Abstract
The soil remediation techniques play an important role when concerned with the environmental pollution caused by landfills, open dumps, mining areas and chemical spills. This paper aims to investigate the efficiency of inorganic and organic amendments to immobilize the heavy metals in a contaminated soil located at the Bingipura landfill site, Bangalore, India. The landfill site soil contaminated with heavy metals, i.e., copper, zinc, iron, chromium, cadmium, nickel and lead was studied with immobilization technique as this technique is relatively easily applicable and low cost. The immobilization efficiencies achieved with inorganic amendments, i.e., lime, cement, sodium hydroxide and organic amendments such as sawdust, arecanut fiber and dry leaves corresponding to pH values of 7.0, 8.5 and 10 were analyzed. The long-term efficiencies of organic and inorganic mixtures were assessed by conducting leaching tests on the stabilized soil for three months. The percentage leaching of various heavy metals from amended soil using different mixtures was observed during this period and the immobilization efficiencies were estimated. The highest immobilization efficiency was obtained using an admixture of lime with 5% sawdust corresponding to a pH value of 10. The leachability orders of different heavy metals from the amended soil were compared with the standard sequences of solubility of their hydroxides and found that the results were similar to these sequences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
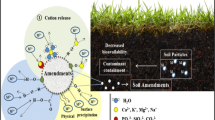
The land, the base and source of comforts for all the living beings on the earth is a non-renewable natural resource which needs to be taken care as a priority. Many lands turned unproductive due to over population and the activities of human beings. The modern agricultural and industrial activities led to the land degradation and turned some of the productive lands into wastelands. The wastelands atlas of India, 2019 [1] states that the total wasteland area of the country was about 5,57,665.51 sq. km. (16.96%) in 2015–16. The disposal of wastes and sludges by industries leads to contamination of soil and groundwater. The commonly available hazardous metals in a contaminated soil include copper (Cu), zinc (Zn), lead (Pb), cadmium (Cd) and chromium (Cr) [2,3,4,5,6]. There are two soil remediation techniques which have in practice been (i) extraction techniques (to remove the contaminants from the soil) and (ii) immobilization technique (to make the contaminants immobile by decreasing their solubility) [7]. The extraction techniques such as soil washing, remove the contaminants from the soil and are more desirable than immobilization technique. But the extraction techniques which make use of leaching solutions to remove contaminants require treatment of wastewater generated after extraction of contaminants from the soil and also need safe disposal of these extracted metals [8,9,10]. Another problem with extraction techniques is that it is not easy to completely remove the contaminants from the fine-grained soil. On the other hand, the immobilization technique avoids the need of excavation of the contaminated soil, treatment of wastewater and disposal of the contaminated fluid. Thus, provides relatively fast and cost-effective solution to treat the contaminated soil [11, 12]. The metal hydroxides formed after immobilization will not migrate because of their low soluble form and reduces the probability of contamination of nearby water bodies [13]. Using this technique, the solubility and mobility of heavy metals are decreased by increasing the pH value or by increasing the adsorption capacity of soils [14].
The immobilization technique if applied to convert the contaminated sites to agricultural fields, it is called as phytostabilization. In phytostabilization, the mobility of contaminants within the vadose zone will be reduced, thereby reducing off-site contamination and reducing contaminant solubility or bioavailability to the food chain. Kosiorek & Wyszkowski [15] have studied the use of manure, clay, charcoal, zeolite, and calcium oxide for phytostabilization. The clay minerals and natural zeolites are very effective to enhance fertility of the soil as well as to remediate the polluted soil. These amendments are appropriate for preserving agricultural sustainability on a long-term basis [16].
