Abstract
The present study addresses the sensitivity of rice species to varying concentrations of Al toxicity. Aluminium salt for plants was used in increasing order (240, 360, 480 µM) during short period to decipher the impact of metal stress on metabolic status with reference to oxidative damages. Interestingly, plants responded well with increase in linear root growth. In a dose dependent manner of metal concentration, plants suffered more from developed ROS (both O2− and H2O2) in root cortex. The histochemical detection of tissue lysis as detected by Evans blue and Hematoxylin was in proportionate to the aluminium concentration over control. In response to peroxide radical accumulated in the tissues, plants were characterized in a variable manner for APX, CAT and GR activities. Still, on protein polymorphism of these genes, the plants responded well with a distinct expression varied over control. In support of decreased activity, a single band expression was key feature to characterize the plants under Al toxicity. Plants though maintained a stable proportion of non-thiol content but a steeper up regulation of GR activity at highest concentration of Al was indicating for more GSH recruitment in oxidative stress. Banding patterns of APX, CAT and GR through Al concentrations appeared as bio-indices under metal reactivity in rice species. Betaine aldehyde dehydrogenase was also in proportionate manner to support the synthesis of osmolyte under metal toxicity. This is more relevant with protein expression of aldehyde dehydrogenase activity and distinct bands favor the gene expression under modulation of metal stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminium (Al), the 3rd abundant metal in earth crust has been a great threat to the plant’s development and productivity, particularly, in soil with high acidity [1]. In the tropics and semi-tropic regions, the problems of Al toxicity become more prone with soil with low pH (< 5.0). This causes tricky to solubilize Al3+ in its exchangeable forms particularly, when lime practices are customized. This is more affected in submerged acid soil where sub-surface abundance of soluble Al (mostly the aluminium silicate) becomes hindered in exchange for the plants [2]. Among the cereal crops, typical symptoms of the Al toxicity are restricted to the root apices to disintegrate the tissues with varying amounts. Thus, the bio-indication studies with Al toxicity are confirmed through some typical phenological, physiological as well as molecular changes in roots. These are always in concomitant to species specific responses according to tolerance and susceptible reactions of the host plants [3]. This result occurs under water relation with differential accumulation of Al3+.
Ample evidences are there, where plants are most adversely affected with development of some reactive oxygen species (ROS). In root tissues, particularly, at the growing meristematic zones are the most sensitive with different ROS induced activity. In practical sense regardless of plant species, the ROS generation is happened to be a trait of sensitivity at cellular damages [4, 5]. The diffusible nature of different ROS could lead to interact with major organelle sitting bio-molecules and consequently manifests the physiological and cellular dysfunctions. Among the ROS a few of those (e.g. hydrogen peroxide, H2O2) appears to be relatively stable and safer also to induce anti-oxidation, however, within a threshold values [6]. Al is established as an important metal to induce H2O2. Rice, the most common cereal is displayed with its wider ranges of tolerance to various abiotic stresses. Upland rice varieties have been seriously in constraints for acidic soil (pH ≤ 4.5) with an ample exchange of Al toxicity [7]. Mostly Boron (B) deficiency in this soil is the results of chemical replacement of that element with few aluminium salts. Therefore, ample chances are prevailed with the aluminium toxicity in upland rice varieties in changes of cellular activities in different dimensions. In this context, aroma bearing moieties are important of those rice cultivars and less discussed to be dealt with Al toxicity. Contextually, aromatic rice is physiologically characterized with betaine aldehyde dehydrogenase (BADH) for aroma biosynthesis as well as with modulation with salinity [8]. With this it would not be irrelevant that aluminium stress and its perception at cellular level may be a determinant for the BADH activity. This is more supported with alteration of BADH functioning under different degrees and duration of salinity stress [9].
