeability and respiratory functions via membrane depolarization, and finally to the disruption of cell integrity and cell death [52]. Uptake of NPs by bacteria occurs via diffusion, endocytosis, phagocytosis, etc. [53].
Further, AgNP uptake is possible via the flip-flop mechanism of a membrane or direct penetration via an ion channel (Fig. 3). Active transport also exists with passive transport. Due to increased membrane permeability and disruption of the cell wall, the cell content, including proteins, enzymes, DNA, ions, metabolites, and energy reservoir, also leaks into the environment. Therefore, the disintegration of the cell wall by adhesion of NPs is believed to be the primary mechanism of antimicrobial action. Also, AgNPs have been shown to cause irregular well formations on the cell wall, allowing NPs to enter the periplasmic space and finally inside the cell [54]. After a brief contact of AgNPs with bacterial cells, peripheral damage and dense pits on the cell surface and structural changes in bacterial cell morphology were observed [52].
Toxicity mechanism behind the antibacterial activity of AgNPs. Ag ions can be entered inside the cell via ion channel or by diffusion or leakage, etc. Once inside, Ag ion can inhibit the electron transport chain, disrupt the double-helical structure of the DNA, inhibit the protein synthesis, and hamper the enzymatic activity. Further, increase in Ag ion concentration can lead to an increase in reactive oxygen species (ROS) and disrupt the signal transduction pathway
Gram-negative bacteria are more sensitive to AgNPs because the cell wall of Gram-negative bacteria is thinner than that of Gram-positive strains. The thick cell wall can reduce the penetration of NPs into cells [55]. Additionally, the role of capping agents acts multidimensionally in a way that they (1) prevent the agglomeration of the NPs, (2) enhance antimicrobial action, and (3) reduce toxicity [52]. Capping agents create electrostatic and electrosteric repulsions between particles to prevent aggregation of AgNPs [56]. When an excess of AgNPs is present in the cells, it interacts with biomolecules such as proteins, lipids, and carbohydrates and triggers the signaling pathway to generate ROS. ROS are oxygen-containing molecules with high redox potential. Under normal conditions, the production of ROS and the antioxidant capacity of the cell are balanced. However, if there is an imbalance between the antioxidant mechanism and excessive ROS production, the redox balance of the cell promotes oxidation, leading to oxidative stress [57]. ROS is responsible for the denaturation of protein and nucleic acid damage due to the strong affinity of silver with sulfur and finally causes apoptosis. After that, inhibition of cell proliferation occurs. The AgNPs release Ag+ in or outside of the bacteria. The smaller size of AgNPs with high surface area results in faster Ag+ release than large size AgNPs with a low surface area. Ag+ can interact with sulfhydryl groups in protein and enzymes [58]. ROS is a primary agent in causing cell membrane disruption and modification of DNA. As sulfur and phosphorus are essential components of protein and DNA, the interaction hinders DNA replication, cell reproduction or even leads to the termination of transcription of microorganisms. Also, Ag+ can inhibit protein synthesis by denaturation of ribosomes in the cytoplasm [50]. AgNPs can interfere with bacterial signal transduction. Bacterial signal transduction is influenced by phosphorylation of protein substrates, and NPs can dephosphorylate tyrosine residues on peptide substrates. Disruption of signal transduction can lead to cell apoptosis and termination of cell multiplication [59].
Techniques used for characterization of nanoparticles
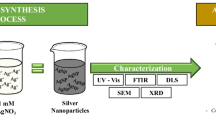
Important properties of NPs such as size, shape, crystal nature, surface properties, aggregation state, solubility, structure, and chemical composition play an important role in downstream application in different biotechnological, biomedical, and environmental fields [60]. Many analytical techniques have been used to assess the synthesized nanomaterials, including the color change of the solution, which is monitored by ultraviolet–visible spectroscopy (UV–Vis spectroscopy). For the characterization of NPs, some techniques that are reported in the scientific literature are transmission electron microscopy, scanning electron microscopy, X-ray diffraction, Fourier transform infrared spectroscopy, zeta potential, etc. We can determine the size and shape and crystal nature by these techniques. Characterization of AgNPs after synthesis, such as shape, size, size distribution, solubility, aggregation, and surface, is necessary because physico-chemical properties of a particle could have a significant impact on their biological properties and should be assessed before assessing toxicity or biocompatibility [3]. In this section, details of these individual techniques are discussed.
