Abstract
Clean label products with natural antioxidants have always attracted great attention due to healthy benefits. In this study, the stability of palm olein oil with various natural antioxidants (cedarwood, citronella, clove, nutmeg and rosemary oil) during the heat treatment was investigated; the acidic value, conjugated diene and triene content, peroxide value, thiobarbituric acid reactive substance value and instrumental color of the palm olein oil were analyzed. Sample with nutmeg oil exhibited the highest oxidation stability. In addition, the higher the nutmeg oil concentration, the better the prevention of oil oxidation during the heat treatment. During the repeated frying with corn extrudate, nutmeg oil concentration of 4 g/kg palm olein oil was able to prevent lipid degradation. The acidic and peroxide value of the palm olein oil in the fryer was statistically similar to that of the extracted oil from the fried extrudate. Nutmeg oil can be considered as a potential natural antioxidant for industrial frying of snack food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the most common methods of food cooking is deep fat frying [1]. Frying operation provides a desirable flavor and texture to the fried food. However, when the oil is repeatedly used at high temperature and in the presence of air, the undesirable flavor can be achieved. Due to thermal oxidation, the formations of volatile and non-volatile decomposition products are observed and the oil gradually deteriorates [2]. Severe decomposition of frying oils compromises the fried food quality as well as creates a potential hazard to the health of the consumers [3]. Many products of oil oxidation such as aldehydes, epoxides, hydroxyketones, dicarboxylic and carbonyl compounds are known as inducers of tissue injury in several pathological conditions including cardiovascular diseases, colon cancer, atherosclerosis, diabetes, neurodegenerative diseases and aging [4]. Different methods have been applied to food frying for prevention of oil oxidation and use of antioxidants is an efficient method. Synthetic antioxidants are widely used in food frying due to low cost and high stability. However, consumer concern regarding food safety has motivated the food producers to use natural antioxidants [5]. Various preparations from plant source have been applied to food frying including majorana extract [6], green tea extract [7], Teucrium polium extract [8], raja banana peel extract [9].
Fried snack foods are consumed worldwide due to great variety in shape, flavor and texture. In South East Asia, palm olein oil is widely used in snack frying due to its low cost and high stability against oxidation. In addition, palm olein oil has a low melting point (22–24 °C) resulting in no waxy or greasy mouth-feel of the fried products [10].
During snack frying, the oil is absorbed by the product and thus has a great impact on the product quality [11]. Currently, natural antioxidant preparations are commercially available in the world market. However, little consideration has been given to them in snack food frying. The objectives of this research were to (i) investigate the effects of commercial natural antioxidants as well as the effects of antioxidant content on the quality of palm olein oil during the heat treatment at elevated temperature, (ii) examine the effects of commercial natural antioxidant on the quality of palm olein oil during the frying of snack food.
Methods and materials
Materials
Palm olein was provided by Tuong An Vegetable Oil Joint Stock Company (Ho Chi Minh City, Vietnam). The peroxide value and free fatty acid were 0.01 meq/kg and 0.6 g/kg, respectively. Six natural antioxidants and a synthetic antioxidant used in the study are shown in Table 1.
High fiber snack for the frying test was produced from 87.8% corn meal, 7.5% polydextrose, 4% sugar and 0.7% salt (The ratio was calculated on dry weight basis). A twin-screw extruder (MPF 80/15 model, APV Baker, Peterborough, The United Kingdom) with the average productivity of 300 kg/h was used [12]. The extruded products were immediately sealed for frying test.
All chemicals used in this study were originated from Merck (Darmstadt, Germany) and of the analytical grade.
Oil heating test
The effects of natural antioxidant preparations on prevention of oil oxidation were evaluated by using oil heating test which was performed according to the procedure of Chammem et al. [13] with slight modification. Mixture of palm olein oil and antioxidant preparation was heated at 180 ± 2 °C for 6 h per day for 5 consecutive days in an electric fryer (OFE-321F model, Henny Penny Corporation, Eaton, The United States). At the end of the daily heating, 100 mL of oil was collected for analysis of free fatty acid content, conjugated dienes and triens, peroxide value, thiobarbituric acid reactive substances and instrumental color.
