Abstract
Asparaginase is a very important antineoplastic drug extensively used for the treatment of acute lymphoblastic leukemia and other tumor malignancies. But intrinsic glutaminase activity of this enzymatic drug is responsible for serious life threatening side effects. In this report, 154 bacterial isolates were isolated from rhizosphere of different plants and river water. All isolates were screened for glutaminase-free periplasmic asparaginase activity, and it was found that only four isolates (i.e., BO1, CO1, CO3 and GG1) lacked detectable glutaminase activity. Their measured asparaginase activities ranged from 0.1 to 0.37 IU/ml. These glutaminase-free asparaginase producing bacterial isolates were identified as Pseudomonas otitidis, Enterobacter cloacae, Ochrobactrum anthropi and Escherichia fergusonii, on the basis of morphological, cultural, biochemical characteristics and 16S rRNA gene sequencing. Crude enzyme extracted from these strains was screened for antitumor activity. Antitumor results showed that asparaginase obtained from P. otitidis and E. cloacae possesses potent antiproliferative effect on human leukemia (MOLT-4) and breast cancer (T47D and MDA-MB-231) cell lines, as compared to standard E. coli asparaginase preparation (Sigma), used in the present study. Based on these results, the asparaginase obtained from P. otitidis and E. cloacae could be used as a potent antiproliferative agent. However, more indepth studies are required for strengthening the current findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteins constitute a major class of biomolecules having application in diverse fields ranging from food and feed industries to pharmaceuticals. Different protein pharmaceutics such as hormones, growth factors, antibodies, enzymes, interferons, blood factors and thrombolytics, etc. perform different activities such as delivering cytotoxic drugs, replacing some abnormal proteins, boost up the pathways and interacting with some molecules in vivo [1]. Asparaginase (l-Asparaginase amidohydrolase, E.C. 3.5.1.1) is a therapeutic enzyme used in treatment of children with acute lymphoblastic leukemia, Hodgkin disease, acute myelocytic leukemia, acute melanomonocyteic leukemia, chronic lymphocytic leukemia, lymphosarcoma, reticlesarcoma and melanosarcoma [2].
The anti-neoplastic action of asparaginase is explained on the fact that certain tumor cells, more specifically leukemic cells are deficient in their ability to synthesize the non-essential amino acid asparagine de-novo due to the absence of asparagine synthetase [3] but they require huge amount of asparagine to keep up their rapid malignant growth. To fulfill their nutritional requirement they use serum asparagine. Asparaginase as a chemotherapeutic drug rapidly hydrolyses serum asparagine into aspartate and ammonia [4]. The nutritional stress induced by asparaginase due to depletion of serum asparagine leads to DNA, RNA and protein biosynthesis inhibition in ALL and other asparagine dependent tumor cells, resulting in subsequent apoptosis due to cell cycle arrest in G0/G1 phase [5]. However, normal cells are unaffected due to the presence of asparagine synthetase [6]. Asparaginases have been reported in plants, animals and several microbial species [2] but particularly gram-negative bacteria have proven to be the most efficient producers of this enzyme. The cell wall of gram-negative bacteria contains periplasmic space between plasma membrane and peptidoglycan layer. That space contains several physiologically different membrane anchored proteins and metabolically essential hydrolyzing enzymes; asparaginase (Asparaginase-II) is one of the most important out of them [7].
Nowdays, asparaginase purified from two bacterial sources viz Escherichia coli and Erwinia chrysanthemi have been used for clinical purposes. However, asparaginases obtained from both these sources possess intrinsic glutaminase activity, which is responsible for several life threatening complications in patients [8]. These complications are restricted to clinical applications of this enzymatic drug. Therefore, to improve the long-term, event-free, survival rates of patients, glutaminase-free asparaginase with potent antitumor activity is highly desirable.
The existing disadvantages related to therapy of this novel drug can be solved with the isolation of glutaminase-free asparaginase producing bacterial strains. In the present investigation, glutaminase free asparaginase producing bacterial strains were isolated and identified. Furthermore, antitumor activity of crude asparaginases was evaluated.
