Abstract
Two newly developed low erucic acid containing varieties of Indian mustard (RLC 3) and rapeseed (GSC 7) were evaluated for cooking quality. Oils extracted from these varieties were analyzed for physicochemical properties, fatty acid composition, antioxidants and oxidative stability. Consequently, GSC 7 had the highest smoke point (234.3 °C) and was the most stable cooking oil in terms of chemical parameters. The highest value of oleic acid was observed in the RLC 3 oil (66.1%), and the lowest value of erucic acid was observed in the GSC 7 oil (0.5%). GSC 7 oil contained higher value of total antioxidants (616.3 mg/kg). The food products prepared using GSC 7 oil were found to be more stable on the basis of analytical parameters. The study recommends that varieties of canola may be promoted to increase its availability at affordable price.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapeseed-Mustard oil is largely consumed in north-eastern, northern and eastern regions of India, as its pungency is a desired and inherent part of the local cuisine. It shares about 27.8 percent of the India’s oilseed economy. More than half of rural inhabitants use this oil as their staple edible oil and monthly consumption varies between two and four liters per family. But, rapeseed-mustard cultivars grown in India have high level of erucic acid and glucosinolates [1]. Erucic acid is a non-branched, long chain fatty acid with 22 carbons and a cis-configuration (double bond on C-13). More than permissible limits dietary intake of erucic acid has been reported to cause impair myocardial conductance, lipidosis in children and high level of blood cholesterol. Glucosinolates are responsible for the characteristic pungency of rapeseed-mustard oil. Though the nutritional advantages of rapeseed-mustard oil available in India outdo many other edible oils (lowest amount of harmful saturated fatty acid (SFA), and contains two essential fatty acids—linoleic and linolenic), the presence of erucic acid is considered undesirable. The maximum content of erucic acid in edible oils should be 2 percent and the suggested daily intake should not be more than 7 mg per kg body weight [2].
Moreover, the Indian varieties of rapeseed-mustard are not much popular in international market due to concerns over erucic acid content. Hence, the efforts to develop low erucic acid containing varieties are gaining interest in the crop improvement program of Rapeseed-Mustard in India. In this context, Punjab Agricultural University, Ludhiana, has released and recommended two varieties of Brassica oilseeds with low erucic acid in the year 2014 and 2015, i.e., gobhi sarson (Rapeseed) GSC 7 (Erucic acid 0.5%) and raya (Indian mustard) RLC 3 (Erucic acid 0.8%), first canola quality varieties in India [3]. The oil content of GSC 7 and RLC 3 is 40.5 and 41 percent, respectively. The oils extracted from rapeseed-mustard cultivars have high smoke point which is very suitable for Indian conventional cooking. Indian conventional cooking is comprised of high temperature cooking methods such as frying, sautéing, boiling and grilling but combination of only 2 or 3 is used in one recipe.
The canola quality oils extracted from these two novel varieties have not been evaluated earlier for its cooking quality and oxidative stability. Therefore, the present work was designed to determine the fatty acid composition, sensory parameters and oxidative stability of the food products prepared using low erucic acid oils.
Material and Methods
Materials
Cooking oils of the two varieties of canola (Experimental), i.e., RLC 3 and GSC 7 with corresponding non-canola (Control), i.e., PBR 357 and GSL 1 were procured from the Oilseed Section, Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana. All the analytical and guaranteed reagents and solvents used were supplied by Himedia (Mumbai, India).
Physicochemical Properties
Physical parameters Fresh oils were analyzed for color, smoke point, frying temperature, frying time and viscosity using standard methods [4].
Chemical parameters Peroxide value (PV), iodine value (IV), saponification value (SV), acid value (AV) and free fatty acid (FFA) of the control and experimental oils were determined by Association of Official Analytical Chemists (AOAC) methods [4].