Effects of natural antioxidants on the palm olein oil quality during the heat treatment
Nutmeg, cedarwood, citronella, clove and rosemary oil samples were used as natural antioxidants in the oil heating process. For comparison, tocopherol and butylated hydroxytoluene (BHT) were also used. Each antioxidant was blended with palm olein oil at the concentration of 2 g/kg. The control sample was performed without antioxidant addition.
Effects of nutmeg oil concentration on the palm olein oil quality during the heat treatment
Nutmeg oil was selected to add to palm olein oil at various concentrations: 1, 2, 3, 4 and 5 g/kg. The heat treatment procedure was similar to that of the previous experiment.
Snack frying test
Effects of nutmeg oil on the palm olein oil quality during the snack frying
Nutmeg oil was added to palm olein oil at the concentration of 4 g/kg. The extrudate was fried with 1 g per 10 g of palm olein oil and the frying was performed for 5 min. After removal of the extrudate from the fryer, the oil temperature was risen up to 180 °C again in 5 min; 15 frying batches were carried out per day for 5 consecutive days and the frying process was repeated three times. At the end of the daily frying process, 100 mL of oil was collected for analysis of free fatty acid content, conjugated dienes and trienes, peroxide value, thiobarbituric acid reactive substances and instrumental color.
Chemical analysis
Acidic value of oil was measured by ISO 660 method (ISO, 2009). The conjugated dienes and conjugated trienes were analyzed by the absorbance at 232 and 268 nm, respectively according to official method 2.206 of the IUPAC (1987) [14]. Peroxide value of oil was determined by ISO 3960 method (ISO, 2007). The thiobarbituric acid reactive species (TBARS) assay was conducted according to the procedure previously described [15].
Instrumental color analysis
Color of oil was determined by using Konica Minolta spectrophotometer (CR 410 model, Konica Minolta Inc, Osaka, Japan). Color data were presented by CIE L*, a*, b* coordinates. Three measurements were taken for each sample and averaged [16].
Statistical analysis
All experiments were carried out in triplicate. The experimental results were expressed as means ± standard deviation (n = 3). Mean values were considered significantly different when the probability was less than 0.05 using multiple range test. One-way analysis of variance was conducted by using the software Statgraphics Centurion XV (Manugistics Inc., Rockville, MD, The United States).
Results and discussion
Effects of natural antioxidants on the palm olein oil quality during the heat treatment
Change in oil quality during the heat treatment is described in Table 2. The measurement of acidity is a frequently used test to assess the oil quality during food frying. Slight increase in acidity during the heating was observed for all oil samples. It can be suggested that triglyceride hydrolysis is not the main reason for the increased acidity since food is not present in the heated oil. According to Chammem et al. [13], increase in acidic value during the oil heating is mainly due to the degradation of secondary products of oil oxidation such as alcohols, ketones and aldehydes [17].
At the end of the heating, the acidity of the control sample was significantly higher than that of all antioxidant added samples. It is clear that addition of antioxidant significantly reduced oil deterioration during the heat treatment. Similar results are reported when the rosemary extract is added to the heated sun flower oil [18].
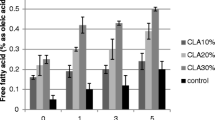
Although the antioxidant quantity was fixed at 2.0 g/kg for all samples, the acidity varied from 0.20 to 0.34% at the end of the treatment. The lowest acidity was reported for nutmeg oil. The acidity of the oil sample with nutmeg oil was 44.4% lower than that of the control. This is an indication that nutmeg oil can effectively prevent lipid oxidation.