Physiologically, BADH gene has two functions: in wild or mutated form. For the former, it synthesizes a stable osmolytes viz glycine betaine (GB) following rate limited enzymatic pathways. But, the mutated form of BADH could produce the aroma generating moieties, 2-acetyl pyrroline (2AP) [10]. In general, high aroma containing varieties are exhibited with more sensitivity to water/salinity stress with suppressed activities of BADH in GB synthesis. Therefore, activity of BADH ought to be more corroborated with GB accumulation for plants’ survival under water deficit stress or conditions allied like ROS development. The ROS is more relevant in varying degrees either in partial or full impairment of cellular metabolism under Al contamination in rice [11]. On the other hand, plants developed dehydration under Al hyper-accumulation and induces to GB in expense of BADH activity [12]. In the present study, the authors have focused the BADH activity for GB synthesis along with other cellular functionales, especially, with ROS metabolism of rice varieties under Al stress. The rice variety Dadshal is aroma containing but moderately salt tolerant, employed to test the validity of BADH activity as bio-marker for Al sensitivity in relation to ROS accumulation.
Material and Methods
The experiment was conducted in the laboratory of Plant Physiology and Molecular Biology section, Department of Botany, University of Kalyani, West Bengal, India. The traditional indica rice variety Dadshal (moderate salt tolerant aromatic rice cv.) was collected from Central Soil Salinity Research Institute, Canning, West Bengal, India. The seeds were germinated in seed germinator (37 ± 1 ºC with relative humidity around 80%, 900–1000 µEm−2 s−1 light intensity) under laboratory condition. 10 days old seedlings were treated with various concentration (240, 360 and 480 µM) of aluminium salt (KAl(SO4)2·12H2O) supplemented with Hoagland nutrient media, pH 4.8 [with 0.1(N) HCl]. The plants were transferred in open aerated condition for 7 days. On each 2 days intervals the solution was renewed to check any shortage of nutrients to the plants. The plant samples were then harvested, dissected into roots and stem, freezed in liquid nitrogen and stored − 80 °C for further biochemical use. After immediate harvesting shoot and root length, fresh weights were recorded with 5 plants from each treatment. For measurement of growth indices plant samples were completely oven dried at 80 °C till the constant weights were not achieved.
Estimation of Al in Plant Tissue
The dried samples were completely digested with a tri mixture (HCl:HNO3:HClO4 = 1:1:1) under constant heat until the color becomes transparent. The clear digested solution was filtered and diluted with deionized water properly and the metal content was analyzed by atomic absorption spectrophotometer (Schimadzu, Model No. A6800) [19]. KAl(SO4)2·2H2O was used as standard and the content was presented on dry weight basis of the tissue. For tissue specific accumulation of Al was detected in vivo by Hematoxylin staining. The tissue is transferred into a solution (10 mg/ml) of hematein (oxidized hematoxylin) to yield the color complex. The individual root samples were stained with hematoxylin solution for 30 min at room temperature. The surface adhered stain in excess was removed by distilled water with several times. Finally the root samples with uniform sizes were selected and observed under stereo microscope.
Histochemical Detection of ROS
Aluminium induced oxidative stress with ROS generation was detected in vivo for both O2− and H2O2. The roots with undamaged tips were placed in an aseptic tubes containing 0.02% Nitro blue solution (0.1 g NBT in 50 mM sodium phosphate buffer, pH = 7.5 and kept in dark condition overnight [13]). The excess solution was drained off, rinsed with warm absolute alcohol to remove the chlorophyll for few times and finally fixed with 70% glycerol (w/v). The blue patches for ROS were observed under microscope for documentation.
For the peroxide accumulation DAB staining was used method [14] with the plant materials. The plant parts were transferred in solution containing 100 mg DAB salt in 100 ml distilled water in glass tubes and kept in dark. The pH of the solution was maintained with 0.1 N HCl. After incubation for overnight the material were gently washed with distilled water followed by absolute alcohol under boiling condition to remove the chlorophyll. The development of brown spots indicating peroxide development was monitored with microscope.
The product of ROS mediated lipid peroxidation was detected as suggested by Pompella et al. [14]. In brief, the roots were stained with Schiff’s reagent [15] for 20 min followed by rinsing with sulfite solution (0.5% [w/v] K2S2O5 in 0.05 M HCl) The retention of the color after washing with Na sulfite solution indicates the lipid peroxide derivatives in root tissue. The loss of integrity of plasma membrane by ROS oxidation was monitored by Evans blue staining [16]. Root slices were transformed in 10 ml solution comprising of 0.05% (w/v) Evans blue in 100 mM CaCl2 at pH 5.6 and incubated for 30 min at room temperature. The excess stain was rapidly washed several times with 100 mM CaCl2, pH = 5.6.