Various organic and inorganic additives can be used to restrict the mobility of contaminants in the soil using immobilization technique [17,18,19,20,21,22,23,24]. The additives such as clay, calcium hydroxide, cement, zeolites, hydroxyapatite, phosphates, organic compost, and microbes are widely used materials to immobilize the metals in soil [25, 26]. Addition of locally available materials such as natural clays would be a relatively economical method [27]. Ordinary Portland cement and fly ash can be used to solidify the metals in sludges [28,29,30]. Kogbara [31] studied the efficiency of immobilization technique using cement and mixtures of cement with fly ash, slag and lime. Lin et al. [22] studied the use of synthesized zeolite to treat a cadmium contaminated soil. Addition of Ca/Cao/NaH2PO4 on cesium contaminated soil was investigated by Mallampati et al. [32]. The efficiency of calcium and calcium oxide (Ca/CaO) to immobilize the metals, arsenic, cadmium, chromium and lead in soil was studied by Mallampati et al. [33]. The organic wastes such as sewage sludge, green waste and compost are also effective to immobilize the metals in soil and to increase the fertility of soil [34].
The chemicals are introduced in the ground by various methods like flooding the surface of the site, forced injection or by permitting slow penetration into the soil. But the frequently used method of mixing additives to the soil has been by tilling operations [27, 35]). Compared to other metals, it is easy to obtain the soluble form of cadmium [36]. Between the pH range of about 5.0–7.5, the solubility of lead was reported as less. At a soil pH of 6.5–7.5, Cu and Ni adsorb strongly on clay minerals. Zinc adsorbs at pH values above 6.0, but easily gets converted to a soluble form [37, 38]. The use of organic amendments in immobilization not only improves the soil structure but also increases the moisture content in the soil which is required for chemical reactions and moisture holding capacity of the soil [39,40,41,42,43,44,45,46,]. Awokunmi [40] found that application of sawdust successfully immobilized potentially toxic metals in a contaminated soil. The use of various organic amendments in soil immobilization was studied by several researchers [41,42,43,44,45,46,47,48,49] and found that the organic amendments are very effective in immobilization remediation of contaminated soils.
As the contaminated soil also contains organic matter, it is effective to use organic amendments along with inorganic amendments for treating the pollutants. There is a limited research work done on the combination of organic and inorganic additives to immobilize the toxic metals in soils at the dump sites. Hence, an attempt was made to use sawdust, arecanut fiber and dry leaves in combination with three inorganic amendments, i.e., lime, cement and sodium hydroxide (NaOH). As the solubility of most of the metals decreases from ‘hydroxides’ to ‘sulphates’ to ‘carbonates’ to ‘chlorides’, it is proposed to convert the heavy metals in soil into stable metal hydroxides, which can be achieved by increasing the pH. The pH that is required to convert the metal into metal hydroxide varies from metal to metal. It is thus proposed to check the efficiency at pH values of 7.0, 8.5 and 10.
The efficiency of immobilization technique also depends on the mixing methods and their application. The soils containing more clay or debris are difficult to mix, especially for in-situ applications. This technique is limited due to the insufficient data available related to the long-term consistency of the treated material [50]. A proper estimate of the field conditions for a longer period needs to be made with caution. It is required to monitor the fate of trace elements and the amendments, over long periods of time as the environmental conditions such as acid rain and pH changes may cause leaching of metals. Decrease in the pH value may incline to solubilize the metals in treated soil [51,52,]. Wang et al. [47] studied the long-term immobilization efficiency of biochar and stated that the factors such as acid rain, floods, changes in soil condition, plant roots and microorganisms in the soil significantly reduce the immobilization effect of biochar. When the physico-chemical properties of a soil change, the immobilized heavy metals may become active (mobile). Therefore, this remediation technique requires permanent monitoring [52]. Long-term stability of the amended soils also depends on the solubility of metals in their converted form [53,54,55]. As the long-term monitoring of the treated site is a costly process, it is advisable to conduct leaching tests to check the stability of the treated soil by simulating the field conditions. Hence, it was proposed to observe the stability and leachability of chemical compounds formed in the amended soil for a period of three months using leaching tests. The results were then compared against the pH changes and standard values of the solubility of metal hydroxides.
