Abstract
The phytase producing bacterial strain, Bacillus licheniformis ONF2 was isolated from the proximal intestine of the freshwater fish, Nile tilapia, Oreochromis niloticus. The bacterial phytase was purified 37.45 fold from the crude supernatant by two step chromatography with an overall yield of 21.3 %. It was a monomeric protein with molecular mass of 40–42 kDa. The enzyme was optimally active at pH 6.5–7.5 and at 50 °C temperature and was quite stable at pH ranging from 5.0 to 9.5. It showed temperature stability range of 20–75 °C. The activity of the enzyme was moderately inhibited by 5 mM Mn2+, Mg2+ and K+ and largely affected by the metal ions Cu2+, Hg2+, Zn2+, Co2+ and EDTA but, in the presence of 1 mM CaCl2, the inhibitory effect was less intense. One unit of purified phytase released 1130.3 ± 40.2 and 720.5 ± 35.2 µg of inorganic phosphate per gram of sesame seed meal and soybean meal respectively. The properties of the presently purified phytase to hydrolyze plant phytate and maintaining stability at high temperature make it suitable for applications in animal feed industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytate or phytic acid is the storage form of phosphorous in plant seeds, grains and cereals where it may constitute up to two-thirds of the total phosphorous (P) content [1]. This phytate phosphorous is poorly available to mono-gastric and agastric animals because they lack the phytate degrading enzyme in their gastrointestinal tract. This limits the dietary incorporation of phytate rich plant ingredients in aquafeeds as this storage form of phosphorous passes unutilized in the feces [2, 3]. Phytic acid also chelates several metal ions such as Ca2+, Mg2+, Zn2+ and Fe2+, which forms complexes with proteins, and thus reduces their digestibility and also inhibits certain digestive enzymes like α-amylase, trypsin, acid phosphatase and tyrosinase [4]. Most of the phytate-P is consequently excreted into water which contributes to the phosphorus pollution problems in areas of intensive livestock production [5, 6].
Phytate degrading enzymes or phytases (myo-inositol hexa-kis-phosphate phosphohydrolase; EC 3.1.3.8) catalyze the degradation of phytate to inorganic monophosphate and myo-inositol and thus eliminate its anti-nutritional properties. Phytases have significant applicability in animal nutrition because of their ability to hydrolyze the phytate content of the plant ingredients which also improves the bioavailability of protein and minerals and minimizes the need for supplemental inorganic phosphate in the diet [6]. Supplemental phytase has been reported to improve phytate phosphorous bioavailability in different animals like pigs, poultry birds and fish [7–9]. Phytase is already being used in food industry for food processing and in US the annual sales of phytase are currently estimated to be about USD 500 million [10].
Phytases are widespread in nature as they are found in plant and animal tissues and also have been reported in several microorganisms including bacteria, yeast and fungi [11]. The filamentous fungus, Aspergillus niger is known as the most potent producer of extracellular phytase and industrial production of phytase currently utilizes Aspergillus, on which considerable research has been conducted [12]. However, due to several properties, such as substrate specificity, resistance to proteolysis and catalytic efficiency, bacterial phytases have some advantages as compared to the fungal enzymes [13].
Phytases from a number of bacteria have been purified and characterized but phytases with thermostability required for application in animal feed has been lacking [14]. In addition, majority of phytases characterized so far, are acidic and exhibit little enzyme activity at neutral and alkaline pH environment while the environment of the digestive tract in most agastric fish is either neutral or alkaline [15]. Therefore, novel phytases with improved properties are of great interest for their probable future applications in animal nutrition. In the present investigation, the main aim was to purify and characterize the extracellular phytase from Bacillus licheniformis ONF2, a fish gastrointestinal isolate [16] and evaluate its efficacy in degradation of plant phytate through in vitro assay.
Material and Methods
Materials Used
Most of the chemicals and reagents used were of the analytical grade and were purchased from Merck, Bangalore, India unless otherwise stated. Phytic acid, as a dodecasodium salt, was obtained from Himedia, India and DEAE-Sepharose CL-6B and Sephadex G-100 (G100120) from Sigma-Aldrich, India. The acrylamide, bis-acrylamide and the molecular weight marker (Genei PPMWM) were from Genei, Merck Milipore, India. The phytic acid rich plant ingredients i.e. soybean meal and sesame oilseed meal used in the phytate hydrolysis assay were obtained from the local market.