Formation of conjugated dienes and trienes is mainly associated to the production of primary oxidation products [18]. The polyunsaturated fatty acid oxidation occurs with the formation of hydroperoxides. After peroxides have been formed, the non-conjugated double bonds presenting in natural unsaturated lipids immediately suffer a rearrangement generating conjugated dienes. When polyunsaturated fatty acids containing three or more double bonds undergo oxidation, the conjugation can be extended to include another double bond resulting in the formation of conjugated trienes [19]. Table 2 shows that the conjugated diene content was low at the beginning of the process but tended to increase with the increase in heating cycles. Notably, the lowest conjugated diene contents at the end of the heat treatment were reported for the oil samples with nutmeg and rosemary oil; these values were 32.0% and 28.0%, respectively lower than that of the control sample. The increase in conjugated diene content is proportional to the uptake of oxygen and formation of peroxides during the early stages of oxidation as well as to the degradation rate of linoleic acid [20].
Table 2 presents gradual augmentation of conjugated triene content for all samples during the heat treatment. According to Guo et al. [21], as the production rate of ketones is higher than their decomposition rate, the conjugated triene content continues to increase. At the end of the heat treatment, the lowest conjugated triene content was reported for the oil sample with nutmeg; this value was 27.7% lower than that of the control sample which had the highest conjugated triene concentration. It can be noted that cedarwood oil is not efficient for prevention of the formation of both conjugated diens and triens since their contents were similar to those of the control sample at the end of the heat treatment.
Peroxide value is an indicator of primary products of oil oxidation [13]. At the beginning of the heating, all oil samples had similar peroxide value. During the first step of the heat treatment (until 18 h), increase in peroxide value was observed for all samples. Nevertheless, the lowest maximum peroxide value was reported for the sample with nutmeg oil and rosemary oil while the control sample and the sample with cedarwood oil had the highest value. The lower the maximum peroxide value during food frying, the greater the delaying effect of oil oxidation and the more effectiveness of antioxidant preparation [13]. During the second step of the heat treatment (after 18 h), the peroxide value reduced sharply for all samples excluding the sample with tocopherol the peroxide value of which remained constant. This could be attributed to the decomposition of secondary products such as aldehydic compounds [22]. It can be noted that the sample treated with tocopherol was still in the early stage of oil oxidation while the other samples were at the later stage of the oxidation process. Similar observation is reported when rosemary ethanol extract was added to palm oil during the potato frying [21].
Secondary products of oil oxidation can be estimated by the 2-thiobarbituric acid test [4]. No statistical difference in malonaldehyde content among all samples (p > 0.05) was observed at the beginning of the heat treatment whereas the malonaldehyde content gradually increased as the heating time increased due to oxidative degradation of the palm olein. At the end of the heat treatment, the malonaldehyde content was reduced in the order: control > cedarwood > clove > citronella > rosemary > BHT = tocopherol > nutmeg. Notably, all oil samples with different antioxidant preparations had a lower malonaldehyde content than the control sample (p < 0.05) due to higher resistance towards generation of oxidative products.
Particularly, the malonaldehyde content of the sample with nutmeg oil was 55.8% lower than that of the control sample. Reduction in malonaldehyde content is also reported when the extracts of pomegranate (Punica granatum L.) peel, oregano and rosemary are separately blended with palm oil during deep-fat frying [23].
Colors are an important physical indicator of oil quality. Changes in the instrumental color of the oil samples during the heat treatment are reflected in Table 2. At the beginning of the heating, the color of the oil samples was slightly different since the antioxidant preparations used in this study had various colors. At the end of the heat treatment, the L* and a* values of all samples were decreased while the b* value was increased. According to Lalas [24], reduction in lightness is due to the formation of dimmers, trimer and tetramers through oxidative and thermal reactions. Moreover, the darkening of oil color is due to the oxidation of phenolic antioxidants in the oil during the heating [25]. However, the L* value of samples with citronella, cedarwood oil and BHT was more reduced than that with nutmeg, tocopherol, clove and rosemary oil. The intensity of yellow color was increased throughout the heating process due to the generation of carotenoids and xanthophylls in the palm olein oil [18]. In addition, the terpenoid in citronella would shift colors upon oxidation by heat treatment [26].