UV–visible spectroscopy
UV–Vis spectroscopy is usually the first technique to confirm the formation of NPs due to change in the chromatic property during synthesis. The synthesis of AgNPs can be confirmed by visual observation of the mixture of pale yellow to dark brown; the color change is due to the variation of the surface of plasmon [61]. The bio-reduction of Ag ions to nano-Ag in the solution has been characterized using UV–visible spectrophotometer at a different wavelength range of 300–700 nm, with the standard peak value for monitoring of synthesis of AgNPs ranges between 415 and 417 nm [62]. NPs with unique optical properties show a surface plasmon resonance (SPR) effect due to the excitation of an electron on the metal surface. The excitation varies depending on the shape, size, and concentration of the metal ions that have been studied by using UV–Vis spectra [63]. Spectrophotometric absorbance peak observed at a specific wavelength, due to the phenomenon of SPR presented by the AgNPs, showed the production of AgNPs [64]. The spectroscopy works dependent on the standard of the light retained or dispersed by the metal NPs in the UV and visible region of the electromagnetic spectrum. The metal NPs with novel optical properties display surface plasmon resonance (SPR) impact because of the excitation of an electron on the metal surface.
X-ray diffraction (XRD) analysis
X-ray beam diffraction is a popular technique used to analyze the geometry of the unknown sample. X-ray diffraction is based on the phenomenon of constructive interference produced by monochromatic X-rays and material. X-rays are produced by a cathode ray tube. These X-rays are filtered to produce monochromatic radiation and then paralleled to concentrate and directed toward a material. Powder forms of NP are analyzed between the range of 2–80 θ, and the corresponding peaks produced are recorded. The crystalline nature of the sample can be recognized from their diffraction patterns according to the law of Bragg [53], and the results are analyzed.
Law of Bragg: 2d sinө = nλ, where d is the spacing between the diffracting planes, ө the incident angle, n any integer, and λ the wavelength of the beam.
This technique works based on the principle that when a beam of X-rays with a wavelength range of 0.5–2 Å is incident on the material, it gets diffracted by its crystalline surfaces. It additionally provides information about the scattering peaks, which corresponded to an interplanar spacing of the crystal lattice. This technique also analyzed the crystal size by using the Scherrer equation [63]:
where 0.9 is the shape factor, λ is the wavelength of X-ray, β is the line broadening at half the maximum intensity in radians, and θ is the Bragg angle.
In one of the studies published recently, the XRD analysis revealed the crystalline nature of the synthesized AgNPs. Four significant Bragg's reflections detected are at 37.53, 44.16, 66.22, and 76.74, corresponding to the planes 111, 200, 220, and 311, respectively. These peaks are well attributed by the Joint Committee on Powder Diffraction Standards (JCPDS) of the AgNPs of the face-centered cubic (FCC) crystal lattice structure (JCPDS no. 01-1164). The average crystallite size of the synthesized AgNPs is considered by using the Debye–Scherer equation [33].
Fourier transform infrared spectroscopy (FTIR)
During FTIR analysis, NPs absorb electromagnetic energy in the infrared region of the spectrum and cause the subatomic molecules to vibrate. These vibrations are quantized at specific positions, and the absorption is designated by a particular wavenumber. This wavenumber has a range from 4000 to 400 cm−1. A recorded spectrum shows the position of bands related to the nature and strength of bonds and specific functional groups [65]. The size of the NP depends on the intensity of the peaks, and the shape of the NPs depends on the position of the band obtained in the FTIR spectrum [63]. Samples are prepared by mixing 1% (w/w) specimens with 100 mg of potassium bromide (KBr) powder and pressing the mixture into a thin slice. The samples are characterized by FTIR using absorbance mode and KBr pellet under 1:99 ratio of sample and potassium bromide [66, 67]. Then, the spectrum is recorded at a resolution of 4 cm−1 [67]. FTIR spectrum reveals the presence of functional groups in the biomolecule. Biomolecules that are responsible for the reduction of the Ag ions and capping of the reduced AgNPs are analyzed by FTIR [68]. FTIR analyses have revealed the presence of functional groups of carbonyl/amine/hydroxyl, which are involved in the reduction and capping of NP during the synthesis process [23].
Transmission electron microscopy (TEM)
This technique is based on the principle that an electron beam transmitted through the surface of the NPs and the interaction of transmitted electrons create an image. This investigation provides information about the particle shape and size [63]. For this purpose, stock solutions are prepared with 10 mg of AgNPs in 100 ml deionized water. Then samples are sonicated for about 5 min. Then two drops of the diluted sample are deposited on a carbon-coated copper TEM grid and allowed to dry before measurement. After that, the high-resolution images obtained with a transmission electron microscope are analyzed for particle size, shape, and morphology of metal NP [69]. The size of the NPs’, mono-dispersity, shape, aggregation state, etc., are detected using TEM. Further, a selected area diffractogram is used to analyze the crystalline structure of the synthesized AgNPs [70].