Antioxidants
Total tocopherols were determined as per the method given by Japan Customs Analysis Methods (JCAM) [5]. Oil sample (2 g) was accurately weighed into a 25 ml volumetric flask, and 10 ml methanol was added. The flask was then sonicated for 10 min. Volume was made up with methanol, and flask was again sonicated for 5 min. Then, the solution was filtered through syringe filter (Merck Millipore) of a 0.45 μm pore-size. The filtrate was used as test solution for HPLC (Waters™) analysis. Further, a 100 μl of the test solution prepared in triplicate was injected into HPLC. The peak area ratios of the tocopherols were calculated in the test solution against standard solution (> 95.0% purity) based on the peak areas of each substance from the obtained chromatogram, and converted them to weight ratios using the calibration curves constructed. Total tocopherols were calculated by using the formula:
Total carotenoids were determined by a spectrophotometric method [6]. Oil sample of 0.5 ml was weighed (0.4244 g) accurately in a conical flask. To which 4 ml diethyl ether was added along with 0.5 ml saturated KOH. The flask was kept in dark for 30 min. Then, 5 ml distilled water was added and centrifuged the solution at 2500 rpm for 5 min. Supernatant was picked up with the help of auto pipette and read at 445 nm (Systronics UV–VIS-108 spectrophotometer, Bangalore, India). The samples were prepared in triplicate for each analysis, and the mean value of absorbance was obtained. Carotenoid content was calculated according to the following formula:
where X is the Carotenoid content, A is the highest absorbency value of 445 nm, Y is the quantity of extracting solution, A%1 cm is the average absorption coefficient 2500 of carotenoid molecule, g is the weight of sample.
Cooking Processes
Common food products such as parantha, poori, panjiri and vegetable (Potato-French beans) were cooked in Brassica oils by conventional cooking methods like shallow frying, deep frying, roasting and sautéing, respectively.
Parantha (an unleavened Indian flatbread) was prepared using 100 g wheat flour with 20 ml water, and the prepared dough (25 g) was rolled out in to circular sheets (diameter 15.5–16.0 cm; thickness 1.8–2.2 mm). A 5 ml of oil was used for shallow frying at 175 ± 5 °C for 2 min on a flat skillet (diameter 26 cm; thickness 4.8 mm). For the preparation of poori (an unleavened Indian flatbread), small portion (12.0–12.5 g) of wheat dough (300 g wheat flour with 60 ml water and 30 ml of oil) was rolled out in to circular sheets (diameter 7.3–7.7 cm; thickness 1.8–2.2 mm). Poori was deep fried using 300 L of oil at 180 ± 5 °C for 20 s until puffed. Panjiri (Indian sweet snack) was prepared by roasting whole wheat flour (75 g) and chickpea flour (25 g) with 20 ml of oil and 30 g of sugar in a pre-heated oven at 190 ± 5 °C for 15 min. Potato-French beans vegetable was prepared by sautéing vegetables (Potato and beans-100 g each). For sautéing, beans and potato were cut into almost equal small bite-sized pieces (about 1 cm; 2 × 2 cm, respectively) and 10 ml oil was pre-heated to 140 ± 5 °C in a skillet for sautéing vegetables (200–10 g each) for 5 min. Thereafter, vegetables were cooked in the closed skillet for 15 min.
Sensory Evaluation
The developed products were evaluated for sensory parameters thrice by a selected panel of 10 judges from the Department of Food and Nutrition, Punjab Agricultural University, Ludhiana. The judges were served each preparation with two control and two test samples. All the samples were prepared from the same ingredients used in the standardized recipes. The samples were coded to avoid any bias. The panelists were asked to score the product for appearance, color, texture, flavor and overall acceptability (OAA) on nine point hedonic scale. On the scorecard, scores 1, 2, 3, 4, 5, 6, 7, 8 and 9 represented dislike extremely, dislike very much, dislike moderately, dislike slightly, neither like nor dislike, like slightly, like moderately, like very much and like extremely, respectively. All panelists were instructed to rinse their plate with mineral water before testing each sample. The product characterization was carried out under “day light” illumination and in isolated booths within a nutrition laboratory. Further, the mean score for each sample was calculated.
Fatty acid Composition and Oxidative Stability
Sample preparation of the cooked products All the products were dried in a hot air oven at 60 °C till constant weight. The dried samples were then powdered and pressed to pass through 5 mm sieve and stored at ambient conditions (20 °C, 60% RH) in air tight containers until analysis.