Effects of nutmeg concentration on the palm olein oil quality during the heat treatment
The effects of nutmeg oil concentration on the palm olein oil quality during the heat treatment are shown in Table 3. During the treatment time, the acidity content of all samples was gradually increased. The higher the concentration of nutmeg oil, the lower the acidity of palm olein oil at the end of the heat treatment. According to Matulyte et al. [27], the major components in the nutmeg oil are the myristicin, safrole, α-pinene, β-pinene and sabinene which have strong antioxidant activity.
The conjugated dienes and trienes content of all samples gradually increased from 0 to 30 h of the heat treatment (Table 3). As the nutmeg concentration in the blended oil was increased, the formation of conjugated dienes and trienes was significantly decreased. The lowest conjugated trienes contents were reported for the sample of 4 and 5 g/kg and these values were 37.5% and 61.1%, respectively lower than that of the control sample. According to Adiani et al. [28], both myristicin and safrole in nutmeg oil are stable even at high temperature and that effectively decelerates the formation of conjugated dienes and trienes during the heat treatment.
Table 3 also shows that the initial peroxide value of all samples was insignificantly different (p > 0.05). When the nutmeg oil concentration varied from 0 to 2 g/kg palm olein oil, the peroxide value increased during the early stage of the heat treatment and then reduced at the later stage. Nevertheless, the maximum peroxide value of the control samples was 1.9 and 2.2 times higher than that of the sample with 1 and 2 g/kg nutmeg oil with palm olein oil, respectively. In addition, as the nutmeg oil concentration increased from 3 to 5 g/kg palm olein oil, gradual increase in peroxide value was clearly observed and degradation of hydroperoxides was therefore not predominant during the heat treatment. It can be inferred that the higher the nutmeg oil concentration, the better the prevention of oil oxidation. However, at the end of the heat treatment, the peroxide value of from 2 to 5 g/kg samples was statistically similar. Similar results are observed when palm oil is blended with nutmeg oil during the high temperature storage [29].
Changes in the malonaldehyde content are also presented in Table 3. As the treatment time increased, the malonaldehyde content of all samples gradually increased. As the nutmeg oil concentration increased from 0 to 5 g/kg, the malonaldehyde content at the end of the heat treatment significantly (p < 0.05) decreased. Similarly, Kaur et al. [15] reports that increase in carotenoid or γ-tocopherol content in soybean oil also reduced the malonaldehyde content when the treated oil samples were preserved at 60 °C.
Changes in the oil color are shown in Table 3. The L* and a* values of all oil samples decreased during the heating process while the b* value increased. Although the nutmeg oil concentration in the samples was different, their difference in instrumental color was minor at the end of the heat treatment.
Effects of nutmeg oil on the palm olein oil quality during the extrudate frying
Table 4 presents the quality of palm olein oil during the extrudate frying. The acidity was gradually increased for the both samples. According to Erickson [30] during the frying of food with high moisture content, triglyceride hydrolysis is mainly responsible for the increased acidity. Similar increase in acidity is also reported when rosemary extract was added to sunflower oil for potato frying [18]. At the end of the 5th day, the acidity of the control sample was 62.5% higher than that of the nutmeg oil added sample. During the extrudate frying, nutmeg oil strongly retards lipolysis due to sabinene components including terpenes groups which are main antioxidants in nutmeg oil [31].
In this experiment, the obtained fried snack was sampled and immediately used for oil extraction; the acidity of the extracted oil was insignificantly different to that of the oil in the fryer (p > 0.05).
Table 4 also showed that the conjugated diene and triene contents were gradually increased from day 0 to day 5. At the end of the extrudate frying, the conjugated diene and triene contents of nutmeg added sample was 22.2% and 41.6%, respectively lower than that of the control sample. Similarly, Jaswir et al. [32] reports that palm olein oil with rosemary or sage extract has significantly lower conjugated diene and triene content than the control sample during potato frying.