Analysis of Antioxidative Enzymes In Vitro and Their Polymorphism Study
The detection of O2− was done following by the method of Shah et al. [5]. Frozen tissue (1.0 g) was extracted with 65 mM phosphate buffer at pH 7.8. The supernatant after centrifugation at 6000×g for 15 min at 4 °C was mixed in a ratio of 1:2 in 10 mM hydroxylamine hydrochloride following incubation at 25 °C for 30 min. The reaction mixture was added 10 mM sulfanilamide and 1 mM alpha naphthylamine. The absorbance was read at 530 nm to deduce the O2− concentration using 12.8 mM−1 cm−1 as extinction coefficient. For endogenous H2O2 concentration, the method described by Bright et al. [17] was adopted. The tissue (1.0 g) was homogenized thoroughly and extracted with 1% (w/v) PCA. The supernatant was collected after centrifugation at 10,000×g for 15 min at 4 °C and reacted with 10 mM potassium phosphate buffer pH 7 and 1 M KI solution following incubation at dark for 30 min for absorbance at 390 nm. The H2O2 content was determined with calibration curve for 30% H2O2 and expressed as µM g−1 FW.
The plants were initially cut into pieces and kept in frozen condition for future use. From the frozen sample, 1.0 g tissue was ground in liquid nitrogen and thoroughly homogenized in limited volume of buffer: 50 mM Tris HCl pH 8.2, 5 mM β-mercapto ethanol, 1 mM CaCl2 and 0.5% PVP. Initially the homogenate was filter through whatman No. 1 filter paper and centrifuged at 14,000×g for 10 min at 4 °C. The soup was collected and used as enzyme source for in vitro assay. The assay of APX was done according to method suggested by Davletova et al. [18] with modification. The assay mixture containing 0.5 M sodium ascorbate with 100 mM Tris-HCl pH 7.8, 10 mM MgCl2, 100 mM EDTA, 10 mM DTT, 0.2 mM H2O2 was added with 100 µg equivalent enzyme protein. The absorbance was read immediately at 290 nm. The activity was expressed with extinction coefficient 2.8 mM cm−1 as µM ascorbate oxidized min−1 mg−1 protein. For analysis of polymorphisms the protein was initially precipitated with 80% ammonium sulfate overnight at 4 °C, collected the soup, dialyzed through membrane and dissolved in buffer containing 100 mM Tris HCl pH 7.8, 10 mM MgCl2, 1 mM PMSF, 100 mM EDTA, 10 mM KCl. An aliquot equivalent to 50 µg protein was loaded in 10% native PAGE which was pre run in 2 mM ascorbate for 15 min at 4 °C. After loading the protein, the gel was run for 4 h at 4 °C in the same ascorbate concentration. Finally gel was equilibrated with 50 mM sodium phosphate pH 7.0 contain 2 mM ascorbate for 30 min followed by 50 mM sodium phosphate pH 7.5 with 4 mM ascorbate and 2 mM H2O2 for 20 min. After completion of the run, the gel was washed with subsequent phosphate buffer. To resolve the protein, the gel was incubated in 50 mM sodium phosphate buffer pH 7.8 containing 2.45 mM NBT for 15 min. The reaction was stopped with 20 mM TEMED solution following washing with water to remove the stain in excess.
Activity of the CAT both in vitro and in-gel staining was followed as per Nahakpam and Shah [19]. The partially purified protein was incubated in assay mixture containing 200 mM phosphate buffer (pH 7.0) and 200 mM H2O2. The activity was instantly monitored against a blank (10 mM H2O2) by decreasing the absorbance at 240 nm along with extinction coefficient of H2O2 (0.036 mM cm−1) [20]. The isozymic protein was separated on non-denaturing 10% polyacrylamide gel with 10 V lane−1 at 4 °C for sufficient time. A solution mixture with 1% w/v FeCl3 along with 0.05% H2O2 as substrate was used to submerge the gel. The gel was fixed in 0.1% HCl to visualize the distinct bands.