Identification of Bacterial Isolate by 16S rRNA Gene Sequence Analysis
The phytase-producing bacteria were isolated from the proximal part of intestine of the freshwater fish, Nile tilapia, Oreochromis niloticus and was identified by partial 16S rRNA gene sequence analysis. In brief, a single chloroform extraction of genomic DNA was done following Roy et al. [17] and polymerase chain reaction (PCR) amplification of the 16S rRNA genes was done using the forward primer 27F ‘AGAGTTTGATCMTGGCTCAG’ and the reverse primer 1492R ‘GGTTACCTTGTTACGACTT’ and a high fidelity PCR polymerase. PCR products were sent to Laragen Inc., Culver city, California, USA for sequencing. The sequenced data were aligned using ClustalW software and analyzed to find the closest homolog of the isolate using a combination of National Center for Biotechnology Information (NCBI) GenBank and RDP (Ribosomal Database Project) database. A phylogenetic tree was made in MEGA 6 software using the Neighbor-joining method with Bootstrap analysis to obtain information on molecular phylogeny.
Preparation of Cell Extract
The modified phytase screening medium (MPSM) was used as the production medium for cultivation of the isolate ONF2. The composition of the MPSM medium [18] was as follows (g/L): glucose, 10.0; l-arginine, 0.2; urea, 1.0; citric acid, 3.0; sodium citrate, 2.0; MgSO4·7H2O, 1.0; sodium phytate, 3.0; FeSO4·7H2O, 0.1; agar, 20.0; 1 M Tris buffer (pH 8.0),100 mL/L; biotin, 50 mg/L; thiamine HCl, 20 mg/L. For preparation of the MPSM, 3 g sodium phytate was dissolved in 100 mL sterile deionized water and then mixed with 900 mL of sterilized sodium phytate-free MPSM, the pH of which was adjusted to 7.0 prior to sterilization. 100 mL of this production media was inoculated with 200 µL of starter culture containing 1 × 106 no of viable bacteria per mL of broth. The production medium was incubated at 37 °C for 96 h with vigorous shaking at 120–140 rpm. After incubation, the bacterial culture was centrifuged at 12,000×g for 15 min at 4 °C; the supernatant was collected and used for purification of the enzyme.
Purification of Phytase
All the purification steps were carried out at 4 °C. The culture supernatant was first fractionated by ammonium sulphate precipitation from 20 to 80 % saturation. The precipitate was collected after centrifugation at 12,000×g for 10 min, dissolved in 20 mL of Buffer A (20 mM Tris-HCl, pH 7.5) and evaluated for phytase activity. The fraction with highest phytase activity was collected and dialyzed against the same buffer overnight with frequent change to remove the residual (NH4)2SO4. The dialyzed suspension was again centrifuged to remove any insoluble particulate matter. The solution was then applied to DEAE-Sepharose anion exchange column (2 cm × 10 cm), equilibrated with buffer A and eluted with a NaCl step gradient (0–1.0 M) prepared in the above mentioned buffer. The fractions with high phytase activity were pooled, concentrated and loaded onto a pre-equilibrated Sephadex G-100 column (2 cm × 30 cm). The enzyme was eluted with buffer A and fractions each of 3 mL were collected. At each stage of purification process phytase activity and total protein content of the fractions were determined.
Procedure for Enzyme Assay
Phytase activity of the culture supernatant and purified enzyme was estimated following the method of Heinonen and Lahti [19] with slight modification. A reaction mixture containing 100 µL of enzyme and 900 µL of substrate solution (4 mM sodium phytate in 0.25 M sodium acetate buffer, pH 5.5) was incubated at 37 °C for 30 min. To this reaction mixture, 2 mL of freshly prepared acetone ammonium molybdate (AAM) reagent [acetone: 5 N H2SO4: 10 mM ammonium molybdate (2:1:1 v/v)] was added. After incubation for another 1 min, the reaction was stopped by adding 200 µL of 1 M citric acid. The color developed was read spectrophotometrically at 405 nm. One unit (U) of phytase activity was defined as the amount of enzyme that released 1 μmol of inorganic phosphate from sodium phytate per minute.