Material and Methods
Anhydrous l-asparagine, l-glutamine, trichloroacetic acid, and Folin-Ciocalteu’s phenol reagent were purchased from HiMedia, Mumbai, India. Peptone was procured from Central Drug House (P) LTD., India. Asparaginase, RPMI-1640, fetal bovine serum (FCS), 3-(4,5,-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT), penicillin, streptomycin, were purchased from Sigma chemical Co., USA. Primers, Taq DNA polymerase, dNTPs and purification kit were obtained from Bangalore Genei (India). All the chemicals used were of analytical grade and purchased from standard sources.
Isolation of Bacterial Isolates
Total 15 rhizospheric soils and 4 water samples were collected from various sites of State Forest Research Institute, agriculture fields and different sites of river Narmada, Jabalpur, Madhya Pradesh, India. Samples were enriched in medium containing (per l) 15 g l-asparagine (1 M, 100 ml) and 10 g yeast extract. Serially diluted enriched samples were plated into Luria–Bertani agar medium and morphologically different colonies were selected and purified on same agar plates.
Culture Conditions and Extraction of Asparaginase
For quantitative estimation of the enzyme activity, modified basal semi-synthetic broth medium [9] containing (per l): 3.0 g glucose, 5.0 g l-asparagine, 6 g Na2HPO4·2H2O, 1.77 g KH2PO4, 0.37 g MgSO4·7H2O, 0.015 g CaCl2·2H2O, 1.0 g yeast extract and 1.0 g peptone (pH-7), was used. The purified and isolated colony of 24 h old bacterial isolate was aseptically transferred in 20 ml pre-autoclaved above mentioned broth medium in 250 ml Erlenmeyer flask and incubated at 37 °C in orbital shaking incubator at 180 rpm for 24 h. Cells were harvested by centrifugation at 10,000 rpm for 5 min at 4 °C. The cell pellet was washed twice with 0.05 M potassium phosphate (KPi) buffer, pH 8.6 and resuspended into 0.05 M KPi buffer, 1 % hexane (v/v) to an OD600 of 5.0 in fermentation tube. The tubes were incubated at room temperature for 1 h with continuous vortexing after every 10 min. The caps of tubes were left open for 5 min in order to evaporate volatile upper phase, centrifuged at 10,000 rpm for 5 min at room temperature, supernatant was collected and subjected to asparaginase activity [10].
Asparaginase and Glutaminase Assay
The asparaginase activity was measured by the standard method of Wriston [11], which is based on the determination of ammonia liberated from l-asparagine by the action of asparaginase. Ammonia produced in the reaction was calculated by using ammonium sulphate as the standard. One international unit (IU) of asparaginase is defined as the amount of enzyme that librates 1 µmol of ammonia under standard assay conditions. Glutaminase activity was determined by Nesslerization reaction as described by Imada et al. [12], using glutamine as the substrate. Total protein was determined colorimetrically [13], using bovine serum albumin as the standard.
Identification of Bacterial Isolates
Taxonomic characterization of glutaminase-free asparaginase producing isolates was done on the basis of cultural, morphological and biochemical characteristics [14] with the help of Bergey’s Manual of Systematic Bacteriology [15] and further confirmed by 16S rRNA gene sequencing. Gene amplification was done by PCR using two universal eubacterial oligonucleotide primers, 8F, 5′-AGAGTTTGATCCTGGCTCAG-3′ 1492R 5-GGTTACCTTGTTACGACTT-3′, according to the conditions previously described by Weisburg et al. [16]. The DNA similarity was determined by BLAST search tool and sequence was submitted in the National Centre of Biotechnology Information (NCBI), GeneBank database and identified strains were submitted in Bacterial Germplasm Collection Centre (BGCC), Rani Durgavati University, Jabalpur, India. Preliminary multiple sequence alignments and phylogenetic tree constructions were performed by using Clustal W and MEGA software version 6 [17].