For GR activity, 1 ml assay buffer containing 50 mM Tris HCl (pH 7.6), 0.5 mM DTT, 3 mM MgCl2, 0.15 mM NAD(P)H and 1 mM oxidized glutathione (GSSG) was added with 100 µg purified protein using the extinction coefficient (6.22 mM cm−1) of NAD(P)H. The unit of enzyme (U) was detected as µM NAD(P)H oxidized min−1 mg−1 protein. The detection of isoform of GR was done by electrophoretic separation of the protein as 50 µg lane−1 in a non SDS PAGE and was run at 60 and 100 V in for stacking and resolving gel respectively. The reaction of glutathione reduction was done on gel in incubation mixture: 0.5 mM NAD(P)H, 3.5 mM GSSG, 0.5 mM EDTA, 25 mM Tris HCl pH 7.6, 0.5 mM DCPIP, 0.1 mM MTT under dark condition until the band was resolved [21, 22].
For BADH activity assay, plant material was crushed in assay mixture containing 50 mM HEPES-KOH pH 8.0, 20 mM sodium metabisulfite, 10 mM sodium borate, 1 mM EDTA, 5 mM ascorbic acid and 5 mM DTT in cold condition as described in Daniell et al. [23]. The crushed material was centrifuged at 10,000×g for 10 min at 4 °C and supernatant was saved. The enzyme activity was assayed in 1 ml assay buffer: 50 mM HEPES-KOH pH 8.0, 1 mM EDTA, 5 mM DTT and 1 mM NAD+. Finally, 1 mM betaine aldehyde (SIGMA-ALDRICH) was added to this assay mixture to initiate the reaction at 22 °C. The reduction of NAD+ was assayed with reading at 340 nm. The extraction buffer containing 250 mM Tris-HCl pH 8.5, 2 mM DTT, 1 mM EDTA and 10 mM GSH was used to homogenize the material and centrifuged at 27,000×g for 10 min at 4 °C. 100 µg equivalent protein was run in 8% native PAGE for sufficient time. To resolve the protein band, gel was incubated in reaction mixture (100 mM Tris-HCl pH 7.5, 0.1 mM PMS, 1 mM MTT, 1 mM indole-3 aldehyde.
Dried and finely powdered leaf material was initially warmed (37 °C) with 20 ml de-ionized water for overnight. The suspension was filtered and soup was distilled with 1:1 2 N H2SO4. An aliquot of 100 µl was incubated on ice rock for couple of hours. A cold solution of potassium iodide-iodine reagent was added in equal volume following agitation gently. The mixture was centrifuged at 15,000×g for 10 min at 4 °C. The supernatant was detected and peridotite crystals were collected and dissolved in 10 ml of 1, 2 dichloro ethane with vigorous shaking. On incubation at 35 °C for 1 h, the absorbance of the mixture was read at 360 nm. Pure glycine betaine was used as standard to calculate the content and expressed mg/g dry tissue [24, 25].
Statistical Analysis
All the observations were recorded using three replications (n = 3) and the experimental data were expressed as mean ± SE. The statistical analysis was performed by ANOVA analysis, taking p ≤ 0.05 levels of significance [26]. Windows Microsoft Excel 2007 software was employed for data analysis.
Results and Discussion
Effects of Al on Growth and Cellular Activities of Root
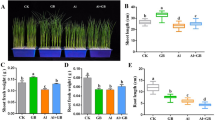
On exposure to Al salt (potassium aluminium silicate; pH = 4.5) for 7 days, rice (cv. Dadshal) had experienced significant changes (p ≤ 0.05) in the root growth. This is more interesting to see unlike other rice land races, it showed a considerable linear increase of roots along with some hairs (Fig. 1a, b). Thus, root’s growth at maximum concentration of Al (i.e. 480 µM) was recorded 1.7 fold over control. Therefore, the effective concentration to maximize the effects of Al may draw the other cellular activities in alteration to this context. In addition, the extent of metal accumulation in root tissues ranged from 1.7 to 3.2 fold in a linear order as compared to control (Fig. 2a). This may possibly give the impetus to probe the metal induced oxidative degeneration of the root tissues. Al accumulation and its effects were more prominent in root tips that dispersed other tissues of the cortex as evident with Hematoxylin (Fig. 2b) and Evans blue (Fig. 3a) staining. The changes in intensities of Hematoxylin staining were more in root tips regardless of Al concentration over