Molecular Mass Determination
The molecular mass of the purified protein was determined by Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). It was carried out according to Laemmli [20] with 12 % poly-acrylamide (w/v) resolving gel. The protein bands were stained and visualized by Coomassie brilliant blue R-250. The molecular weight marker was run in the adjacent lane with the purified protein for molecular mass determination.
Effect of pH and Temperature on Enzyme Activity
The ability of phytase to degrade phytate in the GI tract of animal is determined by its biochemical properties. Therefore, it is necessary to determine the effect of pH (2.0–9.0 with interval of 0.5) and temperature (20–80 °C with interval of 5 °C) on the enzyme’s activity and stability [21]. To determine the optimum pH stability of the purified phytase, the following buffers were used in the previously mentioned phytase assay procedure: 100 mM Glycine–HCl for pH 2.0 and 3.0, 100 mM citrate buffer for pH 4.0–6.0, 100 mM Tris-HCl buffer for pH 7.0–9.0, and 100 mM Glycine–NaOH for pH 10.0. The effect of different temperatures on enzyme activity was determined by carrying out the phytase activity assay at 20–80 °C temperature. The temperature stability of the enzyme was examined by incubating at different temperatures for 15 min, cooled rapidly to 4 °C and assayed for residual phytase activity (at optimal temperature). The pH stability of the purified phytase was also determined by incubating with buffers of different pH for 1 h followed by assessment of the residual enzyme activity.
Protein Determination
Total protein concentration of the fractions and purified enzyme were estimated by the coomassie blue G-250 dye-binding assay using bovine serum albumin as a standard [22].
Effect of Cations and Potential Inhibitors on Enzyme Activity
The effect of cations and other potential inhibitors on the activity of the purified phytase was estimated by pre-incubation of the enzyme with equal volumes of CaCl2, MgCl2, CuCl2, HgCl2, ZnCl2, NaCl, KCl, CoCl2, MnCl2 and EDTA (to a final concentration of 5 mM) for 30 min followed by the standard assay for phytase activity.
In Vitro Phytate Hydrolysis Assay
In vitro assay with soybean meal (Glycine max) and sesame (Sesamum indicum) seed meal at different pH were carried out following the method of Fu et al. [23] to determine the efficiency of the presently purified phytase in hydrolyzing the phytate phosphorous of the oilseed meal. 0.5 g of finely ground oilseed meal was added to 4.5 mL of buffer (0.25 M citrate buffer for pH 5.0, 5.5, 6.0 and 6.5; 0.25 M Tris–HCl buffer for pH 7.0, 7.5 and 8.0.). To this mixture 0.5 mL of purified phytase was added (1 U g−1 of seed meal) and incubated with shaking at 50 °C for 1 h followed by the addition of 5 mL of trichloroacetic acid to terminate the reaction. The released inorganic phosphate was measured by the method of Han et al. [24].
Statistical Analysis
The data were subjected to analysis of variance (ANOVA) using Origin 6.1 software. Duncan’s multiple range test [25] was employed to test differences among means. The significance of differences was tested at the significance level P < 0.05.
Results and Discussion
Identification of the Isolate
On the basis of the partial 16S rRNA sequencing which was followed by nucleotide homology and phylogenetic analysis, the isolate ONF2 (Genbank Accession no. JX912557.1) was identified as B. licheniformis [16]. It showed maximum similarity with the bacterial strain B. licheniformis LCR32 (Accession no. FJ976541.1). The phylogenetic relationship of ONF2 with its close relatives in the NCBI Genbank is shown in the dendrogram (Fig. 1).