Cell Cultures, Growth Conditions and Treatment
Human T-lymphoblast acute lymphoblastic leukemia (MOLT-4) and breast cancer (MDA-MB-231 and T47D) cell lines were obtained from National Cancer Institute, Bethesda, USA. Cells were grown in RPMI-1640 medium containing 10 % FCS, 100U penicillin/100 µg streptomycin/ml, in CO2 incubator (Thermocon Electron Corporation, USA) at 37 °C with 98 % humidity and 5 % CO2 gas environment. Cells were treated with filter sterilized asparaginase solution during logarithmic growth phase.
Cell Proliferation Assay
The anti-proliferation effect of asparaginase was investigated against human leukemic (MOLT-4) and breast cancer (MDA-MB-231 and T47D) cell lines according to the method of Mosmann [18]. Approximately, 1.2 × 104–1.5 × 104 exponentially growing cells were seeded in 96-well microtiter plates, containing 200 µl RPMI-1640 medium, with 10 % FCS. Plates were incubated at 37 °C in CO2 incubator with 5 % CO2. Cells were treated with different concentrations of asparaginase. After 48 h, 10 µl per well of MTT dye (5 mg/ml, stock) was added and plates were further incubated at 37 °C in CO2 incubator for 4 h. The plates were centrifuged at 2000 rpm for 15 min and supernatant was discarded. The formazan blue crystals, formed by viable cells, were dissolved in 150 µl DMSO and the rate of color production was measured at 570 nm with ELISA reader. The O.D of control samples (untreated cells) were considered to be 100 % and accordingly the viability of treated cells were calculated using the following formula.
All experiments were performed in triplicates and results were expressed in terms of percent proliferation inhibition.
Results and Discussion
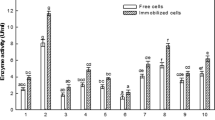
Many proteins and peptides obtained from microbial sources possess biological activity that makes them potential therapeutics. Enzymes represent an important and probably the best investigated group of protein drugs. Isolation and screening of bacteria from natural habitats like soil and water had led to the discovery of many novel and useful secondary metabolites, particularly enzymes, antibiotics, vitamins and enzyme inhibitors. Asparaginase therapy is used all over the world, but several problems occur when this enzyme is used for the treatment of leukemia and other tumor malignancies [19]. Most of the problems arise due to intrinsic glutaminase activity of asparaginase, which is responsible for various side effects including leucopenia, immunosuppression, acute pancreatitis, thromboembolysis, hyperglycaemia and neurological seizures [20]. Therefore, glutaminase-free asparaginase from new sources is highly desirable which can significantly improve therapeutic index of asparaginase therapy. Hence, the search of glutaminase-free asparaginase from new bacterial sources is a challenging goal for scientific communities. In the present investigation, 154 bacterial isolates, isolated from different rhizospheres and river water samples were screened for glutaminase free asparaginase activity. Out of these, glutaminase activity was not determined in 4 bacterial isolates i.e., BO1, CO1, CO3 and GG1 isolated from rhizospheric soil of Brassica oleracea, Calendula officinalis, and river water [Gwari Ghat (river Narmada, Jabalpur, India)], respectively. Hence, these isolates were considered as glutaminase free asparaginase producing isolates, exhibiting asparaginase activity in the range of 0.1–0.37 IU/ml (Table 1).