Materials and Methods
Contaminated Soil
The soil samples were collected from Bingipura dumping yard located at the outskirts of Bangalore, India (Fig. 1). This site, located in South Bangalore close to Electronic city, was spread in 24 acres and overloaded with more than 50,000 tonnes of garbage which contaminated the groundwater of surrounding villages. The soil at the landfill area is highly contaminated with various pollutants and heavy metals. The migration of the pollutants in the soil led to the contamination of water sources near this area and caused potential risk to public health. From the observations of [56], it was found that the nearby surface water body is polluted and showed higher values for salinity, alkalinity, TDS and pH. The contaminated soil collected from the dump site was analyzed to know the quantities of various heavy metals present in it. It was found that the quantities of various metals in the soil ranged from 1.8 to 213 mg/kg. As the concentration levels were relatively low, it was decided to use light treatment technique like immobilization. Since the solubility of metal hydroxides is less, it was aimed to alter the metals into their hydroxide form. It was also proposed to study the influence of pH changes on the immobilization efficiencies.
The soil samples collected from a depth of 0.5 m from different locations at the site were thoroughly mixed to get a representative sample of uniform composition. The soil collected from the site was screened manually to remove debris etc., air dried for one week, sieved through a 2 mm sieve and then oven dried to determine the index properties of soil. The composition of the soil was estimated to be of 52% sand, 19% silt and 29% clay. The soil is classified as clayey sand (SC) as per Indian Standard classification system. The plasticity and compaction characteristics of the contaminated soil are given in Table 1.
Chemical Analysis of Contaminated Soil
The quantities of various metal ions presented in the contaminated soil were estimated with the standard methods of USEPA 3050B [57]. For this, a dry soil sample of about one gram was mixed with 10 ml of 1:1 HNO3 and refluxed for 10 min. The concentrated HNO3 of 5 ml was then added and refluxed for 30 min. The process was repeated till the digestion is completed. The mixture was then reduced to a volume of 5 ml by heating it at 95 °C. It was then mixed with 2 ml of distilled water and 3 ml of 30% hydrogen peroxide (H2O2). The H2O2 was added in 1 ml quantity till bubbling subsides. The mixture was again heated at 95 °C to reduce the volume to 5 ml. When the mixture was cooled, 10 ml of concentrated hydrochloric acid (HCl) was mixed with it and refluxed for 15 min. It was then filtered through 20 µm filter paper and the filtrate was analyzed by atomic absorption spectrometry. The quantities of heavy metals are given in Table 2.
Amended Soil Sample
The contaminated soil sample of about 110 g was taken in a container and the additive/amendment was added to the soil in such a way that the pH value of the mixture achieved the desired value. The samples were prepared with 3 inorganic additives, i.e., lime, cement and NaOH. Each additive was added to adjust the pH of the mixture to maintain a pH value of 7.0, 8.5 and 10. To study the effect of organic amendments, three organic materials, i.e., sawdust, arecanut fiber and dry leaves were used in this research work. The soil was mixed with the inorganic amendment to bring it to the required pH and then the organic amendment was mixed in different proportions (1%, 2% and 5%) corresponding to a pH value of 10. Total 36 samples were prepared out of which 9 samples were prepared with 3 inorganic additives corresponding to pH values of 7.0, 8.5 and 10, and 27 samples were prepared using 3 organic additives of different proportions corresponding to a pH value of 10. The mixtures were subjected to mechanical shaking for 24 h and allowed to react with the pollutants for one week. The amended soil samples thus prepared were tested to evaluate their stability.
