Abstract
Phytases are specialized enzymes meant for phytic acid degradation. They possess ability to prevent phytic acid indigestion, including its attendant environmental pollution. This study was aimed at investigating biochemical properties of purified phytase of B. cereus isolated from Achatina fulica. Phytase produced from Bacillus cereus that exhibited optimal phytate degrading-ability of all the bacteria isolated was purified in a three-step purification. The biochemical properties of the purified enzyme were also determined. The phytase homogeny of approximately 45 kDa exhibited 12.8-purification fold and 1.6% yield with optima phytate degrading efficiency and maximum stability at pH 7 and 50 °C. Remaining activity of 52 and 47% obtained between 60 and 70 °C after 2 h further established thermostability of the purified phytase. Mg2+ and Zn2+ enhanced phytate hydrolysis by the enzyme, while Na+ showed mild inhibition but Hg2+ severely inhibited the enzymatic activity. Km and Vmax were estimated to be 0.11 mM and 55.6 μmol/min/mL, displaying enzyme-high substrate affinity and catalytic efficiency, respectively. Phytase purified from Bacillus cereus, isolated from African giant snails, has shown excellent characteristics suitable for phytic acid hydrolysis and could be employed in industrial and biotechnological applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytic acid (myo-inositol hexakis phosphoric acid) is a secondary metabolite manufactured by plants and are accountable for the total phosphorus in plants (Caliskan-Ozdemir et al. 2021). Phytic acid is abundant in seeds during ripening, and its principal function is to store myo-inositol (Mehta et al. 2006; Nissar et al. 2017). Phytic acid is a major constituent in food crops of the family of legumes, cereals, and oil seed crops. These classes of crops are universally planted for the purpose of food for animals and humans (Nissar et al. 2017). Phytic acid present in these crops has the capability to bind with the available divalent and some monovalent metal ions, such as Ca2+, Mg2+, Fe2+, Na+, and K+, respectively (Sanni et al. 2019; Trivedi et al. 2022). Of all organophosphate molecules, phytic acid is extremely stable owing to its high negative charge and can therefore perform different roles over a wide pH range, which hence favors its anti-nutritive effect (Trivedi et al. 2022). As a result of these anti-nutritive properties, phytic acid in plant-based foods and animal feeds becomes a major problem as it threatens humans’ and animals’ health. It also poses environmental degradation and therefore requires enzymatic degradation so as to reduce its laden on the ecosystem (Singh and Satyanarayana 2008; Nissar et al. 2017; Jatuwong et al. 2020). Hence, hydrolysis of phytate is pertinent in human diets and animal feeds because there are approved daily intakes of phytate, of which if exceeded could result in health problems (Song et al. 2019). Enzymatic hydrolysis of phytic acid by phytase is desirable for effective degradation of phytic acid, though nonenzymatic methods such as cooking, soaking, germinating, fermentation, and addition of vitamin C have been reported (Song et al. 2019).
Phosphatases are sub-class of hydrolases and majorly catalyze hydrolysis of inorganic phosphate in phosphate-containing compounds from various sources. However, hydrolysis of phosphoesters in phytic acid requires more specific enzymes than phosphatases because it has been observed not to be meant for phytic acid catalysis (Sanni et al. 2019). Hence, phytase (myo-inositol hexakisphosphate phosphohydrolases) is a special class of enzyme that is specific for hydrolysis of phytic acid to monophosphate and its derivatives (in some cases to free myo-inositol), releasing inorganic phosphate (Craxton et al. 1997; Haros et al. 2007; Sanni et al. 2019). Presence of phytases has been observed in microorganisms (Coban and Demirci 2014; Sanni et al. 2019), plants, and some animal tissues (Sanni et al. 2019).
Phytases are crucial in both human and animal nutritions (Sadaf et al. 2022). They are used as a feed addition to counteract phytic acid’s antinutritional effects and to promote phosphorus absorption, hence reducing the environmental pollution caused by complex phosphate compounds (Sato et al. 2016; Soman et al. 2020). Thermostability, a wide pH range, and proteolytic resistance of phytase are biochemical characteristics that have been linked to its catalytic efficiency (Song et al. 2019). These factors influence the amino acid groups (Arg, Lys, His, Glu, and Asp) present at the catalytic sites of phytases and frequently control the substrate (phytate) binding and catalysis (Song et al. 2019; Sanni et al. 2019). In comparison to plant and animal sources, microbial phytases have been found to possess exceptional qualities such as thermal and pH stability, inhibitor and metal tolerance, high substrate specificity, and catalytic efficiency for sodium phytate (the substrate). As a result, they have been found to be more effective in managing the environmental threat associated with excess complex phosphate (Fasimoye et al. 2014; Sato et al. 2016; Ajith et al. 2019; Sanni et al. 2019).
Monogastric animals, including pigs, poultry, and fish, find it difficult to make use of phytate phosphorus, and it becomes necessary that they are supplied with extracellular phytase (phytic acid degrader) in their feed in order to meet phosphate requirements. This is important for their growth and development and mitigation of anti-nutritive effect of phytate that could lead to environmental pollution (Roopesh et al. 2006; Sanni et al. 2019). Digestion of phytic acid in monogastric could only be spontaneous if microorganisms inhabiting the gut of the animals are phytate-degraders. Therefore, efforts are being continually made in isolating and screening more microorganisms, including bacteria that are suitable for this purpose (Lan et al. 2002; Sanni et al. 2019). Meanwhile, phytase production has been observed in Bacillus licheniformis (Fasimoye et al. 2014), Geobacillus sp. TF16 (Dokuzparmak et al. 2017), and Lactobacillus coryniformis (Demir et al. 2018), while Jain and Chauhan (2014) and Dahiya and Singh (2016) isolated Bacillus cereus phytase from different sources and reported varying ability of the enzymes in releasing inorganic phosphorus and Danial and Alkhalf (2016) reported physicochemical properties of phytase in Bacillus cereus EME 48. Though previous works from our lab have established phytase in different tissues of African giant snails (Sanni et al. 2017) and physicochemical properties of purified phytase from Aspergillus fumigatus isolated from African giant snails (Sanni et al. 2019).