Scanning electron microscopy (SEM)
SEM is the technique used for studying the morphology and surface topology of the material. In this technique, a sample is made into thin films by placing it on a carbon-coated copper grid. The morphological characters of the synthesized AgNPs are analyzed from SEM [62]. When an electron beam is incident on the material, interaction happens, which causes the emission of secondary electrons, backscattered electrons, and auger electrons. The emission of such electrons depends on the surface geometry of the material and its chemical composition. These collections of electrons are detected in scanning electron microscopy, and the produced signal gives information about the material. The imaging of the surfaces is done with a resolution of about 1 nm, and it depends on the electron probe and their interaction with the material. Due to its high-resolution capacity, this technique has been efficiently utilized to characterize NPs [63].
Energy-dispersive radiographic analysis (EDAX)
The presence of element Ag in the sample can be further characterized by energy-dispersive spectroscopy (EDS) or energy-dispersive radiographic analysis (EDAX) technique [62]. Predictable percentages of carbon, oxygen, chlorine, potassium, etc., indicate the presence of biomolecules, while the carbon-coated copper grids used in the EDX analysis are attributed to the carbon and copper signals [33].
Zeta potential
Zeta potential is an essential parameter to analyze the stability and aggregation of the NPs in a dispersed state using the Zeta Sizer instrument. The electrostatic interaction between the surface charges of the NP with the oppositely charged ions present in the solution creates a double layer of ions, and the electric potential at the boundary of this layer is termed as zeta potential. The values are estimated in the range of + 100 mV −100 mV. NPs with high degrees of stability are detected in the range greater than +25 mV or less than −25 mV [63].
Dynamic light scattering (DLS)
Dynamic light scattering is one of the most common methods used to determine the size of NPs within a suspension. The technique works on changing the Brownian movement of NPs caused by the penetration of laser light. Light transmission causes Doppler shift relative to particle size. The shifts are calculated to assess the particle size by measuring the coefficient of distribution by the autocorrelation function. The average particle size, the polydispersity indicator, and the zeta potential of the synthesized NPs are obtained by DLS [66].
Conclusion
Different algae have been investigated to synthesize AgNPs of various physico-chemical properties with assorted bioactivity such as antimicrobial, anticancerous effect against different cell lines, and so forth. The main advantage of the AgNPs synthesized by algae is the diversity of the size and shape utilizing different algae groups, indicating that distinctive reducing/capping agents are engaged in the synthesis process. This review reasoned that varieties of functional groups in various biomolecules of algae-like proteins (–OH−), carbohydrates (–COO−, OH−, C–O–C), alkynes in lipids, secondary metabolites (alkaloids, flavonoids, polyphenols; –OH−, –C–O–C–, –C–O–, –C = C–, –C = O–), and so forth act as reducing and capping agent. Further, it is discovered that synthesizing AgNPs using green algae (Chlorophyceae group) brings about the formation of relatively smaller NPs, as can be observed in most of the reports (Table 1), while other groups of algae and cyanobacteria ensue synthesis of moderately larger NPs. Our discussion further envisages that diverse algae groups can synthesize AgNPs with greater control over stability, size, shape, and functional groups. Consequently, these AgNPs can be applied more copiously in different applications, including bioimaging, biosensors, gene delivery, catalysis, antimicrobials, antioxidants, and anticancer agents. With the advancement in AgNPs applications, the current issues in medical sciences such as microbial drug resistance, targeted drug delivery, upgradation in the current regime, diagnosis, and imagining technologies have been improved.
Nanotechnology has apparently provided much improvement in many sectors, and it is anticipated that targeted delivery using nanosized materials can completely cure incurable diseases such as HIV and cancers without harming unnecessary cells [71]. However, there are still multiple disadvantages of biologically synthesized AgNPs. Future investigation should focus on mitigating shortcomings of this technique such as different parameters including pH, the concentration of metal solutions, and the concentration of algal extract, which directly affect the synthesis of AgNP synthesis, thus influencing their shape and size [71]. The biological method for the synthesis of AgNP is a slow process compared to the chemical and physical methods. Future studies should focus on reducing the reaction time for the synthesis of nanoparticles, which will make the process easier to scale up [72].
Availability of data and material
Not applicable.