Dendogram showing phylogenetic relationships of Bacillus licheniformis ONF2 with other close homologs based on 16S rRNA gene sequences. The horizontal bars in the phylogenetic tree represent the branch length; similarity and homology of the neighbouring sequences are indicated by the bootstrap values
Phytase activity has been detected previously in a number of bacteria such as, Aerobacter aerogenes [26], Bacillus subtilis [27], Bacillus sp. DS 11 [2], Bifidobacterium sp. [28], Citrobacter braakii [29], Enterobacter sp. [30], Escherichia coli [31], Klebsiella pneumoniae [32], Lactobacillus sanfranciscensis [33] Pseudomonas sp. [34], Selenomonas ruminantium [35] and Mitsuokella jalaludinii [36]. Several species of Bacillus namely, B. subtilis, Bacillus pumilus, Bacillus megaterium, and Bacillus coagulans have been reported to produce the enzyme phytase [21]. The bacterial phytases are generally cell associated in nature with the exception from those of the genera Bacillus, Enterobacter and Lactobacillus amylovorus [37]. The phytase produced by the presently reported B. licheniformis ONF2 was also detected to be extracellular in nature.
Purification and Molecular Properties of the Phytase
The highest phytase production by B. licheniformis ONF2 (1.05 U/mg of total protein) was obtained after optimization of nutritional parameters and culture conditions which include incubation for 96 h at 37 °C with MPSM (pH 7.0) as the production medium containing l-arginine as the alternative nitrogen source (Data not shown). The phytase activity of the culture supernatant was low when any medium other than MPSM was used as the production media. The enzyme was first concentrated by (NH4)2SO4 fractionation and the target protein was detected in the precipitate after 40 % saturation. After removal of residual (NH4)2SO4 by dialysis, the protein was purified by ion-exchange chromatography followed by gel filtration. A summary about the purification scheme is given in Table 1. In each of the purification step the specific activity of the enzyme was increased by several folds. In ion-exchange chromatography the target protein was detected in the fractions after eluting the column with 0.2 M NaCl. Three fractions with highest activity were collected, concentrated and applied to Sephadex G-100 gel filtration chromatography column. The elution profile of the fractions has been represented in the relative chromatograms in Figs. 2A, B. At the end of the three step purification process, the phytate degrading enzyme was purified 37.4 fold with a recovery of 21.3 %. The specific activity was increased up to 39.3 U mg−1 of total protein. The purified phytase was determined to be a monomeric protein with molecular mass of 40–42 kDa as it developed a single band after electrophoresis and coomassie blue staining of the polyacrylamide gel (Fig. 2C). Phytases are generally known to be of high molecular mass proteins with their molecular mass ranging from 38 to 700 kDa [38]. Phytases from several species of Bacillus such as, B. subtilis (37 kDa), Bacillus amyloliquefaciens (44 kDa) have been shown to be monomeric proteins with their molecular mass ranging from 38 to 46 kDa [4]. Bacterial phytases are generally slightly smaller than fungal phytases, including those from Aspergillus fumigatus (75 kDa), A. niger (84 kDa) and Aspergillus ficuum (85–100 kDa) [27].
A Ion-exchange chromatography of the purified phytase by DEAE-Sepharose column. The column was pre equilibrated with 20 mM Tris Hcl (pH 7.5) and was eluted with a stepwise gradient of 0–1 M NaCl at a flow rate of 0.8 mL/min. No protein was eluted after 0.4 M NaCl. [U = The amount of enzyme required to liberate 1 µmol of inorganic phosphate per minute]. B Purification of phytase by gel filtration chromatography (Sephadex G-100). The column was equilibrated with 20 mM Tris Hcl (pH 7.5) and fractions of 3 mL each were collected with a flow rate of 0.4 mL/min. C SDS-PAGE analysis of the phytase from ONF2. Lane 1 crude supernatant (20 µg), lane 2 sample after ion-exchange chromatography (8 µg), lane 3 protein markers (molecular masses in kDa), lane 4 purified phytase (5 µg)
Effect of pH and Temperature on the Activity and Stability of the Purified Phytase
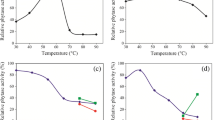
Phytate-degrading enzymes or phytases are special kind of phosphatases which are able to release orthophosphate from phytate and other phosphorylated compounds and depending on the source of origin they may have discrete catalytic properties. In comparison to plant enzymes, microbial phytases are much more pH and thermo stable as they are active at pH below 3.0 and above of 8.0 [39]. The purified phytase in the present study has an optimal pH of 6.5–7.5 and the enzyme was stable at pH values ranging from 5.0 to 9.5 (Fig. 3). The enzyme retained about 65 % of its activity after incubation for 1 h at pH 9.0. Most of the microbial phytases characterized so far have pH optima in the acidic region (4.5–6.0), but the phytases from Bacillus genera are generally seen to have optimum pH of 6.5–8.0 [40]. The purified phytase from B. licheniformis ONF2 retained 50 % of its activity after incubation at pH 4.5 for 1 h. Carps generally do not have an acid secreting stomach and have neutral to alkaline pH environment in their GI tract [41]. Therefore, phytases to be used as feed additive for carps, should have higher activities in the neutral to alkaline pH [15] which complied with the pH optima (pH 6.5–7.5) of the presently purified phytase.