(a) Fatty acid composition The food products were investigated for fatty acid composition by gas–liquid chromatography (model 7820A series, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector and a fused silica capillary column (50 m × 0.25 mm i.d.), coated with CP-SIL 88 as the stationary phase [7, 8]. Fatty acids are the group of lipids most commonly analyzed by gas–liquid chromatography (GLC). Temperatures of the oven, detector and injector were set at 210, 240 and 230 °C, respectively. A sample of 2 µl was injected at a split ratio of 10:1. Single fatty acids were expressed in percentages of the total fatty acids. The samples were analyzed for saturated fatty acids, i.e., SFAs (Palmitic acid-C16:0 and Stearic acid-C18:0), monounsaturated fatty acids, i.e., MUFA (Oleic acid-C18:1and Erucic acid-C22:1), polyunsaturated fatty acid, i.e., PUFA (Linoleic acid-C18:2, α-linolenic acid-C18:3).
(b) Oxidative Stability Peroxide value and free fatty acids were determined by using AOAC methods [4].
Statistical Analysis
All the determinations were executed in triplicate. The obtained figures have been presented as mean ± standard error. A computer program package (CPCS1) was used to ascertain statistical significance for statistical test such as one way analysis of variance (CRD). Tukey’s HSD test was applied to determine significant differences between the means (physicochemical parameters and antioxidants) using Statistical Package for the Social Sciences (SPSS) version 16.0. Student t test was applied to compare the fatty acid composition, sensory evaluation scores and oxidative stability of food products prepared in control and experimental oils of low erucic acid Brassica varieties. The statistical significance was expressed at p < 0.05 and p < 0.01.
Results and Discussion
Physical Parameters
The color is expressed as the sum total of the yellow and red slides used to match the color of the oil in a cell of the specified size in the Lovibond Tintometer. The readings for the color of oil extracted from PBR 357 and RLC 3 were recorded as 35.1 and 32.3, respectively (Table 1). The corresponding figures for rapeseed oils were observed as 35.2 (GSL 1) and 37.3 (GSC 7), respectively. The smoke point of oils extracted from GSC 7 and RLC 3 was recorded as 234.3 °C and 223.7 °C, respectively (Table 1). This meets the standard requirement for edible oils used for high temperature cooking as these oils should have a smoke point above 200 °C [9]. A significant difference (p < 0.05) was observed in the color and smoke point of the control and experimental oils of both Brassica cultivars. The frying temperature was recorded as 164.7 and 173.3 °C in GSL 1 and GSC 7 oils, whereas almost similar values were recorded for frying temperature in PBR 357 as well as RLC 3 such as 172.3 and 172.7 °C, respectively. Frying temperature should not exceed 180 °C as high frying temperature accelerates thermal oxidation and polymerization of oils. High frying temperature also results in formation of free fatty acids [10]. With respect to viscosity, the higher value (42 centipoise (cp)) was recorded in PBR 357 as compared to RLC 3 (35cp) at 32 °C room temperature. Similar trend was observed in control and experimental oil of rapeseed varieties. The viscosity of fresh oil in GSL 1 and GSC 7 was recorded as 40 and 37cp, respectively (Table 1). A significant difference (p < 0.05) was observed in the values of control and experimental oils.
Chemical Parameters
Peroxide value (PV) is a universal method to assess the peroxide formation in the initial stages of oxidation. At fresh conditions, PV was observed as 0.4 and 0.2 meq/kg in the oil extracted from PBR 357 and RLC 3, respectively. Comparatively, the corresponding figures for rapeseed varieties were found little lower such as 0.3 and 0.1 meq/kg in GSL 1 and GSC 7 oil (Table 1). According to Prevention of Food and Adulteration Act (PFA), fresh oils usually have PV well below 10 meq/kg. A rancid taste often begins to be noticeable when the PV is > 20 meq/kg. IV is also used to represent oxidation in fats and oils. At fresh condition, the IV of oils extracted from RLC 3 and GSC 7 were recorded as 114.7 and 116.1 g. SV is an indication of the molecular weights of triglycerides in oil. It is inversely proportional to the average molecular weight or chain length of the fatty acids [11]. The SV of oils extracted from PBR 357 and RLC 3 was found as 168.2 and 188.5 mg KOH/g. In rapeseed varieties, the figures were recorded as 172.9 and 190.6 mg KOH/g in the oils extracted from GSL 1 and GSC 7 (Table 1). AV is a measure of free fatty acids (FFA) present in cooking oil. Rahman et al. reported that the quality of fats and oils is reciprocal with the acid value [12]. Permissible levels of AV for all the cooking oils should be below 0.6 mg KOH/g. AV of PBR 357 and RLC 3 oil was observed as 0.2 mg KOH/g at fresh conditions. The analogous values for rapeseed varieties (GSL 1 and GSC 7) were recorded as 0.3 and 0.1 mg KOH/g (Table 1). A significant difference (p < 0.05) was observed in the chemical parameters of the control and experimental oils of both Brassica cultivars.