All selected strains, i.e., BO1, CO1, CO3, and GG1 were rod shaped, gram negative, motile and catalase positive except CO3. All the biochemical tests which are listed in Table 2 were similar to those described for Escherichia, Pseudomonas, Enterobacter, and Ochrobactrum in biochemical tests for identification of Medical Bacteria (14) and Bergey’s Manual of Systematic Bacteriology [15]. By performing the 16S rRNA gene sequencing confirmed the identity of these bacterial isolates as P. otitidis (BO1), E. cloacae (CO1), O. anthropi (CO3), and E. fergusonii (GG1). Further, phylogenetic dendrogram (Fig. 1) of these identified bacterial strains was constructed by the neighbor-joining method. The 16S rRNA gene sequences were submitted to the GeneBank database under accession number KF607093, KF607097, KF607094 and KF607096, respectively. Further, identified bacterial strains were submitted into Bacterial Germplasm Collection Centre (BGCC), Department of P.G. Studies and Research in Biological Science, R. D. University, Jabalpur and assigned their BGCC number as BGCC#: 2385 (E. fergusonii), BGCC#: 2388 (P. otitidis), BGCC#: 2389 (E. cloacae) and BGCC#: 2390 (O. anthropi) (Table 2).
The antitumor activity of asparaginase is well established but the antitumor efficacy of asparaginase varies with organism to organism. It has been experimentally suggested that the therapeutic efficacy of asparaginase depends on the genetic nature of the organism. Asparaginase obtained from chicken liver [21], yeast [22] and Bacillus coagulans [23] does not possess antitumor activity while, Bacillus subtilis [24], Saccharomyces cerevisiae [25], Mycobacterium tuberculosis [26] and E. coli [27] possesses two types of asparaginase among which only one of them exhibited antiproliferative activity. Therefore, in order to find potent antiproliferative asparaginase producers the authors investigated the antitumor potential of crude glutaminase-free asparaginases against solid (T47D and MDA-MB-231) and lymphatic (MOLT-4) cancer cell lines, through MTT assay. The MTT assay is commonly used to assess cell viability. This assay is based on the conversion of MTT to formazan by reduction of NADH or NADPH dehydrogenases. For investigation of antitumor activity, cells were treated with 0, 0.1, 0.5 and 1 IU/ml concentrations of enzyme for 48 h and anticancer potency has been evaluated in terms of half maximal inhibitory concentration (IC50). The standard E. coli asparaginase procured from Sigma chemical Co. St. Louis, USA, was used as a reference preparation. Quantitative analysis shown in Fig. 2a and b, indicated that crude asparaginase extracted from P. otitidis was able to potentially and significantly inhibit the proliferation of T47D, MDA-MB-231, and MOLT-4 cells with IC50 values of approximately, 0.05, 0.7, and 0.1 IU/ml, respectively. In addition, crude asparaginase obtained from E. cloacae also possesses significant antitumor activity with IC50 values of 0.1, 1, and 0.1 IU/ml which was recorded against MDA-MB-231, T47D, and MOLT-4 cells, respectively. Quantitative analysis shown in Fig. 2c and d indicated that E. fergusonii and O. anthropi showed effective antitumor activity against T47D (IC50 = ~0.1 IU/ml) and MDA-MB-231 (IC50 = ~0.3 IU/ml) cells only. On the other hand, E. coli asparaginase (Sigma) showed antitumor activity against MOLT-4 cells (IC50 = ~0.9 IU/ml) and IC50 values were not recorded against T47D and MDA-MB-231 cells till 1 IU/ml concentration of enzyme. These observations indicated that crude asparaginases extracted from P. otitidis and E. cloacae induce apoptosis with different kinetics in tested human cancer cell lines and anticancer potency of both bacterial asparaginases is significantly high as compared to the commercial E. coli asparaginase.