Changes in peroxide value of the control sample were divided into two stages while only one stage was recorded for the nutmeg oil added sample. The peroxide value of the control sample was increased from day 0 to day 3 which was the end of the first stage; then it came to the second stage when the peroxide value was gradually decreased by the end of the frying test. Whereas, the peroxide value of the nutmeg oil added sample was gradually increased from the beginning to the end of the frying test. Notably, the maximum peroxide value of the control sample was 76.1% higher than that of the nutmeg oil added sample. In the 32 h potato frying, Che Man and Jaswir [33] reports that the addition of rosemary extract at a percentage of 0.4% prevents the increase in peroxide value which was reduced by 37% in comparison with that of the control. In the present study, the peroxide value of the extracted oil from the fried snack was statistically similar to that of the fried oil (p > 0.05).
During the extrudate frying, the malonaldehyde content in the oil was gradually increased from day 0 to day 5 for both the control and nutmeg oil added sample. At the end of the frying, the malonaldehyde content of the palm olein oil sample added with nutmeg oil was reduced by 40.7% compared to that of the control sample. Similar observation is reported when oleoresin rosemary and sage extract are added to palm olein oil during the potato frying process [34].
Slight change in color was recorded during the repeated frying process. The brightness of oil (L*) was decreased from 86.0 to 84.3 for the control while that was reduced from 86.8 to 85.9 for the nutmeg oil added sample. Likewise, the redness (a*) was decreased from − 1.9 to − 4.1 and from − 1.7 to − 3.6 for the control and nutmeg oil added sample, respectively. However, the yellowness (b*) was increased from 6.8 to 16.5 for the control while it was raised from 7.2 to 14.5 for the nutmeg oil added sample at the end of the frying. This may be due to the accumulation of non-volatile decomposed compounds such as oxidized triacylglycerols and free fatty acid. In addition, the darkening of oils is partly due to the absorption of color from the fried food [35]. Similar color changes in lightness (L*) and chromatic coordinates (a* and b*) are also reported when palm olein oil with BHT or ascorbyl palmitate is repeatedly fried with churros at 180 °C [36].
Conclusions
In this study, various natural antioxidants were used to enhance the oxidative stability of the palm olein oil during the heat treatment. Citronella, nutmeg, clove and rosemary oil preparations significantly retarded the formation of conjugated dienes and trienes as well as primary and secondary oxidation products of palm olein oil. Notably, addition of nutmeg oil to palm olein oil resulted in the best oxidation stability among the tested natural antioxidant preparations. The higher the nutmeg oil concentration, the better the prevention of oil oxidation during the heat treatment. The nutmeg oil concentration of 4 g/kg palm olein oil was able to maintain oxidation stability of palm olein oil when the extrudate was daily fried for 15 batches and for 5 consecutive days. The quality of palm olein oil in the fryer and the quality of extracted palm olein oil from the fried extrudate was insignificantly different. Further studies are required to understand the mechanism by which phenolics compounds in nutmeg oil inhibit or retard the oxidative process during the snack frying with palm olein oil.
Data availability
Data and material are available on request.