Abbreviations
- AgClNPs:
-
Silver chloride nanoparticles
- AgNO3 :
-
Silver nitrate
- AgNPs:
-
Silver nanoparticles
- AuNPs:
-
Gold nanoparticles
- DLS:
-
Dynamic light scattering
- DNA:
-
Deoxyribonucleic acid
- EDAX:
-
Energy-dispersive radiographic analysis
- EDS:
-
Energy-dispersive spectroscopy
- EDX:
-
Energy-dispersive X-ray
- FCC:
-
Face-centered cubic
- FTIR:
-
Fourier-transform infrared spectroscopy
- HR-TEM:
-
High-resolution transmission electron microscopy
- ICP-AES:
-
Inductively coupled plasma atomic emission spectroscopy
- JCPDS:
-
Joint Committee on Powder Diffraction Standards
- KBr:
-
Potassium bromide
- MBC:
-
Minimum bactericidal concentration
- MIC:
-
Minimum inhibitory concentration
- NaBH4 :
-
Sodium borohydride
- NMs:
-
Nanomaterials
- NPs:
-
Nanoparticles
- ROS:
-
Reactive oxygen species
- S:
-
Spectrophotometry
- SAED:
-
Selective area electron diffraction
- SEM:
-
Scanning electron microscopy
- SPR:
-
Surface plasmon resonance
- TEM:
-
Transmission electron microscopy
- TGA:
-
Thermogravimetry
- UV–Vis spectroscopy:
-
Ultraviolet–visible spectroscopy
- XRD:
-
X-ray diffraction
References
Chugh, H., Sood, D., Chandra, I., Tomar, V., Dhawan, G., Chandra, R.: Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 46, 1210–1220 (2018)
Arya, A., Mishra, V., Chundawat, T.S.: Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chem. Data Collect. 20, 100190 (2019)
Abdelghany, T.M., Al-Rajhi, A.M.H., Al Abboud, M.A., Alawlaqi, M.M., Magdah, A.G., Helmy, E.A.M., Mabrouk, A.S.: Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A review. Bionanoscience 8, 5–16
Patel, V., Berthold, D., Puranik, P., Gantar, M.: Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 5, 112–119 (2015)
Pulit-Prociak, J., Banach, M.: Silver nanoparticles–a material of the future…? Open Chem. 14, 76–91 (2016)
Jeevanandam, J., Barhoum, A., Chan, Y.S., Dufresne, A., Danquah, M.K.: Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 9, 1050–1074 (2018)
Valodkar, M., Modi, S., Pal, A., Thakore, S.: Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: a green approach. Mater. Res. Bull. 46, 384–389 (2011)
Varghese, R.A., Anandhi, P., Arunadevi, R., Boovisha, A., Sounthari, P., Saranya, J., Parameswari, K., Chitra, S.: Satin leaf (Chrysophyllum oliviforme) extract mediated green synthesis of silver nanoparticles: antioxidant and anticancer activities. J. Pharm. Sci. Res. 7, 266 (2015)
Dhuper, S., Panda, D., Nayak, P.L.: Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano Trends J. Nanotech. Appl. 13, 16–22 (2012)
Nindawat, S., Agrawal, V.: Fabrication of silver nanoparticles using Arnebia hispidissima (Lehm.) A. DC. root extract and unravelling their potential biomedical applications. Artif. Cells Nanomed. Biotechnol. 47, 166–180 (2019)
Netala, V.R., Bukke, S., Domdi, L., Soneya, S., Reddy, S., Bethu, M.S., Kotakdi, V.S., Saritha, K.V., Tartte, V.: Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif Cells Nanomed. Biotechnol. 46, 1138–1148 (2018)
Vincy, W., Mahathalana, T.J., Sukumaran, S., Jeeva, S.: Algae as a source for synthesis of nanoparticles—a review. Int. J. Latest Trends Eng. Technol. 5, 005-009 (2017)
Shanmuganathan, R., Karuppusamy, I., Saravanan, M., Muthukumar, H., Ponnuchamy, K., Ramkumar, V.S., Pugazhendhi, A.: Synthesis of silver nanoparticles and their biomedical applications—a comprehensive review. Curr. Pharm. Des. 25, 2650–2660 (2019)
Venkatesan, J., Kim, S.-K., Shim, M.S.: Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 6, 235 (2016)
Carbone, M., Donia, D.T., Sabbatella, G., Antiochia, R.: Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 28, 273–279 (2016)
Gajbhiye, S., Sakharwade, S.: Silver nanoparticles in cosmetics. J. Cosmet. Dermatol. Sci. Appl. 6, 48–53 (2016)
Zewde, B., Ambaye, A., Stubbs Iii, J., Raghavan, D.: A review of stabilized silver nanoparticles–synthesis, biological properties, characterization, and potential areas of applications. Nanomedicine 4, 1–14 (2016)
Rajan, R., Chandran, K., Harper, S.L., Yun, S.-I., Kalaichelvan, P.T.: Plant extract synthesized silver nanoparticles: an ongoing source of novel biocompatible materials. Ind. Crops Prod. 70, 356–373 (2015)