Dose-dependent antiproliferative effects on the cell viability of human breast cancer (T47D and MDA-MB-231) and T-lymphoblast acute lymphoblastic leukemia (MOLT-4) cell lines. Approximately, 1.5 × 104 cells/ml were cultured at 37 °C and treated with 0, 0.1, 0.5 and 1 IU/ml concentrations of P. otitidis (a), E. cloacae (b), E. fergusonii (c), O. anthropi (d) and E. coli (e) asparaginase, separately. After 48 h of treatments, cell viability was determined by MTT assay and IC50 values were determined by regression analysis. Each value represents the mean ± SD for three determinations
In a previous investigation, Ohnuma et al. [21] reported that the crude asparaginase obtained from chicken liver did not possess antitumor activity at 0.7–1.0 IU/ml of enzyme treatment. Furthermore, in an other previous study Oza et al. [28] reported that asparaginase obtained from Withania somnifera L inhibited only 2 and 26 % proliferation of human leukemic cells at 0.01 and 1 IU/ml of enzyme treatment, respectively. Therefore, as compared to E. coli asparaginase as well as of the above mentioned previous reports, the cytotoxic effect induced by P. otitidis, and E. cloacae asparaginases suggested that even crude enzyme from these bacterial isolates have the potential to inhibit the proliferation of tested cancer cell lines and can induce apoptotic cell death. Further, the cytotoxic effect of asparaginase from these bacterial strains can be increased many fold after purification and characterization of the enzyme and glutaminase-free potent antitumor property can be more advantageous in the field of asparaginase therapy. More interestingly, in the present study it was found that enzyme extracted from O. anthropi did not exhibit significant cytotoxic effect on T47D and MOLT-4 cells and only approximately 35 % proliferation inhibition was noted against both cell lines till 1 IU/ml concentration of enzyme. In contrast, at this concentration approximately 85 and 70 % proliferation inhibition against T47D and MOLT-4 cells were recorded with crude asparaginase extracted from P. otitidis. Therefore, the authors suggest that low cytotoxic potential of O. anthropi asparaginase might be due to high Km of enzyme while, for strong antitumor activity the Km of enzyme should be below the concentration of substrate in the medium or circulating system [29]. However, more indepth studies are required for strengthening the current findings. To further evaluate the effectiveness of anticancer potential of P. otitidis and E. cloacae asparaginases, the purification and in vitro and in vivo studies of anticancer activity of both asparaginases are under progress.
Conclusion
In the present study, the authors have reported isolation of glutaminase-free asparaginase producing bacterial strains from natural habitat and identified them by cultural, morphological, biochemical and 16S rRNA gene sequencing. The crude asparaginase extracted from P. otitidis and E. cloacae significantly inhibited the proliferation of breast as well as lymphatic cancer cell lines, as compared to commercial E. coli asparaginase used in the present study. Crude asparaginase obtained from the isolated strains does not exhibit intrinsic glutaminase activity. In this respect the present study is an important finding and can be used as new approach in the field of asparaginase therapy but more intensive studies are still required to validate antitumor potential of P. otitidis and E. cloacae asparaginases.
References
Kang TS, Stevens RC (2009) Structural aspects of therapeutic enzymes to treat metabolic disorders. Hum Mutat 30:1591–1610
Verma N, Kumar K, Kaur G, Anand S (2007) L-Asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol 27:45–62
Asselin BL, Ryan D, Frantz CN et al (1989) In vitro and in vivo killing of acute lymphoblastic leukemia cells by l-asparaginase. Cancer Res 49:4363–4368
Lubkowski J, Palm GJ, Gilliland GL, Derst C, Rohm KH (1996) Crystal structure and amino acid sequence of Wolinella succinogenes l-asparaginase. Eur J Biochem 241:201–207
Gong SS, Basilico C (1990) A mammalian temperature-sensitive mutation affecting G1 progression results from a single amino acid substitution in asparagine synthetase. Nucleic Acids Res 18:3509–3513