Antioxidants
Antioxidants influence quality of edible oils to a greater extent during cooking processes. Depending on structural features, antioxidants, individually or synergistically, can scavenge free radicals, inactivate pro-oxidant metals, quench singlet oxygen and inactivate sensitizers. Antioxidants such as total tocopherol and total carotenoids present in the low erucic Brassica oils investigated in this study have been presented in Fig. 1. Tocopherol contributes to oil stability and shelf life of the product. The total tocopherol content present in PBR 357 and RLC 3 oil was reported as 233.5 and 379.0 mg/kg, respectively. Likewise, Richards et al. also reported an average total concentration of total tocopherol as 400 to 700 mg/kg in canola oil [13]. The present findings were in line with earlier studies [14, 15] wherein total tocopherol content ranged between 80 to 1000 mg/kg in canola oil. The highest value of total tocopherol was found in the oil extracted from GSC 7, i.e., 460.6 mg/kg. National Academy of Sciences also documented canola oil as a good source of Vitamin E as compared to soybean oil (116 mg/kg) as it contains twice as much α-Tocopherol (270 mg/kg) [16].
Carotenoids are the natural pigments which are present in a variety of plants. In the present study, the total carotenoid content was recorded as 140.8 mg/kg and 146.3 mg/kg in PBR 357 and RLC 3 oil, respectively (Fig. 1), whereas the corresponding figures for GSL 1 and GSC 7 were 139.8 mg/kg and 155.7 mg/kg. Carotenoids shield the oil from thermal oxidation but only in synergistic manner with α-Tocopherol. A combination of antioxidants gives canola oil an important role in prevention and treatment of breast cancer and colon cancer [17, 18]. A significant difference (p < 0.05) was observed in the antioxidant content present in control and experimental oils extracted from Indian mustard and rapeseed varieties.
Sensory Evaluation
The OAA score of shallow fried food product (Parantha) prepared using GSC 7 oil (8.9) was significantly (p < 0.05) higher than the product prepared using GSL 1 oil (7.5) (Table 2), whereas there was no significant difference in the shallow fried product prepared using oils extracted from Indian mustard. Likewise, the OAA scores depicted that deep-fried product (Poori) prepared using GSC 7 oil (8.4) was significantly (p < 0.05) more acceptable to the panellists than the one prepared in GSL 1 oil (7.9). Further, there was a significant difference (p < 0.05) in appearance and color of the food product prepared by roasting (Panjiri) in the PBR 357 oil (7.7 and 7.4) and RLC 3 oil (8.1 and 7.9), while no significant difference was observed in the texture and flavor of the Panjiri roasted in both oils. However, a significant difference (p < 0.05) in OAA was observed at 5 percent level in both the GSL 7 (8.1) and GSL 1 (7.7) oils. The data revealed that there was no significant difference between all the sensory parameters of potato-beans sautéed in oils of both control and experimental Indian mustard varieties. All the sensory parameters were at par with each other. The panellists liked the vegetable sautéed in both oils.