Siddiqi, K.S., Husen, A.: Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Res. Lett. 11, 363 (2016)
Thakkar, K.N., Mhatre, S.S., Parikh, R.Y.: Biological synthesis of metallic nanoparticles. Nanomedicine 6, 257–262 (2010)
Rauwel, P., Küünal, S., Ferdov, S., Rauwel, E.: A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mater. Sci. Eng. 2015, 1–9 (2015)
Schexnailder, P., Schmidt, G.: Nanocomposite polymer hydrogels. Colloid Polym. Sci. 287, 1–11 (2009)
Das, C.G.A., Kumar, V.G., Dhas, T.S., Karthick, V., Govindaraju, K., Joselin, J.M., Baalamurugan, J.: Antibacterial activity of silver nanoparticles (biosynthesis): a short review on recent advances. Biocatal. Agric. Biotechnol. 27, 101593 (2020)
Sathishkumar, R.S., Sundaramanickam, A., Srinath, R., Ramesh, T., Saranya, K., Meena, M., Surya, P.: Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. 23, 1180–1191 (2019)
Husain, S., Afreen, S., Yasin, D., Afzal, B., Fatma, T.: Cyanobacteria as a bioreactor for synthesis of silver nanoparticles—an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J. Microbiol. Methods. 162, 77–82 (2019)
Adebayo-Tayo, B., Salaam, A., Ajibade, A.: Green synthesis of silver nanoparticle using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon 5, e02502 (2019)
Kathiraven, T., Sundaramanickam, A., Shanmugam, N., Balasubramanian, T.: Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 5, 499–504 (2015)
Satapathy, S., Shukla, S.P., Sandeep, K.P., Singh, A.R., Sharma, N.: Evaluation of the performance of an algal bioreactor for silver nanoparticle production. J. Appl. Phycol. 27, 285–291 (2015)
Edison, T.N.J.I., Atchudan, R., Kamal, C., Lee, Y.R.: Caulerpa racemosa: a marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess. Biosyst. Eng. 39, 1401–1408 (2016)
Salari, Z., Danafar, F., Dabaghi, S., Ataei, S.A.: Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity. J. Saudi Chem. Soc. 20, 459–464 (2016)
Mahajan, A., Arya, A., Chundawat, T.S.: Green synthesis of silver nanoparticles using green alga (Chlorella vulgaris) and its application for synthesis of quinolines derivatives. Synth. Commun. 49, 1926–1937 (2019)
Arya, S.S., Sharma, M.M., Das, R.K., Rookes, J., Cahill, D., Lenka, S.K.: Vanillin mediated green synthesis and application of gold nanoparticles for reversal of antimicrobial resistance in Pseudomonas aeruginosa clinical isolates. Heliyon. 5, e02021 (2019)
Borah, D., Das, N., Das, N., Bhattacharjee, A., Sarmah, P., Ghosh, K., Chandel, M., Rout, J., Pandey, P., Ghosh, N.N.: Alga-mediated facile green synthesis of silver nanoparticles: photophysical, catalytic and antibacterial activity. Appl. Organomet. Chem. 34, e5597 (2020)
Salaam, A., Adebayo-Tayo, B.C., Ajibade, A.: Phycosynthesis of silver nanoparticles using Chlorella vulgaris metabolites: its antibacterial, anti-biofilm and in-vitro cytotoxicity potential and effect of optimized conditions on biosynthesis: Chlorella vulgaris silver nanoparticles. Afr. J. Biomed. Res. 23, 17–23 (2020)
Dhas, T.S., Kumar, V.G., Karthick, V., Angel, K.J., Govindaraju, K.: Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 120, 416–420 (2014)
Rajeshkumar, S., Malarkodi, C., Paulkumar, K., Vanaja, M., Gnanajobitha, G., Annadurai, G.: Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int. J. Met. 2014, 1–8 (2014)
Annamalai, J., Nallamuthu, T.: Green synthesis of silver nanoparticles: characterization and determination of antibacterial potency. Appl. Nanosci. 6, 259–265 (2016)
Khalifa, K.S., Hamouda, R.A., Hanafy, D., Hamza, A.: In vitro antitumor activity of silver nanoparticles biosynthesized by marine algae. Dig. J. Nanomater. Biostruct. 11, 213–221 (2016)
Vieira, A.P., Stein, E.M., Andreguetti, D.X., Colepicolo, P., da Costa Ferreira, A.M.: Preparation of silver nanoparticles using aqueous extracts of the red algae Laurencia aldingensis and Laurenciella sp. and their cytotoxic activities. J. Appl. Phycol. 28, 2615–2622 (2016)
Sánchez-López, E., Gomes, D., Esteruelas, G., Bonilla, L., Lopez-Machado, A.L., Galindo, R., Cano, A., Espina, M., Ettcheto, M., Camins, A.: Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10, 292 (2020)