References
R. Sayyad, J. Food Sci. Technol. 54(8), 2224–2229 (2017)
M. Kasprzak, M. Rudzińska, R. Przybylski, D. Kmiecik, A. Siger, A. Olejnik, LWT 123, 109078 (2020)
F. Esfarjani et al., Food Sci. 7(7), 2302–2311 (2019)
D.J. Charles, Antioxidant Properties of Spices, Herbs and Other Sources (Springer, New York, 2012)
V. Raikos, EC Nutrition 8(2), 33–34 (2017)
G. Al-Bandak, V. Oreopoulou, Int. J. Food Sci. Technol. 46(2), 290–296 (2011)
B. Aydenız, E. Yilmaz, Food Technol. Biotechnol. 54(1), 21–30 (2016)
Y. Asadi, R. Farahmandfar, Food Sci. Nutr. 8(2), 1187–1196 (2020)
D.Y. Purwaningsih, D.R. Zuchrillah, I. Nurmala, IOP Conf. Ser.: Mater. Sci. Eng. 462, 012034 (2019)
O.I. Mba, M.-J. Dumont, M. Ngadi, Food Biosci. 10, 26–41 (2015)
J.C. Lumanlan, W.M.A.D.B. Fernando, V. Jayasena, Int. J. Food Sci. Technol. 55(4), 1661–1670 (2020)
J.H. Yang, T.T.T. Tran, V.V.M. Le, LWT-Food Sci. Technol. 96, 1–6 (2018)
N. Chammem et al., Ind. Crops. Prod. 74, 592–599 (2015)
Y. Zhong, T. Madhujith, N. Mahfouz, F. Shahidi, Food Chem. 104(2), 602–608 (2007)
D. Kaur, D.S. Sogi, A.A. Wani, Int. J. Food Prop. 18(12), 2605–2613 (2015)
M. Kowalska, M. Woźniak, A. Żbikowska, M. Kozłowska, Biomolecules 10(1), 115 (2020)
K. Warner, in Advances in Deep-Fat Frying of Foods, ed. by S.G. Sumnu, S. Sahin (CRC Press, Florida, 2008), pp. 201–214
S. Urbančič, M.H. Kolar, D. Dimitrijević, L. Demšar, R. Vidrih, LWT-Food Sci. Technol. 57(2), 671–678 (2014)
M.-A. Poiana, Int. J. Mol. Sci. 13(7), 9240–9259 (2012)
Y.B. Che Man, J.L. Liu, B. Jamilah, R. Abdul Rahman, J. Food Lipids 6(3), 181–193 (1999)
Q. Guo, S. Gao, Y. Sun, Y. Gao, X. Wang, Z. Zhang, Ind. Crops. Prod. 94, 82–88 (2016)
M. Manral, M.C. Pandey, K. Jayathilakan, K. Radhakrishna, A.S. Bawa, Food Chem. 106(2), 634–639 (2008)
T. Hemachandra, R. Jayathilake, W. Madhujith, Trop. Agric. Res. 28(3), 247–255 (2017)
S. Lalas, in Advances in Deep-Fat Frying of Foods, ed. by S.G. Sumnu, S. Sahin (CRC Press, Florida, 2008), pp. 57–80
F.M. Nor, S. Mohamed, N.A. Idris, R. Ismail, Food Chem. 110(2), 319–327 (2008)
C. Turek, F.C. Stintzing, Compr. Rev. Food Sci. Food Saf. 12(1), 40–53 (2013)
I. Matulyte et al., Foods 9(1), 37 (2020)
V. Adiani, S. Gupta, S. Chatterjee, P.S. Variyar, A. Sharma, J Food Sci Technol 52(1), 221–230 (2015)
P.E. Eze-Steven, C.N. Ishiwu, S.C. Udedi, B.O. Ogeneh, Int. J. Curr. Microbiol. Appl. Sci 2, 373–383 (2013)
M.C. Erickson, Food Lipids (CRC Press, Boca Raton, 2002), pp. 382–429
I. Rodianawati, P. Hastuti, M.N. Cahyanto, Procedia Food Sci. 3, 244–254 (2015)
I. Jaswir, Y.B. Che Man, D.D. Kitts, J. Am. Oil Chem. Soc. 77(11), 1161–1168 (2000)
Y.B. Che Man, I. Jaswir, Food Chem. 69(3), 301–307 (2000)
Y.B. Che Man, C.P. Tan, J. Am. Oil Chem. Soc. 76(3), 331–339 (1999)
Y. Srivastava, A.D. Semwal, J. Food Sci. Technol. 52(2), 984–991 (2015)
R. Castro-Lopez, J.A. Gómez-Salazar, A. Cerón García, M.E. Sosa-Morales, Presented at the CSBE/SCGAB 2017 Annual Conference Winnipeg, Manitoba, Canada, 6–10 August 2017, Paper No CSBE17101 p. 1–7
Funding
This study was not funded by any source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J.H., Tran, T.T.T. & Le, V.V.M. Effects of natural antioxidants on the palm olein quality during the heating and frying. Food Measure 14, 2713–2720 (2020). https://doi.org/10.1007/s11694-020-00517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00517-x