control (Fig. 2b). Al accumulation with its other fates in development of peroxide derivatives through lipid peroxidation was marked with Evans blue staining [27]. This is an indirect indication for exposures of oxidative stress and thereby loss of bio-molecules [28]. In a linear and dose dependant manner plants showed a gradual increase in Schiff’s conjugates with metal concentrations (Fig. 3b). The Schiff’s reagent has been convincingly a marker for lipid peroxides and other organic moieties containing carbonyl groups and thus, bears almost direct relationship of metal induced oxidative damages [14a]. The intensity of color, however, was more confined to the root tips; still, it was also variable in remaining parts of cortical cells under different concentrations. This is more interesting to note that a discriminating behavior exist in Al induced root growth and its underlying cellular metabolic facts. The plants recorded a well sustenance of root growth, however, also prone to oxidative damages on cellular metabolites. In general, roots are most targeted organs for Al toxicity and thus scored for its vulnerability to the metal [15]. This gives the impetus for the varietal selection for water stress tolerance under metal stress to avoid the depletion of root growth in cereal crops [29]. Physiologically the variety Dadshal was not marked any significance changes under Al stress in its canopy development (leaves, seeds). This otherwise also becomes promising traits to maintain photosynthetic activities under metal stress for tolerant species [30]. However, at cellular level, these varieties display specific bio-molecules in degenerated forms of lipids. Evans blue staining in the root tissues, particularly, more in cortical zones is the evidence for escaping the meristem against ROS activities. In earlier studies, it has also been confirmed that the incongruity between root development and ROS accumulation may be a selective trait for rice plants under salt concentrations. The similar observation of the root growth inhibition is also substantiated with degeneration of auxin and cytokinin as well as loss of microtubules in root meristem. ROS being the most suitable for Al toxicity also indicates the tissue susceptibility for degenerative processes of bio-molecules.
Accumulation of Al in roots and shoots (a), variation of hematoxylin staining for Al detection in roots (b) of 7 day old rice seedlings. Data presented herein are the means of five replication and significantly variable at each concentration denoted by different letters through t-test (p ≤ 0.05). For histochemical staining the best documented root samples are presented to show the variations under Al treatment
Effects of Al on Generation of ROS and Its Cellular Impact
In the present experiment, the generation of O2− and H2O2 were detected both in vivo and in vitro under variable Al concentrations. In a concentration dependant manner, the rice variety recorded a steeper up-regulation for O2− as compared to control (Fig. 4a–c). The ranges were in order of 1.25 fold to 2.5 fold under Al concentrations over control. At cellular level, the accumulation of O2− was more relevant to monitor the impact of oxidative stress. Interestingly, the roots and leaf sheath accumulated a fair amount of O2− as detected by NBT staining. Still, for leaf sheath the pattern of accumulation was more diffused than same in roots. This may advocate the more sensitivity of root to Al than shoot in rice. On the other hand, the spontaneous generation of peroxide (H2O2) from O2− is a common consequence for metal induced oxidative stress. So, plants in concomitant generation of H2O2 had also experienced a differential coloration with DAB staining (Fig. 5a–c). This apparently appears the differential accumulation of H2O2 in leaf sheath and root tissue as found in in vivo staining, however, not quantified exactly. The accumulation of H2O2 had maximized at 360 µM of Al for leaf, whereas for root it was with highest concentration (i.e. 480 µM).