For being suitable for incorporation into feed, thermal stability of the enzyme is very important as commercial aquaculture feeds are subjected to pelleting which involves high temperature (60–80 °C) and steam [21]. The presently purified phytase was fairly stable at temperatures from 20 to 75 °C and had maximum activity at 50 °C at neutral pH. Results of enzyme activity at varying temperatures are presented in Fig. 4. Residual phytase activity of 85 % was detected after incubation of the enzyme at 60 °C for 15 min. The purified phytase in the present study required 1 mM CaCl2 for its activity and in its absence the enzyme activity and stability was decreased at high temperature. Similar temperature stability studies have been carried out with phytase from other species of Bacillus. The phytase from Bacillus sp. DS11 had an optimum temperature at 70 °C and optimal pH of 7, and retained about 50 % of its activity after incubation at 90 °C for 10 min in the presence of 5 mM CaCl2 [2]. Gulati et al. [42] also showed that the partially purified phytase from Bacillus laevolacticus with an optimal activity temperature of 70 °C retained 80 % of its residual activity after 3 h of incubation at 60 °C in the presence or absence of CaCl2. The presently purified phytase from B. licheniformis exhibited 50 % of residual activity at 80 °C after 15 min of incubation.
Effect of Metal Ions on the Phytase Activity
The effects of various chemicals on enzyme activity were tested in the presence or absence of 1 mM CaCl2. The phytase activity was determined with sodium phytate as substrate. The enzyme activity was observed to be greatly inhibited by EDTA in the absence of CaCl2. The phytase activity was also largely inhibited by the metal ions Cu2+, Hg2+, Zn2+, Co2+ and moderately by Mn2+, Mg2+ and K+ at 5 mM concentration. The residual phytase activities of the purified enzyme after incubation with metal ions are given in Table 2. The inhibitory effects might be because of the fact that phytate forms insoluble complexes with metal ions, which may decrease the active concentration of the substrate in the phytase assay [30]. It was also noticed that the enzyme activity was increased by the presence of 1 mM CaCl2 and in its absence the inhibitory effect of the tested chemicals on purified phytase was more intense. Wang et al. [15] suggested that besides stabilizing effect on phytase Ca2+ also may play an important role as substrate activator. Ca2+ dependencies on enzyme activity and stability of phytases also have been reported from other species of Bacillus such as B. subtilis and B. amyloliquefaciens [43]. This is quite dissimilar to the properties of acid phytases from Yersinia kristeensenii and Citrobacter braakii which were neither stimulated by calcium ions, nor EDTA have any inhibitory effect on their activity [23, 29] and in fact, the enzyme activity of phytase from K. pneumoniae 9-3B was slightly stimulated by EDTA [32].