Leaching Tests for Amended Soil
To evaluate the long-term stability of the soil mixtures, leaching tests were conducted by passing water through the soil placed in the containers. The arrangement of containers for leaching test is shown in Fig. 2. Since the mobility of most of the metals in the soil decreases with increase in pH, it was proposed to mix the additives to adjust the pH of the mixture to 7.0, 8.5 and 10. The mixture was placed in the containers with light compaction and left for four weeks under moist condition. The distilled water was then passed through the columns (to simulate rainfall on amended soil in the field) and the effluent was collected in a container. The effluent samples were collected from the container at every 12 h time interval. The concentrations of heavy metals in effluents were found by atomic absorption spectroscopy (AAS) to know the amounts of various contaminants leached out after solidification and to assess the capabilities of these solidifying agents. The sample tested was mixed with the remaining effluent, and the concentrations determined were corresponding to the cumulative effluent leached from the soil. The effluent concentrations were monitored for a period of 120 days to know the long-term efficiency of the amendments under study in retarding the migration of metal ions. From the effluent concentrations estimated, the cumulative percentage leached and the immobilization efficiency were estimated for each metal ion (Table 3).
Results and Discussion
The immobilization efficiencies obtained with different additives are compared with respect to each metal ion as shown in Figs. 3, 4, 5, 6, 7, 8, 9. From the results, it was observed that the increase in pH value increases the immobilization efficiencies and the efficiencies also increased with the addition of organic amendments. The addition of all the three organic amendments increased the immobilization efficiencies, but the sawdust gave the best results. The highest and lowest leaching rates were registered for cadmium and copper metals, respectively. Among the additives studied, the soil treated with lime + 5% sawdust gave the best results. Out of the metals present in this mixture, only 0.62% of Cu, 14.74% of Cr, 10.33% of Fe, 22.72% of Pb, 19.36% of Zn, 5.71% of Ni and 24.08% of Cd leached out from the treated soil. All the metal ions achieved highest immobilization for lime with 5% sawdust at a pH value of 10. The immobilization efficiencies obtained with lime alone at a pH value of 10 were in the range of 51–78%, whereas these values ranged from 75 to 99% when sawdust is added to the lime at same pH value of 10. It was also observed that the immobilization efficiencies of all the three additives are high at a pH value of 10 when combined with 5% saw dust. Hence, it was identified that the addition of 5% sawdust increased the efficiencies approximately by 20% for all the three inorganic additives studied.
From the leaching test results on lime stabilized soil, the orders of leachability from different mixtures were observed as follows.
Lead > Cadmium > Copper > Nickel > Zinc > Chromium > Iron (pH = 7.0).
Cadmium > Lead > Nickel > Zinc > Copper > Chromium > Iron (pH = 8.5).
Cadmium > Lead > Iron > Nickel > Zinc > Copper > Chromium (pH = 10.0).
Cadmium > Lead > Zinc > Chromium > Iron > Nickel > Copper (pH = 10.0 with 5% sawdust).
The leachability orders of the metals from NaOH treated soil were observed as under:
Lead > Nickel > Cadmium > Zinc > Copper > Chromium > Iron (pH = 7.0).
Cadmium > Lead > Nickel > Zinc > Copper > Chromium > Iron (pH = 8.5).
Cadmium > Lead > Nickel > Iron > Zinc > Copper > Chromium (pH = 10.0).
Cadmium > Lead > Zinc > Chromium > Nickel > Copper > Iron (pH = 10.0 with 5% sawdust).
From the results of leaching tests on cement stabilized soil, the orders of leachability from different mixtures were observed as follows.
Cadmium > Lead > Nickel > Zinc > Copper > Chromium > Iron (pH = 7.0).
Cadmium > Lead > Nickel > Zinc > Copper > Chromium > Iron (pH = 8.5).
Cadmium > Lead > Iron > Nickel > Zinc > Copper > Chromium (pH = 10.0).
Cadmium > Lead > Zinc > Chromium > Nickel > Iron > Copper (pH = 10.0 with 5% sawdust).
Chaturvedi et al. [58] found the leachability sequence as Cd > Pb > Zn for soil stabilized with humus soil and hydroxyapatite. This sequence is similar to the experimental results of the present study. In general, the metal type, its form and pH value of the mixture influences the leachability of metals from soil. The standard sequences of solubility of metal hydroxides with pH value are in the following order (Digital Analysis Corp [59]. and Snoeyink et al., [60]) (Fig. 10).