Histochemical detection of superoxide by NBT staining in leaf sheath (a), roots (b) and accumulation of O2− (c) of 7 days old rice seedlings under varying concentration of Al. Data presented herein are the means of five replication and significantly variable at each concentration denoted by different letters through t-test (p ≤ 0.05)
Histochemical detection of H2O2 through DAB staining in root (a) and leaf sheath (b) and accumulation of H2O2 (c) of 7 days old rice seedlings under varying concentration of Al. Data presented herein are the means of five replication and significantly variable at each concentration denoted by different letters through t-test (p ≤ 0.05)
Antioxidative Responses of Plants Under Al Toxicity
To lysis the ROS, plants are tuned with different enzymatic reactions by specific enzymes. In the present experiment, it was also evident through analysis and expression of both in vitro and in vivo assay of peroxidase and catalase. It started with a discriminated regulation of APX activity, with the peak at 240 µM of Al concentration by 1.3 fold over control. Under subsequent concentration, the activities were no longer consistent to interact in a dose dependant manner (Fig. 6a, b). On the contrary, activity of CAT was rather inhibited, however, variably all through the metal concentrations against control. The decline in activity of CAT through Al toxicity was subdued regardless of doses by 46% over control. This also holds true for more sensitivity of CAT to metal than other enzymes like APX (Fig. 7a, b). The variability in APX through gene regulation may also be supported for other cellular processes like lignifications as reported in other species [16]. This is also evident that the cv. Dadshal displayed an isozymic form which has been reported a few in number in other rice variety [1]. Therefore, over expression of APX both in activities and polymorphism may be served as reliable indices for this species. In contrast to APX, the reduction of H2O2 by CAT is mediated by without any electron donor (phenolic/organic residues). This is another trait for anti oxidation cascades of the plants. CAT activity following APX strongly advocates the complex nature of minimization of H2O2 stress under Al toxicity. Still, the dampening activity of CAT through Al concentration may be accounted either by down-regulation of synthesis or turn over of its enzymatic proteins. The trend of anti-oxidizing enzymes in the present experiment, appeared not in concomitant to the ongoing concentrations of aluminium; moreover, the accumulation of ROS (O2− and H2O2) is also variable significantly as the plants proceed through metal accumulation. Therefore, the un-parallelism between ROS and its detoxification is deviated from the enzymatic capabilities of plant species (which is in general agreement) is turn aside in Dadshal as in present case. So, the tolerance to aluminium concentrations with sustained growth even under highest aluminium concentration may circumvent the other paths besides enzymatic antioxidation. The later may include any potent antioxidant residues that can quench the ROS and save the plants. In accordance to metal toxicity, plants develop some anti-oxidizing moieties with its conjugate redox system to quench the oxidizing potential of ROS [20]. This is also in agreement that the development of non-thiol compounds like glutathione engaged in quenching of ROS. Instead of measuring the ratio of glutathione (in its oxidized and reduced form), the authors have assayed the GR activity in vitro. GR can transfer electron through NAD(P)H to oxidized glutathione retrieves the reduced form which undergoes depletion in oxidative state. Plants recorded a significant (p ≤ 0.05) modulation of enzyme activity with maximum value of 1.6 fold only at highest concentration of Al (Fig. 8a). In the present experiment, this indicates the varietal insensitivity to the depletion of GSH:GSSG within some threshold concentration of metal stress. It also assumes that present rice variety, Dadshal might have other sources for stabilizing cellular redox other than glutathione turnover by some non-enzymatic antioxidation pathway. To replenish the reduced form of glutathione (GSH); it is the GR activity that indexes the genotypic potential to maintain the cellular redox. Therefore, the cv. Dadshal has qualified to retrieve the reduced redox with the stable activity for GR althrough the Al concentration. Moreover, cv. Dadshal is expected to be a well defined tolerant species to Al toxicity when it recorded a distinct polymorphic form of GR. It is evident from few polymorphic bands for GR protein even passing through variable concentrations of metal (Fig. 8b). More importantly, there recorded hardly any changes of protein intensities along Al concentrations.
In vitro activity of APX (a) and polypeptide polymorphisms of APX through in gel staining (b) of 7 days old rice seedling under varying Al concentrations. Data presented herein are the means of five replication and significantly variable at each concentration denoted by different letters through t-test (p ≤ 0.05)
Activity of catalase in vitro (a) and polypeptide polymorphism of catalase through in gel staining (b) of 7 days old rice seedling under varying Al concentrations. Data presented herein are the means of five replication and significantly variable at each concentration denoted by different letters through t-test (p ≤ 0.05)
The activity of GR in vitro (a) and polypeptide polymorphism of GR through in gel staining (b) of 7 days old rice seedling under varying Al concentrations. Data presented herein are the means of five replication and significantly variable at each concentration denoted by different letters through t-test (p ≤ 0.05)
Effects of Al on Osmoticum Generation and Its Impact
Plants under Al toxicity are expected to develop osmotic stress at the cellular level. Therefore, accumulation of GB, a quaternary amine, osmolyte in nature had scored gradual up regulation. It was maximized by 1.77 fold over control (Fig. 9a). On account of the biosynthesis of GB, the key enzyme activity as BADH was opposite in trend. The incompatibility of BADH activity and GB accumulation may offer to search other pathways to tolerate the aluminium as metal inducing osmotic deficit stress. Still, it is the inherent capability of the Dadshal genotype; moderate to low aroma containing can survive well despite of non-adequate BADH activity. This possibility might have also expressed through down regulation by 48.08% regardless of Al concentration (Fig. 9b). The expression potential of aldehyde dehydrogenase was obtained in partial purification of the protein following its separation of non denaturing gel stained with specific substrate (Fig. 9c). A distinct polypeptide band variable in concentration was recorded through Al doses. This polypeptide may not necessarily pinpoint the responsible isoforms in reaction for GB biosynthesis and thus not justified to claim for tolerance. Still, it remains more in quest whether any multigenic form of ALDH is required to impart specific roles either in osmolyte biosynthesis of GB or other cellular functionales to quench the metal stress that yet to be studied.