In Vitro Phytate Hydrolysis by the Purified Phytase
Two phytate rich plant oilseed meals, sesame and soybean were used for the in vitro hydrolysis assay with the purified phytase from B. licheniformis strain ONF2. The assay was carried out at pH from 5.0 to 8.0 as the purified phytase was maximally active at the pH of 7.0 with sodium phytate as substrate. The highest release of inorganic phosphate (1130.3 ± 40.2 µg g−1) was detected at pH 7.0 for sesame seed meal whereas with soybean meal 720.5 ± 35.2 µg of inorganic P was released with 1 U of phytase per gram of seed meal (Fig. 5). Being protein rich the soybean meal has high prospect because of its ability to partially replace the fish meal in aquafeed [44]. The sesame oilseed meal is rich source of phosphorous but most of its P is present in the form of phytic acid which is not digestible and thus unavailable to non-ruminants [9].
Phytate hydrolyzing ability of the purified phytase at different pH (5–8.0) with soybean and sesame seed meal as substrate. The reaction was carried out at the optimal temperature of 50 °C. Hydrolysis activity was measured as the amount (µg) of inorganic P released per gram of oilseed meal. Data (mean ± SE) with different letters are significantly different (n = 3) (P < 0.05)
Phytate is considered as an important anti-nutritional factor in plant material because of its low bioavailability and when combined with other nutrients, without phytase, it leads to reduced utilization efficiency by monogastric or agastric aquatic animals [45]. Therefore, the phytate content of plant material needs to be hydrolyzed before its incorporation in the diet of aquatic animals. Fu et al. [23] reported inorganic P release from soybean meal by the phytase from Y. kristeensenii employing the pH range of 1.5–5.5 to simulate the conditions of the digestive tract of monogastric animals. The maximal inorganic phosphorus release was obtained at pH 4.5 when either 0.25 or 1 U of enzyme was added per gram of soybean meal. In the present study, the plant phytate hydrolysis activity was carried out at pH 5, 5.5, 6, 6.5, 7, 7.5 which is similar to the pH conditions in the digestive tract of most agastric fish like carps. The efficiency of the presently purified phytase in releasing inorganic P indicates that it might be useful in degrading the phytate compounds of these plant ingredients resulting in improved nutritional value.
Conclusion
In the present study, high activity of the purified phytase from B. licheniformis in the pH range of 6.5–7.5 makes it an interesting and suitable candidate for application in animal feed industry. The results of the in vitro hydrolysis test indicated that the enzyme was able to release inorganic P from phytate rich sesame and soybean oilseed meal at pH 6.5–7.0. The enzyme was also seen to have high stability spanning broad temperature range from 20 to 75 °C with activity maxima at 50 °C. These properties of the presently purified phytase indicate that this enzyme also might have probable applications in fish feed industry for improving nutritional value of phytate rich plant feedstuffs. To the authors’ knowledge this is the first report regarding purification and characterization of phytase from a fish gastrointestinal isolate. Several commercial phytases like Natuphos, Ronozyme P, Biophos-TS etc. are being used as animal feed additives but the phytase producing bacteria offer some advantages as they can be utilized as live probiotic supplement in feed. The isolate used in the present study was isolated from the intestine of Nile tilapia and is able to survive and colonize in the GI tract environment of the fish. If used as probiotic, they offer permanent solution to the regular application of phytase feed additives. Further research should be conducted to evaluate the potential of the phytase from fish GI tract bacteria in improving the growth and health of commonly cultured freshwater fish.