Cd(OH)2 > Pb(OH)2 > Ni(OH)2 > Zn(OH)2 > Cu(OH)2 > Cr(OH)3 > Fe(OH)2 (pH = 7.0).
Cd(OH)2 > Pb(OH)2 > Fe(OH)2 > Ni(OH)2 > Zn(OH)2 > Cr(OH)3 > Cu(OH)2 (pH = 8.5).
Cd(OH)2 > Pb(OH)2 > Zn(OH)2 > Cr(OH)3 > Fe(OH)2 > Ni(OH)2 > Cu(OH)2 (pH = 10.0).
The leachability orders of metals from the amended mixtures of this study were observed to be similar to these sequences with small variations. These variations may be due to the solid matrix created by cementing agents and cation exchange with soil.
Conclusions
This study analyzed the immobilization efficiencies of inorganic amendments with and without organic amendments in reducing the mobility of heavy metals in contaminated soil. The immobilization efficiencies of heavy metals were studied with three inorganic additives, i.e., lime, NaOH and cement corresponding to three pH values, i.e., 7.0, 8.5 and 10.0 and with three organic amendments, i.e., sawdust, arecanut fiber and dry leaves. Leaching tests were conducted for a period of 120 days to know the long-term efficiency of amended mixtures. It was observed that with the increase in pH value, the leachability of metals decreased drastically and the immobilization efficiencies increased. The immobilization efficiencies have also increased with the addition of organic amendments and out of the organic amendments tested at different proportions, addition of 5% sawdust yielded the best results. The leachability rates of metals were observed to be the lowest with the addition of 5% sawdust to lime at a pH value of 10. The highest immobilization efficiencies obtained with this amendment were 99.4%, 94.3%, 89.7%, 85.3%, 80.6%, 77.3% and 75.9% for the metals of Cu, Ni, Fe, Cr, Zn, Pb and Cd, respectively. It was identified that the addition of 5% sawdust increased the efficiencies by about 20% for all the three inorganic additives studied. The leachability orders of the metals from the amended soil varied with pH and were in different orders, but were observed to be in good agreement with the standard sequences of solubility of metal hydroxides. The small differences indicated the differences in cation exchange and matrix cementation in soil with lime and cement. The identified amendment mixtures in this study are thus useful to treat the dumpsite of this case study.
References
Wasteland Atlas of India, Department of Land Resources, MRD, Government of India, URL: https://dolr.gov.in/documents/wasteland-atlas-of-india (accessed on 26th Feb 2021) (2019)
J. Aslam, S.A. Khan, S.H. Khan, Heavy metals contamination in roadside soil near different traffic signals in Dubai, United Arab Emirates. J. Saudi Chem. Soc. 17(3), 315–319 (2013)
C.R. Evanko, D.A. Dzombak, Remediation of Metals-Contaminated Soils and Groundwater (Groundwater Remediation Technologies Analysis Center, Pittsburg, 1997)
K. Hange, O.R. Awofolu, Assessment of anthropogenic influence on the level of selected heavy metals (Cu, Zn, Cd and Pb) in soil. J. Soil Sci. Environ. Manag 8(6), 113–121 (2017)
K. Tahar, B. Keltoum, Effects of heavy metals pollution in soil and plant in the industrial area West Algeria. J. Korean Chem. Soc. 55(6), 1018–1023 (2011)
F. Zhang, X. Yan, C. Zeng, M. Zhang, S. Shrestha, L.P. Devkota, T. Yao, Influence of traffic activity on heavy metal concentrations of roadside farmland soil in mountainous areas. Int. J. Environ. Res. Public Health 9(5), 1715–1731 (2012)
N. Bolan, A. Kunhikrishnan, R. Thangarajan, J. Kumpiene, J. Park, T. Makino, K. Scheckel, Remediation of heavy metal(loid)s contaminated soils–to mobilize or to immobilize. J. Hazard. Mater. 266, 141–166 (2014)