Fatty Acid Composition
The fatty acid composition of control and experimental oil of both Indian mustard and rapeseed varieties is given in Table 3. The data revealed that oils extracted from both, i.e., PBR 357 and GSL1 varieties were found higher in erucic acid (51.1 and 36.5%, respectively). The findings were in agreement with the recent studies [19, 20]. The consumption of mustard oil rich in erucic acid has resulted into myocardial lipidosis as erucic acid is known to cause health issues in human beings, whereas oils extracted from RLC 3 and GSC 7 were higher in oleic acid (66.1 and 64.2%, respectively) and lower in erucic acid (1.3 and 0.5%, respectively). Oleic acid is the principal lipid of a class that makes low density lipo-proteins (LDLs) resistant to oxidation and thereby lowers the chances of atherosclerosis [21]. Recent dietary guidelines have also suggested the desirability to substitute cooking oil used in daily diets with MUFA rich oils. MUFA rich cooking oils do not oxidize at a faster rate like PUFA rich oils due to single carbon bond and unlike animal fats these do not exaggerate the SFA levels in the blood stream. Olive oil is considered as the most prominent source of MUFA. India is only a consumer and not a producer of olive oil which makes this oil a costlier and thereby inaccessible to the large section of population in India. Our results corroborated with the results documented by earlier researchers [22]. A significant (p < 0.01) difference was observed in the levels of fatty acids such as palmitic acid (2.2 & 4.2%, respectively), oleic acid (10.1 & 66.1%, respectively) and erucic acid (51.1 & 1.3%, respectively) of PBR 357 and RLC 3 oils, whereas no significant difference was observed in the levels of stearic acid, linoleic acid, α-linolenic acid, MUFA and PUFA of both the PBR 357 and RLC 3 oils. Similarly, the fatty acid composition of GSL 1 and GSC 7 oil showed a significant (p < 0.01) difference in the fatty acid such as oleic acid (24.1 and 64.2%, respectively) and erucic acid (36.5 and 0.5%, respectively). The mean values of stearic acid were found significantly (p < 0.05) different, i.e., 1.2 and 1.9 percent in GSL 1 and GSC 7 oil, respectively, while no significant difference was observed in terms of palmitic aid, linoleic acid, α-linolenic acid, SFA, MUFA and PUFA among oils extracted from rapeseed varieties. The total amounts of SFA were lower in all the oils of both Brassica varieties.
Fatty acid Composition of the Food Products Prepared Using Conventional Cooking Methods
The fatty acid composition of shallow fried (Parantha) and deep fried product (Poori) is given in Table 4. In terms of shallow fried product, a significant (p < 0.05) difference was observed in the PUFA content of the product prepared using PBR 357 (23.8%) and RLC 3 (27.4%) oils, whereas there was no significant difference in the SFA and MUFA levels present in the food product. The rationale behind minute changes in the overall fatty acid profile of the samples could be less time for frying [23, 24]. Further, a significant difference was observed in the percentage of oleic acid and erucic acid present in the product prepared using PBR 357 (20.0 and 36.3%, respectively) and RLC 3 oils (58.7 and 0.4%, respectively). In case of rapeseed varieties, no significant difference was observed in the palmitic, stearic, linoleic acid content present in the GSL 1 and GSC 7 food product, whereas the level of α-linolenic acid showed a significant (p < 0.05) difference between GSL 1 (6.5%) and GSC 7 (8.5%) product prepared by shallow frying. Also, oleic and erucic acid showed a significant (p < 0.01) difference in both GSL 1 (25.7 and 18.3%, respectively) and GSC 7 (64.2 and 0.4%, respectively) shallow fried product.
Further, the fatty acid composition of deep fried product in PBR 357 and RLC 3 oil showed significant (p < 0.05) difference in C16:0, C18:2 and total SFA content, whereas no significant difference was observed in C18:0, C18:3, MUFA and PUFA in the deep fried product prepared using PBR 357 and RLC 3 oils. However, a reduction was observed in the α-linolenic acid level of both shallow fried and deep fried product as compared to fresh oils of both Brassica varieties. This may be due low stability of α-linolenic acid at high temperature [24]. Still, a significant amount of α-linolenic acid was present in both control and experimental products prepared using oils extracted from Indian mustard and rapeseed varieties. The present research findings were in concordance with earlier studies which documented heat resistance attribute of mustard oil [24, 25]. Overall, a less deterioration and change in fatty acid composition was observed during the frying process. Also, the oil uptake in deep fried product was not high as continuous frying was done. The viscosity of the oil did not get affected, which resulted in less adherence of oil with the product [24].