References
NRC (National Research Council) (1993) Nutrient requirements of fish. National Academy Press, Washington, DC
Kim YO, Kim HK, Bae KS, Yu JH, Oh TK (1998) Purification and properties of a thermostable phytase from Bacillus sp. DS11. Enzyme Microb Tech 22:2–7
Baruah K, Sahu NP, Pal AK, Debnath D (2004) Dietary phytase: an ideal approach for a cost-effective and low polluting aqua feed. NAGA World Fish Center Q 27:15–19
Singh B, Kunze G, Satyanarayana T (2011) Developments in biochemical aspects and biotechnological applications of microbial phytases. Biotechnol Mol Biol Rev 6:69–87
Yoon SJ, Choi YJ, Min HK, Cho KK, Kim JW, Lee SC, Yeon Hoo Jung YH (1996) Isolation and identification of phytase-producing bacterium, Enterobacter sp. 4, and enzymatic properties of phytase enzyme. Enzyme Micro Technol 18:449–454
Cao L, Wang W, Yang C, Yang Y, Diana J, Yakupitiyage A, Luo Z, Li D (2007) Application of microbial phytase in fish feed. Enzyme Micro Technol 40:497–507
Igbasan FA, Simon O, Miksch G, Männer K (2001) The effectiveness of an Escherichia coli phytase in improving phosphorus and calcium bioavailabilities in poultry and young pigs. Arch Anim Nutr 54:117–126
Liebert F, Portz L (2005) Nutrient utilization of Nile tilapia Oreochromis niloticus fed plant based low phosphorus diets supplemented with graded levels of different sources of microbial phytase. Aquaculture 248:111–119
Roy T, Banerjee G, Dan SK, Ghosh P, Ray AK (2014) Improvement of nutritive value of sesame oilseed meal in formulated diets for rohu, Labeo rohita (Hamilton), fingerlings after fermentation with two phytase producing bacterial strains isolated from fish gut. Aquacult Int 22:633–652
Abelson PH (1995) A potential phosphate crisis. Science 283:2015
Vohra A, Satyanarayana T (2003) Phytases: microbial sources, production, and potential biotechnological applications. Crit Rev Biotechnol 23:29–60
Pandey A, Szakacs G, Soccol CR, Rodriguez-Leon JA, Soccol VT (2001) Production, purification and properties of microbial phytases. Bioresour Technol 77:203–214
Konietzny U, Greiner R (2004) Bacterial phytase: potential application, in vivo function and regulation of its synthesis. Braz J Microbiol 35:11–18
Lei XG, Stahl CH (2001) Biotechnological development of effective phytases for mineral nutrition and environmental protection. Appl Microbiol Biotechnol 57:474–481
Wang Q, Fu SJ, Sun JY, Weng XY (2011) Characterization of a thermostable alkaline phytase from Bacillus licheniformis ZJ-6 in Pichia pastoris. World J Microbiol Biotechnol 27:1247–1253
Dan SK, Ray AK (2014) Characterization and identification of phytase producing bacteria isolated from the gastrointestinal tract of four freshwater teleosts. Ann Microbiol 64:297–306
Roy T, Mondal S, Ray AK (2009) Phytase-producing bacteria in the digestive tracts of some freshwater fish. Aquacult Res 40:344–353
Howson SJ, Davis RP (1983) Production of phytate-hydrolyzing enzyme by some fungi. Enzyme Micro Technol 5:377–382
Heinonen JK, Lahti RJ (1981) A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem 113:313–317
Laemmli UK (1970) Cleavage of structural protein during the assembly of the lead of bacteriophage T4. Nature 227:680–685
Dechavez RB, Serrano AE Jr, Nuñal S, Caipang CMA (2011) Production and characterization of phytase from Bacillus spp. as feed additive in aquaculture. ELBA Bioflux 4:394–403
Bradford M (1976) A rapid and sensitive method of the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Fu D, Huang H, Luo H, Wang Y, Yang P, Meng K, Bai Y, Wu N, Yao B (2008) A highly pH-stable phytase from Yersinia kristeensenii: cloning, expression, and characterization. Enzyme Microb Technol 42:499–505
Han Y, Wilson DB, Lei XG (1999) Expression of an Aspergillus niger phytase gene (phyA) in Saccharomyces cerevisiae. Appl Environ Microbiol 65:1915–1918
Duncan DB (1955) Multiple range and multiple F-tests. Biometrics 11:1–42
Greaves MP, Anderson G, Webley DM (1967) The hydrolysis of inositol phosphates by Aerobacter aerogenes. Biochim Biophys Acta 132:412–418