K. Chair, A. Bedoui, N. Bensalah, C. Sáez, F.J. Fernández-Morales, S. Cotillas, M.A. Rodrigo, Treatment of soil-washing effluents polluted with herbicide oxyfluorfen by combined biosorption–electrolysis. Ind. Eng. Chem. Res. 56(8), 1903–1910 (2017)
C.N. Mulligan, R.N. Yong, B.F. Gibbs, Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng. Geol. 60(1–4), 193–207 (2001)
S. Satyro, M. Race, R. Marotta, M. Dezotti, M. Guida, L. Clarizia, Photocatalytic processes assisted by artificial solar light for soil washing effluent treatment. Environ. Sci. Pollut. Res. 24(7), 6353–6360 (2017)
D.M. Hamby, Site remediation techniques supporting environmental restoration activities: a review. Sci. Total Environ. 191(3), 203–224 (1996)
R.A. Wuana, F.E. Okieimen, Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. (2011). https://doi.org/10.5402/2011/402647
N.T. Basta, S.L. McGowen, Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Pollut. 127(1), 73–82 (2004)
USEPA, Guide to the Disposal of Chemically Stabilized and Solidified Waste, SW-872 (Office of Water and Waste Management, Washington DC, 1982)
M. Kosiorek, M. Wyszkowski, Remediation of cobalt-contaminated soil using manure, clay, charcoal, zeolite, calcium oxide, main crop (Hordeum vulgare L), and after-crop (Synapis alba L). Minerals 10(5), 429 (2020)
K.M. Manjaiah, R. Mukhopadhyay, R. Paul, S.C. Datta, P. Kumararaja, B. Sarkar, Clay minerals and zeolites for environmentally sustainable agriculture, in Modified clay and zeolite nanocomposite materials, ed. by Mariano Mercurio (Elsevier, Binoy Sarkar and Alessio Langella, 2019), pp. 309–329
D.E. Abbott, M.E. Essington, M.D. Mullen, J.T. Ammons, Fly ash and lime-stabilized biosolid mixtures in mine spoil reclamation. J. Environ. Qual. 30(2), 608–616 (2001)
C. Brunori, C. Cremisini, P. Massanisso, V. Pinto, L. Torricelli, Reuse of a treated red mud bauxite waste: studies on environmental compatibility. J. Hazard. Mater. 117(1), 55–63 (2005)
X. Cao, L.Q. Ma, A. Shiralipour, Effects of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator Pteris vittata. L. Environ. Pollut. 126(2), 157–167 (2003)
R. Clemente, D.J. Walker, A. Roig, M.P. Bernal, Heavy metal bioavailability in a soil affected by mineral sulphides contamination following the mine spillage at Aznalcóllar (Spain). Biodegradation 14(3), 199–205 (2003)
W. Friesl, E. Lombi, O. Horak, W.W. Wenzel, Immobilization of heavy metals in soils using inorganic amendments in a greenhouse study. J. Plant Nutr. Soil Sci. 166(2), 191–196 (2003)
C.F. Lin, S.S. Lo, H.Y. Lin, Y. Lee, Stabilization of cadmium contaminated soils using synthesized zeolite. J. Hazard. Mater. 60(3), 217–226 (1998)
D.J. Walker, R. Clemente, M.P. Bernal, Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 57(3), 215–224 (2004)
G. Guo, Q. Zhou, L.Q. Ma, Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ. Monit. Assess. 116(1–3), 513–528 (2006)
N. Finzgar, B. Kos, D. Lestan, Bioavailability and mobility of Pb after soil treatment with different remediation methods. Plant Soil Environ. 52(1), 25 (2006)
T.A. Martin, M.V. Ruby, Review of Insitu remediation technologies for lead, zinc, and cadmium in soil. Remediat. J. J. Environ. Cleanup Costs Technol. Tech. 14(3), 35–53 (2004)
G. Czupyrna, R.D. Levy, A.I. MacLean, H. Gold, Insitu immobilization of heavy-metal contaminated soil (No.AFE-0302-FMI-8472–68). Foster-MillerInc Waltham Ma (1988)
C.Y. Ong, P.C. Chui, Solidification of industrial waste sludge with incineration fly ash and ordinary portland cement, proc. 9th National Undergraduate Research Opportunities Programme Congress 2003 (NUROP2003), Nanyang Technological University, Singapore, 13th Sept., 1215–1230 (2002).