Accumulation of GB as osmolite in vitro (a), activity of BADH (b) and protein polymorphism of aldehyde dehydrogenase (ALDH) (c) of 7 days old rice seedling under varying Al concentrations. Data presented herein are the means of five replication and significantly variable at each concentration denoted by different letters through t-test (p ≤ 0.05)
Conclusion
This experiment with its all available facts and figures concludes that rice variety with their better adaptability to water stress may evade the Al toxicity. This also holds true with the genotypic plasticity for glycine betaine biosynthesis coupled with ROS induced oxidative stress tolerance. The overall cellular mechanism either by sequestering the metal in non-invading manner or/and inducing the redox system by enzymatic paths could characterize the plant. The histochemical reactions to Al to point out its efficacy along with polymorphisms in gene expression might also pose the bio indicator of rice land races.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GR:
-
Glutathione reductase
- ROS:
-
Reactive oxygen species
- H2O2 :
-
Hydrogen peroxide
- O2 − :
-
Superoxide
- BADH:
-
Betaine-aldehyde dehydrogenase
- GB:
-
Glycine betaine
- 2AP:
-
2-acetyl pyrroline
- NBT:
-
Nitrotetrazolium blue chloride
- DAB:
-
3, 3′-Diaminobenzidine
- PCA:
-
Perchloric acid
- PVP:
-
Polyvinylpyrrolidone
- EDTA:
-
Ethylenediaminetetraacetic acid
- DTT:
-
Dithiothreitol
- PMSF:
-
Phenylmethylsulfonyl fluoride
- TEMED:
-
Tetramethylethylenediamine
- NAD(P)H:
-
Reduced Nicotinamide adenine dinucleotide phosphate
- GSSG:
-
Oxidized glutathione
- DCPIP:
-
2,6-Dichlorophenolindophenol
- PMS:
-
Phenazonium metho sulphate
- MTT:
-
3-(4,5- Dimethyl-2 thiazolyl 2,5 diphenyl-tetrazolium bromine)
- ALDH:
-
Aldehyde dehydrogenase
References
Sen G, Eryilmaz IE, Ozakca D (2014) The effect of aluminium-stress and exogenous spermidine on chlorophyll degradation, glutathione reductase activity and the photo system ii d1 protein gene (psba) transcript level in lichen Xanthoria parietina. Phytochemistry 98:54–59
Delhaize E, Ryan PR (1995) Aluminium toxicity and tolerance in plants. Plant Physio 107(31):5–321
Mossor-Pietraszewska T (2001) Effect of aluminium on plant growth and metabolism. Acta Biochemica Polonica 48(3):673–686
Duan JJ, Guo SR, Kang YY, Zhou GX, Liu XE (2009) Effects of exogenous spermidine on active oxygen scavenging system and bound polyamine contents in chloroplasts of cucumber under salt stress. Acta Ecol Sin 29:0653–0661
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. J Plant Sci 161:1135–1144
Cheeseman John M (2007) Hydrogen peroxide and plant stress: a challenging relationship. Plant Stress 1(1):4–15
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publisher, Boca Raton, pp 85–107
Hasthanasombut S, Paisarnwipatpong N, Triwitayakorn K, Kirdmanee C, Supaibulwatana K (2011) Expression of OsBADH1 gene in Indica rice (Oryza sativa L.) in correlation with salt, plasmolysis, temperature and light stresses. Plant Omics J 4(7):400–407
Cha-um S, Kirdmanee C, Supaibulwatana K (2004) Biochemical and physiological responses of Thai jasmine rice (Oryza sativa L. ssp. indica cv. KDML105) to salt stress. Sci Asia 30:247–253
Liu JH, Moriguchi T (2006) ADC pathway plays an important role in salt stress response of apple in vitro callus. Plant Genomics China 124:1315–1325
Cha-um S, Supaibulwatana K, Kirdmanee C (2007) Glycinebetaine accumulation, physiological characterizations and growth efficiency in salt-tolerant and saltsensitive lines of indica rice (Oryza sativa L. ssp. indica) in response to salt stress. J Agron Crop Sci 193:157–166