Hong SW, Chu IH, Chung KS (2011) Purification and biochemical characterization of thermostable phytase from newly isolated Bacillus subtilis CF92. J Korean Soc Appl Biol Chem 54:89–94
Tamayo-Ramos JA, Sanz-Penella JM, Yebra MJ, Monedero V, Haros M (2012) Novel phytases from Bifidobacterium pseudocatenulatum ATCC 27919 and Bifidobacterium longum spp infantis ATCC 15697. Appl Environ Microbiol 78:5013–5015
Kim HW, Kim YO, Lee JH, Kim KK, Kim YJ (2003) Isolation and characterization of a phytase with improved properties from Citrobacter braakii. Biotechnol Lett 25:1231–1234
Farouk A, Greiner R, Hussin ASM (2012) Purification and properties of a phytate-degrading enzyme produced by Enterobacter sakazakii ASUIA279. J Biotechnol Biodivers 3:1–9
Greiner R, Konietzny U, Jany KD (1993) Purification and characterization of two phytases from Escherichia coli. Arch Biochem Biophys 303:107–113
Escobin-Mopera L, Ohtani M, Sekiguchi S, Sone T, Abe A, Tanaka M, Meevootisom V, Asano K (2012) Purification and characterization of phytase from Klebsiella pneumoniae 9-3B. J Biosci Bioeng 113:562–567
De Angelis M, Gallo G, Corbo MR, McSweeney PLH, Faccia M, Giovine M, Gobbetti M (2003) Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Food Microbiol 87:259–270
Park I, Cho J (2011) The phytase from antarctic bacterial isolate, Pseudomonas sp. JPK1 as a potential tool for animal agriculture to reduce manure phosphorus excretion. Afr J Agric Res 6:1398–1406
Yanke LJ, Bae HD, Selinger LB, Cheng KJ (1998) Phytase activity of anaerobic ruminal bacteria. Microbiology 144:1565–1573
Lan GQ, Abdullah N, Jalaludin S, Ho YW (2011) Purification and characterization of a phytase from Mitsuokella jalaludinii, a bovine rumen bacterium. Afr J Biotechnol 10:12796–12806
Sreeramulu G, Srinivasa DS, Nand K, Joseph R (1996) Lactobacillus amylovorus as a phytase producer in submerged culture. Lett Appl Microbiol 23:385–388
Konietzny U, Greiner R (2002) Molecular and catalytic properties of phytase degrading enzymes (phytases). Int J Food Sci Technol 37:791–812
Caipang CMA, Dechavez RB, Apines-Amar MJS (2011) Potential application of microbial phytase in aquaculture. ELBA Bioflux 3:55–66
Huang H, Luo H, Wang Y, Fu D, Shao N, Wang G, Yang P, Yao B (2008) A novel phytase from Yersinia rohdei with high phytate hydrolysis activity under low pH and strong pepsin conditions. Appl Microbiol Biotechnol 80:417–426
Schafer A, Koppe WM, Meyer-Burgdorff KH, Gunther KD (1995) Effects of microbial phytase on the utilization of native phosphorus by carp in a diet based on soybean meal. Water Sci Technol 31:149–155
Gulati HK, Chadha BS, Saini HS (2007) Production and characterization of thermostable alkaline phytase from Bacillus laevolacticus isolated from rhizosphere soil. J Ind Microbiol Biotechnol 34:91–98
Farhat A, Chouayekh H, Farhat MB, Bouchaala K, Bejar S (2008) Gene cloning and characterization of a thermostable phytase from Bacillus subtilis US417 and assessment of its potential as a feed additive in comparison with a commercial enzyme. Mol Biotechnol 40:127–135
Silva-Carrillo Y, Hernández C, Hardy RW, González-Rodríguez B, Castillo-Vargasmachuca S (2012) The effect of substituting fish meal with soybean meal on growth, feed efficiency, body composition and blood chemistry in juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquaculture 364:180–185
Cheng W, Chiu CS, Guu YK, Tsai ST, Liu CH (2013) Expression of recombinant phytase of Bacillus subtilis E20 in Escherichia coli HMS 174 and improving the growth performance of white shrimp, Liptopenaeus vannamei, juveniles by using phytase-pretreated soybean meal containing diet. Aquacult Nutr 19:117–127
Acknowledgments
The authors are grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi [Project No. 37(1415)/10/EMR-II] for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dan, S.K., Nandi, A., Banerjee, G. et al. Purification and Characterization of Extracellular Phytase from Bacillus licheniformis Isolated from Fish Gut. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 85, 751–758 (2015). https://doi.org/10.1007/s40011-015-0571-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0571-4