The fatty acid composition of roasted (Panjiri) and sautéed (Potato-French bean vegetable) food products cooked in Brassica oils is presented in Table 5. Panjiri prepared in PBR 357 and RLC 3 oil showed a significant (p < 0.05) difference in C16:0 and total SFA, whereas C18:0, C18:1, C18:3, C22:1, total MUFA and PUFA showed a significant difference at one percent level of significance. Surprisingly, the levels of C18:3 were very low in the panjiri prepared in PBR 357 oil (2.9%) as compared to RLC 3 oil (12.0%). In contrast, the amount of C18:3 was found at par in the roasted product prepared in GSL 1 (4.7%) and GS 7 oil (4.5%). Moreover, the levels of oleic acid were found higher in the roasted product prepared using GSC 7 oil (60.5%) as compared to product (46.3%) prepared using RLC 3 oil. This may be due to the higher smoke point of GSC 7 (234.3 °C) as compared to RLC 3 (223.7 °C). Also, MUFA (oleic acid) is oxidized at much slower rate as compared to PUFA. Moreover, high amounts of oleic acid are connected with a reduced risk of coronary heart disease. Thus, MUFA rich oil encourages a desirable result pertaining to health benefits [26].
The Potato-French bean vegetable sautéed in PBR 357 and RLC 3 oils showed a significant (p ≤ 0.01) difference in palmitic acid, oleic acid, α-linolenic acid (C18:3) and erucic acid (C22:1). Overall, there was no significant difference in the SFA, MUFA and PUFA content of vegetable cooked in both PBR 357 and RLC 3 oils. On the other hand, the vegetable, sautéed in GSL 1 and GSC 7 oil showed a significant (p < 0.01) difference in C18:1, C22:1 and total SFA. Whereas, C16:0, C18:2 and C18:3 showed a significant difference at five percent level of significance. Overall, the total MUFA content was found at par in the vegetable cooked in all oils of both Indian mustard and rapeseed varieties, whereas the level of erucic acid was found lower in the sautéed product prepared by GSC 7 oil (0.6%) as compared to the product prepared using RLC 3 oil (0.8%). The consumption of 15 g rapeseed oil with 2 percent erucic acid content still provides 300 mg erucic acid [2]. In our study, all the food products prepared by GSC 7 and RLC 3 oils contained low erucic acid content which illustrated that these low erucic acid oilseed varieties were safe for human consumption.
Oxidative Stability of Food Products
Two indicators namely peroxide value and free fatty acids were used to evaluate the oxidative stability of the food products prepared using conventional cooking methods using Brassica oils (Table 6). The shallow fried product (Parantha) prepared using PBR 357 and RLC 3 oil showed no significant difference in the peroxide value, whereas a significant (p < 0.05) difference was observed in the PV of products prepared using GSL 1 and GSC 7 oils. The average PV values were recorded as 1.5 and 1.4 meq/kg in the products cooked in GSL 1 and GSC 7 oils, respectively. Though the PV was found less than the codex standard peroxide value (< 10 meq/kg), still, thermal oxidation occurred but to a lesser extent. This may be due to the less time used in shallow frying. In case of deep fried product (Poori), a significant (p < 0.05) difference was observed in the PV of products prepared using PBR 357 and RLC 3 oils (0.4 and 0.3 meq/kg, respectively) as well as GSL 1 and GSC 7 (0.3 and 0.2 meq/kg, respectively) oils. Comparatively, a lesser PV was observed in the food product deep fried in the RLC 3 and GSC 7 oils than PBR 357 and GSL 1 oils. This can be attributed to the presence of high oleic acid which has good oxidative stability [27].
The PV of roasted food product (Panjiri) prepared in PBR 357 and GSC 7 oil showed a significant (p < 0.01) difference with an average value of 0.4 and 0.3 meq/kg, respectively, whereas there was no significant difference in the corresponding figures of the product prepared using oils of rapeseed varieties. The PV was found less in experimental oils as compared to control counterpart but in agreement with the standard peroxide value, i.e., < 10 meq/kg. This may be due to presence of comparatively higher total antioxidants present in the oils extracted from RLC (525.3 mg/kg) and GSC 7 (616.3 mg/kg). Both of these antioxidants help shielding the oil from oxidation in a synergistic manner. Similar trends were also observed in the sautéed food product prepared using both Brassica oils.