R. Malviya, R. Chaudhary, Evaluation of leaching characteristics and environmental compatibility of solidified/stabilized industrial waste. J. Mater. Cycle Waste Manage. 8(1), 78–87 (2006)
H. Patel, S. Pandey, Exploring the reuse potential of chemical sludge from textile wastewater treatment plants in India-A hazardous waste. Am. J. Environ Sci. 5(1), 106 (2009)
R.B. Kogbara, A review of the mechanical and leaching performance of stabilized/solidified contaminated soils. Environ. Rev. 22(1), 66–86 (2013)
S.R. Mallampati, Y. Mitoma, T. Okuda, S. Sakita, M. Kakeda, High immobilization of soil cesium using ball milling with nano-metallic Ca/CaO/NaH 2 PO 4: implications for the remediation of radioactive soils. Environ. Chem. Lett. 10(2), 201–207 (2012)
S.R. Mallampati, Y. Mitoma, T. Okuda, S. Sakita, M. Kakeda, Solidification and immobilization of heavy metals in soil using with nano-metallic Ca/CaO Dispersion Mixture in E3S Web of Conferences. Vol 1, pp. 350–361 (2013)
V.P. Gadepalle, S.K. Ouki, R.V. Herwijnen, T. Hutchings, Immobilization of heavy metals in soil using natural and waste materials for vegetation establishment on contaminated sites. Soil & Sediment Contam. 16(2), 233–251 (2007)
S.G. Liao, D.W. Li, Review of contaminated sites remediation technology, in Advanced Materials Research. Trans Tech Publ 414, 1–4 (2012). https://doi.org/10.4028/www.scientific.net/amr.414.1
R.P. Gambrell, Trace and toxic metals in wetlands—a review. J. Environ. Qual. 23(5), 883–891 (1994)
O. Abollino, M. Aceto, M. Malandrino, C. Sarzanini, E. Mentasti, Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res. 37(7), 1619–1627 (2003)
R.D. Harter, Effect of soil pH on adsorption of lead, copper, zinc, and nickel 1. Soil Sci. Soc. Am. J. 47(1), 47–51 (1983)
K.N. Palansooriya, S.M. Shaheen, S.S. Chen, D.C. Tsang, Y. Hashimoto, D. Hou, Y.S. Ok, Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ. Int. 134, 105046 (2020)
E.E. Awokunmi, Impact of saw dust application on the distribution of potentially toxic metals in contaminated soil. Bull. Environ. Contam. Toxicol. 99(6), 765–770 (2017)
N. Baruah, N. Gogoi, M. Farooq, Influence of biochar and organic soil amendments on bioavailability and immobilization of copper and lead to common cocklebur in acidic sandy loam soil. J Environ Chem Eng 8(6), 104480 (2020)
L. Gong, J. Wang, T. Abbas, Q. Zhang, M. Cai, M. Tahir, H. Di, Immobilization of exchangeable Cd in soil using mixed amendment and its effect on soil microbial communities under paddy upland rotation system. Chemosphere 262, 127828 (2021)
I. Naeem, N. Masood, V. Turan, M. Iqbal, Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicol. Environ. Safet 208, 111723 (2021)
M.I. Rafique, A.R. Usman, M. Ahmad, M.I. Al-Wabel, Immobilization and mitigation of chromium toxicity in aqueous solutions and tannery waste-contaminated soil using biochar and polymer-modified biochar. Chemosphere 266, 129198 (2021)
M.S. Rizwan, M. Imtiaz, J. Zhu, B. Yousaf, M. Hussain, L. Ali, H. Hu, Immobilization of Pb and Cu by organic and inorganic amendments in contaminated soil. Geoderma 385, 114803 (2021)
P. Saengwilai, W. Meeinkuirt, T. Phusantisampan, J. Pichtel, Immobilization of cadmium in contaminated soil using organic amendments and its effects on rice growth performance. Expo Health 12(2), 295–306 (2020)