Madkour, L.H.: Ecofriendly green biosynthesized of metallic nanoparticles: bio-reduction mechanism, characterization and pharmaceutical applications in biotechnology industry. Glob. Drugs Ther. 3 (2018)
Güzel, R., Erdal, G.: Synthesis of silver nanoparticles. In: Khan, M., (ed) Silver Nanoparticles-Fabrication, Characterization and Applications, pp. 3–20. IntechOpen (2018). https://doi.org/10.5772/intechopen.75363
Zhang, W., Niu, X., Meng, S., Li, X., He, Y., Pan, J., Qiu, F., Zhao, H., Lan, M.: Histidine-mediated tunable peroxidase-like activity of nanosized Pd for photometric sensing of Ag+. Sens. Actuators B Chem. 273, 400–407 (2018)
Siddiqi, K.S., Husen, A., Rao, R.A.K.: A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 16, 14 (2018)
Rahman, A., Kumar, S., Bafana, A., Dahoumane, S.A., Jeffryes, C.: Individual and combined effects of extracellular polymeric substances and whole cell components of Chlamydomonas reinhardtii on silver nanoparticle synthesis and stability. Molecules 24, 956 (2019)
El Shafey, A.M.: Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: a review. Green Process. Synth. 9, 304–339 (2020)
Madkour, L.H.: Biogenic–biosynthesis metallic nanoparticles (MNPs) for pharmacological, biomedical and environmental nanobiotechnological applications. Chronicles Pharm. Sci. J. 2, 384–444 (2018)
Gurunathan, S., Kang, M.-H., Qasim, M., Kim, J.-H.: Nanoparticle-mediated combination therapy: two-in-one approach for cancer. Int. J. Mol. Sci. 19, 3264 (2018)
Mumtaz, T., Qindeel, M., Tarhini, M., Ahmed, N., Elaissari, A.: Exploiting proteases for cancer theranostic through molecular imaging and drug delivery. Int. J. Pharm. 587, 119712 (2020)
Yin, I.X., Zhang, J., Zhao, I.S., Mei, M.L., Li, Q., Chu, C.H.: The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 15, 2555 (2020)
Abbaszadegan, A., Ghahramani, Y., Gholami, A., Hemmateenejad, B., Dorostkar, S., Nabavizadeh, M., Sharghi, H.: The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J. Nanomater. 2015, 1–8 (2015)
Roy, A., Bulut, O., Some, S., Mandal, A.K., Yilmaz, M.D.: Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 9, 2673–2702 (2019). https://doi.org/10.1039/c8ra08982e
Murugan, K., Samidoss, C.M., Panneerselvam, C., Higuchi, A., Roni, M., Suresh, U., Chandramohan, B., Subramaniam, J., Madhiyazhagan, P., Dinesh, D.: Seaweed-synthesized silver nanoparticles: an eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol. Res. 114, 4087–4097 (2015)
Ninganagouda, S., Rathod, V., Singh, D., Hiremath, J., Singh, A.K., Mathew, J.: Growth kinetics and mechanistic action of reactive oxygen species released by silver nanoparticles from Aspergillus niger on Escherichia coli. Biomed Res Int. 2014, 1–9 (2014)
Meikle, T.G., Dyett, B.P., Strachan, J.B., White, J., Drummond, C.J., Conn, C.E.: Preparation, characterization, and antimicrobial activity of cubosome encapsulated metal nanocrystals. ACS Appl. Mater. Interfaces. 12, 6944–6954 (2020)
Akter, M., Sikder, M.T., Rahman, M.M., Ullah, A.K.M.A., Hossain, K.F.B., Banik, S., Hosokawa, T., Saito, T., Kurasaki, M.: A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J. Adv. Res. 9, 1–16 (2018). https://doi.org/10.1016/j.jare.2017.10.008
Haase, A., Tentschert, J., Jungnickel, H., Graf, P., Mantion, A., Draude, F., Plendl, J., Goetz, M.E., Galla, S., Maši, A.: Toxicity of silver nanoparticles in human macrophages: uptake, intracellular distribution and cellular responses. J. Phys. Conf. Ser. 304, 12030 (2011)
Yun’an Qing, L.C., Li, R., Liu, G., Zhang, Y., Tang, X., Wang, J., Liu, H., Qin, Y.: Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 13, 3311 (2018)
Li, L., Li, L., Zhou, X., Yu, Y., Li, Z., Zuo, D., Wu, Y.: Silver nanoparticles induce protective autophagy via Ca2+/CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology 13, 369–391 (2019)
Mourdikoudis, S., Pallares, R.M., Thanh, N.T.K.: Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 10, 12871–12934 (2018)
Sahoo, C.R., Maharana, S., Mandhata, C.P., Bishoyi, A.K., Paidesetty, S.K., Padhy, R.N.: Biogenic silver nanoparticle synthesis with cyanobacterium Chroococcus minutus isolated from Baliharachandi sea-mouth, Odisha, and in vitro antibacterial activity. Saudi J Biol Sci. (2020)