Narta UK, Kanwar SS, Azmi W (2007) Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol 61:208–221
Geckil H, Gencer S, Ates B, Ozer U, Uckun M, Yilmaz I (2006) Effect of Vitreoscilla hemoglobin on production of a chemotherapeutic enzyme, l-asparaginase, by Pseudomonas aeruginosa. Biotechnol J 2:203–208
Kravchenko OV, Kislitsin YA, Popov AN, Nikonov SV, Kuranova IP (2008) Three dimensional structures of l-asparaginase from Erwinia carotovora complexed with aspartate and glutamate. Acta Crystallogr D 64:248–256
Kumar S, Dasu VV, Pakshirajan K (2011) Purification and characterization of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour Technol 102:2077–2082
Geckil H, Ates B, Gencer S, Uckun M, Yilmaz I (2005) Membrane permeabilization of gram-negative bacteria with a potassium phosphate/hexane aqueous phase system for the release of l-asparaginase: an enzyme used in cancer therapy. Process Biochem 40:573–579
Wriston JC Jr (1070) Asparaginase. Methods Enzymol XVII:732–742
Imada A, Igarasi S, Nakahama K, Isono M (1973) Asparaginase and glutaminase activities of microorganisms. J Gen Microbiol 76:85–99
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mac-Faddin FJ (1980) Biochemical tests for identification of medical bacteria. Williams and Wilkins, Baltimore
Kreig RN, Holt G (1984) Bergey’s manual of systematic bacteriology. William and Wilkins, Baltimore
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods 65:55–63
Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui C (2011) l-Asparaginase treatment in acute lymphoblastic leukemia. Cancer 15:238–249
Jakubas PB, Kulis MK, Giebel S et al (2008) Use of l-asparaginase in acute lymphoblastic leukemia: recommendations of the Polish Adult Leukemia Group. Pol Arch Med Wewn 118:664–669
Ohnuma T, Bergel F, Bray RC (1967) Enzymes in cancer: asparaginase from chicken liver. Biochem J 103:238–245
Broome JD (1965) Antilymphoma activity of l-asparaginase in vivo: clearance rates of enzyme preparation from guinea pig serum and yeast in relation to their effect on tumor growth. J Natl Cancer Inst 35:967–974
Mashbum LT, Wriston JC (1964) Tumor inhibitory effect of l-asparaginase from Escherichia coli. Arch Biochem Biophys 105:450–452
Yano S, Minato R, Thongsanit J, Tachiki T, Wakayama M (2008) Overexpression of type-I L-asparaginase of Bacillus subtilis in Escherichia coli, rapid purification and characterisation of recombinant type-I l-asparaginase. Ann Microbiol 58:711–716
Kim KW, Kamerud JQ, Livingston DM, Roon RJ (1988) Asparaginase II of Saccharomyces cerevisiae: characterization of the ASP3 gene. J Biol Chem 263:11948–11953
Reddy VVS, Jayaram HN, Sirsi M, Ramakrishnan T (1969) Inhibitory activity of l-asparaginase from Mycobacterium tuberculosis on Yoshida ascites sarcoma in rats. Arch Biochem Biophys 132:262–267
Cedar H, Schwartz JH (1968) Production of l-asparaginase II by Escherichia coli. J Bacteriol 96:2043–2048
Oza VP, Parmar PP, Kumar S, Subramanian RB (2010) Anticancer properties of highly purified l-asparaginase from Withania Somnifera L. against Acute Lymphoblastic Leukemia. Appl Biochem Biotechnol 160:1833–1840
Davidson L, Brear DR, Wingard P, Hawkins J, Kitto GB (1977) Purification and properties of an l-glutaminase-l-asparaginase from Pseudomonas acidovoranans. J Bacteriol 129:1379–1386
Acknowledgments
Authors are thankful to Head, Department of P.G. Studies and Research in Biological Science, Rani Durgavati University, Jabalpur for providing Laboratory facilities and Dr. Fayaz A. Malik, Scientist, Department of Pharmacology and Cancer Biology, Indian Institute of Integrative Medicine (IIIM-CSIR), Jammu, India, for providing Laboratory facilities for screening of antitumor activity of enzymes. Authors are also thankful to Madhya Pradesh Biotechnology Council, Bhopal, for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, A., Husain, I. Evaluation of Antitumor Activity of Glutaminase-Free Periplasmic Asparaginase from Indigenous Bacterial Isolates as Candidates for Cancer Therapy. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 87, 997–1004 (2017). https://doi.org/10.1007/s40011-015-0681-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0681-z