J. Wang, L. Shi, L. Zhai, H. Zhang, S. Wang, J. Zou, Y. Chen, Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: a review. Ecotoxicol. Environ. Safety 207, 111261 (2021)
Q. Wang, J. Wen, X. Hu, L. Xing, C. Yan, Immobilization of Cr (VI) contaminated soil using green-tea impregnated attapulgite. J. Clean. Prod. 278, 123967 (2021)
X. Yang, H. Pan, S.M. Shaheen, H. Wang, J. Rinklebe, Immobilization of cadmium and lead using phosphorus-rich animal-derived and iron-modified plant-derived biochars under dynamic redox conditions in a paddy soil. Environ. Int. 156, 106628 (2021)
C. Ma, J. Kingscott, M. Evans, Recent developments for in situ treatment of metal contaminated soils, Prepared for U S Environmental Protection Agency, Office of Solid Waste and Emergency Response Technology Innovation Office, Washington, DC (1997)
S.K. Gupta, T. Herren, K. Wenger, R. Krebs, T. Hari, In situ gentle remediation measures for heavy metal-polluted soils, in Phytoremediation of Contaminated Soil and Water, ed. by Norman Terry, Gary Bañuelos (CRC Press, 2000), pp. 303–322
S. Khalid, M. Shahid, N.K. Niazi, B. Murtaza, I. Bibi, C. Dumat, A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 182, 247–268 (2017)
S.L. McGowen, N.T. Basta, G.O. Brown, Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J. Environ. Qual. 30(2), 493–500 (2001)
M. Valipour, K. Shahbazi, A. Khanmirzaei, Chemical immobilization of lead, cadmium, copper, and nickel in contaminated soils by phosphate amendments. CLEAN –Soil Air Water 44(5), 572–578 (2016)
G. Zeng, J. Wan, D. Huang, L. Hu, C. Huang, M. Cheng, D. Jiang, Precipitation, adsorption and rhizosphere effect: the mechanisms for phosphate-induced Pb immobilization in soils—a review. J. Hazard. Mater. 339, 354–367 (2017)
B. Paruti, B. Santhaveeranagoud, Impact on groundwater and soil due to solid waste dump-A case study of S. Bingipur in Bangalore. Ann. Fac. Eng. Hunedoara 17(4), 167–174 (2019)
US Environmental Protection Agency (USEPA), Method 3050B. EPA 660 13–75–009, acid digestion of sediments, sludges and soils, Revision 2, Washington, DC (1996)
P.K. Chaturvedi, C.S. Seth, V. Misra, Sorption kinetics and leachability of heavy metal from the contaminated soil amended with immobilizing agent (humus soil and hydroxyapatite). Chemosphere 64(7), 1109–1114 (2006)
Digital Analysis Corp., NY. http://www.phadjustment.com/TArticles/Heavy_Metal_Reduction.html
V.L. Snoeyink, D. Jenkins, Water Chemistry, vol. 91 (Wiley, New York, 1980)
Funding
The authors declare that there is no funding and no financial support from any agency in conducting the research work and publishing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no confict of interest. The work is not published in any other journal nor is under review in any other journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sumalatha, J., Sivapullaiah, P.V. & Prabhakara, R. Immobilization Remediation of a Heavy Metals Contaminated Soil: A Case Study of Dump Site at Bangalore, India. J. Inst. Eng. India Ser. A 103, 105–114 (2022). https://doi.org/10.1007/s40030-021-00590-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40030-021-00590-5