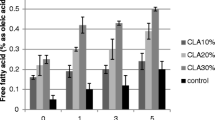
FFA is an indicator of freshness, storage time and stability of fat rich foods. The recommended level of free fatty acid in processed vegetable oil is 0.5 percent [28]. The maximum tolerable limit of FFA for mustard oil has been given as 3 percent (% oleic acid) [29]. All the food products prepared by different cooking methods using all Brassica oils evaluated in the present study depicted FFA levels within the permissible limits. The highest value of FFA (0.6%) was observed in the shallow fried product prepared using GSL 1 oil. This may be attributable to the immediate application of oil on the parantha on a pre-heated hot griddle. Even though the heating time is short, but shallow frying allows free access to air which leads to oxidation [30]. Besides, the lowest value of FFA (0.3%) was recorded in the roasted food product prepared using GSC 7 oil. This may be due to the presence of high level of oleic acid (60.5%) in the product.
Conclusion
Among all Brassica oils, GSC 7 found to be the most stable oil for cooking. The fatty acid composition of RLC 3 and GSC 7 canola oil was consistent with nutrition recommendations aimed at reducing the amount of saturated fat in the diet (< 7% of total fatty acids). Both low erucic varieties contained high level of oleic acid (64–66%) and an intermediate level of PUFA (32%) of which α-linolenic acid made up approximately one-third of total fatty acids. In terms of product development, GSC 7 oil exposed to all conventional cooking methods reported as the best medium for cooking based on fatty acid composition and oxidative stability. And, sautéing was observed as the least harmful cooking method. Hence, the varieties of canola oil may be promoted to increase local as well as national production so as to make the canola oil easily available and affordable to the consumers.
References
Kumar A, Sharma P, Thomas L, Agnihotri A, Banga SS (2009) Canola cultivation in India: scenario and future strategy. In: 16th Australian research assembly on Brassicas, Ballarat Victoria, pp 1–5
Vetter W, Darwisch V, Lehnert K (2020) Erucic acid in Brassicaceae and salmon—an evaluation of the new proposed limits of erucic acid in food. NFS Journal 19:9–15
Sandhu S, Kaur G (2018) Punjab state—the potential canola oil hub of country. Presented at SEA Rapeseed-Mustard conclave, Jaipur, India
AOAC (2000) Oils & fats. In: William H (ed) Official methods of analysis of AOAC international. USA, AOAC International, pp 1–69
JCAM (2013) Quantitative analysis of vitamin E in vegetable oils. Jpn Customs Anal Methods 107:R3
Gao G, Xiaoming WU, Guangyuan LU, Biyun C, Kun XU (2007) Analysis of carotenoid in seed of several oil crops. In: Proceedings of the 12th international rapeseed congress, V, pp 82–84
Choudhary M, Grover K, Kaur G (2015) Development of rice bran oil blends for quality improvement. Food Chem 173:770–777
Appleqvist LÅ (1968) Rapid methods of lipid extraction & fatty acid ester preparation for seed & leaf tissue with special remarks on preventing the accumulation of lipid contaminants. Ark Kenci 28:351–370
AOCS (2003) Sampling & analysis of commercial fats & oils. AOCS official method Cd 8-53 surplus peroxide value acetic acid–chloroform method definition. USA, AOCS Cold Spring Harbour
Berger GK (2005) Frying oil series—the use of palm oil in frying. Malaysia, Malaysian palm oil promotion council pp 4–113
Muhammad N, Bamishaiye E, Bamishaiye O, Usman L, Salawu M, Nafiu M, Oloyede O (2011) Physicochemical properties and fatty acid composition of cyperus esculentus (Tiger nut) Tuber Oil. Biores Bull 5:51–54
Rahman MH, Rahman SS, Nasreen L, Begum UH, Rahman YM, MN, (2017) Biochemical properties and chromatographic investigation of rape seed oils in Bangladesh. Int J Nutr Metabol 9:48–57