Gomathi, A.C., Rajarathinam, S.R.X., Sadiq, A.M., Rajeshkumar, S.: Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug Deliv. Sci. Technol. 55, 101376 (2020)
Devi, G.K., Suruthi, P., Veerakumar, R., Vinoth, S., Subbaiya, R., Chozhavendhan, S.: A review on metallic gold and silver nanoparticles. Res. J. Pharm. Technol. 12, 935–943 (2019)
Singh, K., Panghal, M., Kadyan, S., Chaudhary, U., Yadav, J.P.: Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi drug resistant strains of Pseudomonas aeruginosa isolated from burn patients. J. Nanomed. Nanotechnol. 5, 1 (2014)
Blanco, A. C.: Sodium carbonate mediated synthesis of iron oxide nanoparticles to improve magnetic hyperthermia efficiency and induce apoptosis (2014). Doctoral thesis, UCL (University College London). https://discovery.ucl.ac.uk/id/eprint/1430360. Accessed 26 Apr 2022
Saharan, V., Sharma, G., Yadav, M., Choudhary, M.K., Sharma, S.S., Pal, A., Raliya, R., Biswas, P.: Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 75, 346–353 (2015)
Jalilian, F., Chahardoli, A., Sadrjavadi, K., Fattahi, A., Shokoohinia, Y.: Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. (2020)
Abdel-Raouf, N., Al-Enazi, N.M., Ibraheem, I.B.M., Alharbi, R.M., Alkhulaifi, M.M.: Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J. Biol. Sci. 26, 1207–1215 (2019)
Deshmukh, A.R., Gupta, A., Kim, B.S.: Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activities. Biomed. Res. Int. 2019, 1–14 (2019)
Balakrishnan, S., Sivaji, I., Kandasamy, S., Duraisamy, S., Kumar, N.S., Gurusubramanian, G.: Biosynthesis of silver nanoparticles using Myristica fragrans seed (nutmeg) extract and its antibacterial activity against multidrug-resistant (MDR) Salmonella enterica serovar Typhi isolates. Environ. Sci. Pollut. Res. 24, 14758–14769 (2017)
Garg, D., Sarkar, A., Chand, P., Bansal, P., Gola, D., Sharma, S., Khantwal, S., Mehrotra, R., Chauhan, N., Bharti, R.K.: Synthesis of silver nanoparticles utilizing various biological systems: mechanisms and applications—a review. Prog. Biomater. 9(3), 81–95 (2020)
Kumari, S., Tyagi, M., Jagadevan, S.: Mechanistic removal of environmental contaminants using biogenic nano-materials. Int. J. Environ. Sci. Technol. 16, 7591–7606 (2019)
Yousefzadi, M., Rahimi, Z., Ghafori, V.: The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen). J. Agardh. Mater Lett. 137, 1–4 (2014)
Sangeetha, N., Saravanan, K.: Biogenic silver nanoparticles using marine seaweed (Ulva lactuca) and evaluation of its antibacterial activity. J. Nanosci. Nanotechnol. 2, 99–102 (2014)
Murugan, K., Benelli, G., Ayyappan, S., Dinesh, D., Panneerselvam, C., Nicoletti, M., Hwang, J.-S., Kumar, P.M., Subramaniam, J., Suresh, U.: Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol. Res. 114, 2243–2253 (2015)
Chokshi, K., Pancha, I., Ghosh, T., Paliwal, C., Maurya, R., Ghosh, A., Mishra, S.: Green synthesis, characterization and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae Acutodesmus dimorphus. RSC Adv. 6, 72269–72274 (2016)
Aboelfetoh, E.F., El-Shenody, R.A., Ghobara, M.M.: Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess. 189, 349 (2017)
Ramkumar, V.S., Pugazhendhi, A., Gopalakrishnan, K., Sivagurunathan, P., Saratale, G.D., Dung, T.N.B., Kannapiran, E.: Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 14, 1–7 (2017)
Dixit, D., Gangadharan, D., Popat, K.M., Reddy, C.R.K., Trivedi, M., Gadhavi, D.K.: Synthesis, characterization and application of green seaweed mediated silver nanoparticles (AgNPs) as antibacterial agents for water disinfection. Water Sci. Technol. 78, 235–246 (2018)
Öztürk, B.Y.: Intracellular and extracellular green synthesis of silver nanoparticles using Desmodesmus sp.: their antibacterial and antifungal effects. Caryologia. Int. J. Cytol. Cytosystemat. Cytogenet. 72, 29–43 (2019)
Duygu, D.Y., Erkaya, I.A., Erdem, B., Yalcin, B.M.: Characterization of silver nanoparticle produced by Pseudopediastrum boryanum (Turpin) E. Hegewald and its antimicrobial effects on some pathogens. Int. J. Environ. Sci. Technol. 16, 7093–7102 (2019)