Chen S, Yang Y, Shi W, Ji Q, He F, Zhang Z, Cheng Z, Liu X, Xu M (2008) Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 20:1850–1861
Kumar D, Yusuf M A, Singh P, Sardar M, Sarin NB (2014) Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 4(8). http://www.bio-protocol.org/e1108
Pompella A, Maellaro E, Casini AF, Comporti M (1987) Histochemical detection of lipid peroxidation in the liver of bromobenzene–poisoned mice. Am J Pathol 129:295–301
Ruzin Steven E (1999) Plant microtechnique and microscopy, vol 198. Oxford University Press, New York
Sasaki M, Yamamoto Y, Ma JF, Matsumoto H (1997) Early events induced by aluminium stress in elongating cells of wheat root. Soil Sci Plant Nutri 43:1009–1014
Bright J, Desikan R, Hancock JT, Weir IS, Neil JS (2006) ABA-induced no generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122. https://doi.org/10.1111/j.1365-313x.2005.02615.x
Davletova S, Rizhsky L, Liang HJ, Zhong SQ, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17:168–281
Nahakpam S, Shah K (2012) Expression of Key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedling. Plant Growth Regul 63:23–35
Aebi H (1983) Catalase in vitro. Methods Enzymol 105:121–126
Ghosh N, Das SP, Mandal C, Gupta S, Das K, Dey N, Adak MK (2012) Variations of antioxidative responses in two rice cultivars with polyamine treatment under salinity stress. Physiol Mol Biol Plants 18(4):301–313
Mandal C, Ghosh N, Maiti S, Das K, Gupta S, Dey N, Adak MK (2013) Antioxidative responses of Salvinia (Salvinia natans Linn) to aluminium stress and it’s modulation by polyamine. Physiol Mol Biol Plants 19(1):91–103
Daniel H, Muthukumar B, Lee SB (2001) Markerfree transgenic plants: engineering the chloroplast genome without the use of antibiotic selection. Curr Genet 39:109–116
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Biol 44(1):357–384
Younis ME, Hasaneen MNA, Tourky SMN (2009) Plant growth, metabolism and adaptation in relation to stress conditions. XXIV. Salinity-biofertility interactive effects on proline, glycine and various antioxidants in Lactuca sativa. Plant Omics J 2:197–205
De Mendiburu F (2014) Agricolae: statistical procedures for agricultural research. R Package Version 1 pp 1–2
Yamamoto Y, Kobayashi Y, Kobayashi H (2001) Lipid peroxidation is an early symptom triggered by aluminium, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian J Med Res 128(4):501
Ali B, Hasan S, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ Exp Bot 62:153–159. https://doi.org/10.1016/j.envexpbot.2007.07.014
Boutraa T, Akhkha A, Al-Shoaibi AA, Alhejeli AM (2010) Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. JTUSCI 3:39–48
Acknowledgement
The present work was supported by DST-PURSE program activated to University of Kalyani and one of the authors acknowledges to UGC for Rajib Gandhi National Fellowship as a research fellow to conduct the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest in publishing this research work.
Additional information
Significance statement
The work is embodied with some interesting findings that a rice variety may satisfy the aspects of aluminium toxicity in crop plants and its preliminary interactive responses for tolerance. It is also sensitised with some findings that may vary from a growth responses under aluminium toxicity and discussed.
Rights and permissions
About this article
Cite this article
Bera, S., De, A.K. & Adak, M.K. Modulation of Glycine Betaine Accumulation with Oxidative Stress Induced by Aluminium Toxicity in Rice. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 291–301 (2019). https://doi.org/10.1007/s40011-017-0948-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0948-7