Richards A, Wijesundera C, Salisbury P (2008) Genotype and growing environment effects on the tocopherols and fatty acids of Brassica napus and Brassica juncea. J Am Oil Chem Soc 85:159–168
Abidi S, List G, Rennick K (1999) Effect of genetic modification on the distribution of minor constituents in canola oil. J Am Oil Chem Soc 76:463–467
Dolde D, Vlahakis C, Hazebroek J (1999) Tocopherols in breeding lines and effects of planting location, fatty acid composition and temperature during development. J Am Oil Chem Soc 76:349–355
National Academy of Sciences (2000) Report of the panel on dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. Food and Nutrition Board, National Academy Press, Washington, p 187
Cho K, Mabasa L, Fowler AW, Walsh DM, Park CS (2010) Canola oil inhibits breast cancer cell growth in cultures and in vivo and acts synergistically with chemotherapeutic drugs. Lipids 45:777–784
Bhatia E, Doddivenaka C, Zhang X (2011) Chemo-preventive effects of dietary canola oil on colon cancer development. Nutr Cancer 63:242–247
Antova GA, Angelova-Romova MI, PetkovaZh Y, Teneva OT, Marcheva P (2017) Lipid composition of mustard seed oils (Sinapis alba L.). Bulgar Chem Commun 49:55–60
Dorni C, Sharma P, Saikia G, Longvah A (2018) Fatty acid profile of edible oils and fats consumed in India. Food Chem 238:9–15
Kotlyarov S, Kotlyarova A (2022) Involvement of fatty acids and their metabolites in the development of inflammation in atherosclerosis. Int J Mol Sci 23:1308
Nikzad N, Ghavami M, Seyedain-Ardabili M, Akbari-Adergani B, Azizinezhad R (2021) Effect of deep frying process using sesame oil, canola and frying oil on the level of bioactive compounds in onion and potato and assessment of their antioxidant activity. Food Sci Technol Campinas 41:545–555
Szabo Z, Marosvölgyi T, Szabo E, Koczka V, Verzar Z, Figler M, Decsi T (2022) Effects of repeated heating on fatty acid composition of plant-based cooking oils. Foods 11:192
Choudhary M, Grover K, Javed M (2015) Effect of deep-fat frying on fatty acid composition and iodine value of rice bran oil blends. Proc Natl Acad Sci India Sect B Biol Sci 85:211–218
Burton WA, Pymer SJ, Salisbury PA, Kirk JTO, Oram RN (1999) Performance of Australian canola quality Indian mustard breeding lines. In: Proceedings 10th international rapeseed congress, Canberra, Australia
Alireza S, Tan CP, Hamed M, Che Man YB (2010) Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Int Food Res J 17:295–302
Fullana A, Carbonell-Barrachina AA, Sidhu S (2004) Volatile aldehyde emissions from heated cooking oils. J Sci Food Agric 84:2015–2021
Mariod AA, Ali RT, Ahmed YM, Abdelwahab ST, Abdul AB (2010) Effect of the method of processing on quality oxidative stability of anhydrous butter fat (Samm). Afr J Biotechnol 9:1046–1051
Ranganna S (2005) Handbook of analysis and quality control for fruit and vegetable products. Mc-Graw Hill, New Delhi
Pokorny J, Panek J (2012) The effect of natural antioxidants in herbs and spices on food and shelf-life. In: Peter KV (ed) Handbook of herbs and spices. Woodhead Publishing, Sawston, pp 51–71
Acknowledgements
The authors are thankful to the Oilseed section, Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana, for providing infrastructural facilities for fatty acid analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement Low erucic GSC 7 Brassica oil found as the most stable and healthiest cooking oil among PBR 357, RLC 3and GSL 1 cultivars.
Rights and permissions
About this article
Cite this article
Saklani, S., Grover, K., Choudhary, M. et al. Fatty Acid Composition and Oxidative Potential of Food Products Prepared Using Low Erucic Brassica Oils. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 93, 107–117 (2023). https://doi.org/10.1007/s40011-022-01415-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-022-01415-6