El Kassas, H.Y., Attia, A.A.: Bactericidal application and cytotoxic activity of biosynthesized silver nanoparticles with an extract of the red seaweed Pterocladiella capillacea on the HepG2 cell line. Asian Pac. J. Cancer Prev. 15, 1299–1306 (2014)
Selvam, G.G., Sivakumar, K.: Phycosynthesis of silver nanoparticles and photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Hypnea musciformis (Wulfen) JV Lamouroux. Appl. Nanosci. 5, 617–622 (2015)
Roni, M., Murugan, K., Panneerselvam, C., Subramaniam, J., Nicoletti, M., Madhiyazhagan, P., Dinesh, D., Suresh, U., Khater, H.F., Wei, H.: Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol. Environ. Saf. 121, 31–38 (2015)
Omar, H.H., Bahabri, F.S., El-Gendy, A.M.: Biopotential application of synthesis nanoparticles as antimicrobial agents by using Laurencia papillosa. Int. J. Pharmacol. 13, 303–312 (2017)
Vadlapudi, V., Amanchy, R.: Synthesis, characterization and antibacterial activity of silver nanoparticles from red algae, Hypnea musciformis. Adv. Biol. Res. (Rennes) 11, 242–249 (2017)
Deepak, P., Sowmiya, R., Ramkumar, R., Balasubramani, G., Aiswarya, D., Perumal, P.: Structural characterization and evaluation of mosquito-larvicidal property of silver nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh 1848. Artif. Cells Nanomed. Biotechnol. 45, 990–998 (2017)
Pugazhendhi, A., Prabakar, D., Jacob, J.M., Karuppusamy, I., Saratale, R.G.: Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 114, 41–45 (2018)
Hamouda, R.A., Abd El-Mongy, M., Eid, K.F.: Comparative study between two red algae for biosynthesis silver nanoparticles capping by SDS: Insights of characterization and antibacterial activity. Microb. Pathog. 129, 224–232 (2019)
Ramamoorthy, R., Vanitha, S., Krishnadev, P.: Green synthesis of silver nanoparticles using red seaweed Portieria hornemannii (Lyngbye) PC silva and its antifungal activity against silkworm (Bombyx mori L.) Muscardine pathogens. J. Pharmacogn. Phytochem. 8, 3394–3398 (2019)
Fatima, R., Priya, M., Indurthi, L., Radhakrishnan, V., Sudhakaran, R.: Biosynthesis of silver nanoparticles using red algae Portieria hornemannii and its antibacterial activity against fish pathogens. Microb. Pathog. 138, 103780 (2020)
Madhiyazhagan, P., Murugan, K., Kumar, A.N., Nataraj, T., Dinesh, D., Panneerselvam, C., Subramaniam, J., Kumar, P.M., Suresh, U., Roni, M.: Sargassum muticum-synthesized silver nanoparticles: an effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res. 114, 4305–4317 (2015)
Princy, K.F., Gopinath, A.: Eco-friendly synthesis and characterization of silver nanoparticles using marine macroalga Padina tetrastromatica. Int. J. Sci. Res. 4, 1050–1054 (2015)
Ravichandran, A., Subramanian, P., Manoharan, V., Muthu, T., Periyannan, R., Thangapandi, M., Ponnuchamy, K., Pandi, B., Marimuthu, P.N.: Phyto-mediated synthesis of silver nanoparticles using fucoidan isolated from Spatoglossum asperum and assessment of antibacterial activities. J. Photochem. Photobiol. B 185, 117–125 (2018)
Subbiah, M., Pandithurai, M., Vajiravelu, S.: Spatoglossum asperum J Agardh mediated synthesis of silver nanoparticles, characterization and evaluation antifungal activities. J. Pharmacogn. Phytochem. 8, 1991–1995 (2019)
Bhuyar, P., Rahim, M.H.A., Sundararaju, S., Ramaraj, R., Maniam, G.P., Govindan, N.: Synthesis of silver nanoparticles using marine macroalgae Padina sp and its antibacterial activity towards pathogenic bacteria. Beni Suef Univ. J. Basic Appl. Sci. 9, 1–15 (2020)
Acknowledgements
SC wishes to acknowledge the support of the Council of Scientific and Industrial Research (CSIR), New Delhi, for the award of JRF (19-06-2016-352860). Part of this work was supported by a grant from the Department of Science and Technology, New Delhi (SERB File Number: EEQ/2020/000011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choudhary, S., Sangela, V., Saxena, P. et al. Recent progress in algae-mediated silver nanoparticle synthesis. Int Nano Lett 13, 193–207 (2023). https://doi.org/10.1007/s40089-022-00390-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-022-00390-0