The present study reports biochemical properties of purified phytase from Bacillus cereus isolate associated with gastrointestinal tracts of African giant snails.
Materials and methods
Materials
Sample collection
Bacillus cereus (a bacterium with phytate-degrading capacity) isolated from gastrointestinal tract of African giant snails was obtained from the Department of Biochemistry, Federal University of Technology, Akure, and was maintained on agar slant.
Chemicals
DEAE Sephadex A-50, Sephadex G-100, sodium acetate, hydrochloric acid, sodium hydroxide, Tris–HCl Buffer, bovine serum albumin (BSA), sodium chloride, magnesium chloride, mercury chloride, aluminum chloride, copper chloride, manganese chloride, glycine, sodium hydroxide, hydrochloric acid, EDTA, molybdate, and trichloroacetic acid (TCA) were products of Sigma-Aldrich Laborchemikallen, EC label C.O.O. Germany. All other reagents were of analytical grade.
Methods
Sample preparation, isolation, and identification procedure
Pure strains of different nine bacteria isolated from gastrointestinal tract of African giant snails according to Holt et al. (1994) and Olopoda et al. (2022) were suspended on sterile MacConker agar, Mannitol salt agar, and Deoxycholate citrate agar. Pure isolates of the organisms were identified using different biochemical tests and maintained on nutrient agar slant at 4 °C.
Screening of isolated microorganisms for phytase activity
Pure strains of bacterial isolates were screened for phytate-degrading ability. Phytase was produced using modified phytase screening medium (PSM) consisting 5 g Na phytate, 10 g of sucrose, 2 g (NH4)2SO4, 3 g peptone, 2 g yeast extract, 0.5 g MgSO4, 0.01 g MnSO4.5H2O, 0.01 g of FeSO4, 1 mL triton X-100, and 15 g agar at pH 5.5. The holes were created using sterile borer in the midst of plates consisting nutrient agar, and the holes were filled with PSM. Each pure strain of the bacteria was transferred into the respective plate and incubated for 24 h. The diameter of zone of inhibition of each plate was then measured to quantify phytate-degrading ability of each bacterium.
Growth pattern and phytase activity of Bacillus cereus
Culture preparation was done according to the method of Sung et al. (2011) with little modifications and consisted 5 g of Na phytate, 10 g of sucrose, 2 g (NH4)2SO4, 3 g tritone, 2 g yeast extract, 0.5 g MgSO4, 0.01 g MnSO4.5H2O, 0.01 g of FeSO4, 1 g triton X-100, pH 5.5 incubated at 30 °C for 48 h and was autoclaved at 120 °C for 15 min. Under a well-sterile environment, the media were inoculated with the freshly prepared seed culture of Bacillus cereus using a wire loop and incubated for 48 h at 37 °C using orbital shaker agitating at 210 rpm. One mL of the cell culture sample was withdrawn with the aid of a sterilized syringe at 6 h intervals throughout the incubation period of 48 h. The growth of the organism was monitored at 600 nm, while phytase activity and protein concentration were measured at 660 and 595 nm, respectively, using supernatant obtained after the cell culture was centrifuged at 6000 rpm.
Preparation of seed culture and isolation of phytase
The seed culture preparation was done according to the method of Sung et al. (2011) and Olopoda et al. (2022) with little modification by dissolving 0.5 g of sucrose, 0.3 g of peptone, 2 g of NaCl, and 0.05 g of CaCl2 in 100 mL of distilled water. The peptone was allowed to dissolve before the addition of the salts while pH was adjusted to 5.5. The broth was sterilized by autoclaving it at 120 °C for 15 min. Under a well-sterilized environment, the media were inoculated with Bacillus cereus using a wire loop and incubated for 24 h in an orbital incubator at 210 rpm and temperature of 37 °C.
The working medium was made up of 18 g sucrose, 6 g NaNO3, 0.6 g KCl, 0.6 g MgSO4.7H20, 0.012 g FeSO4.7H2O, and 0.5% Na-phytate, pH 5.5 and was scaled up to 1200 mL. The culture medium was thereafter autoclaved at 120 °C for 15 min. The seed culture was subsequently inoculated into the sterile medium and incubated at 37 °C for 48 h with agitation rate of 125 rpm. The culture fluid was centrifuged at 15,000 g for 20 min to obtain supernatant. The supernatant was then stored as source of crude phytase and used for purification process.
Phytase assay
Phytase activity was determined according to the method of Sanni et al. (2019). The reaction mixture was made up of 1 mL 0.2 M sodium acetate buffer (pH 5.5) containing 0.5% of sodium phytate and 1 mL of enzyme solution. The assay mixture was incubated for 30 min at 37 °C. Addition of 1 mL TCA (15% w/v) was done to terminate the enzyme reaction, followed by the addition of 2 mL color reagent containing 3.66 g FeSO4.7H2O, 0.5 g (NH4)6MO7O24.4H2O, and 1.6 mL concentrated H2SO4 in 50 mL of distilled water. Phytase activity was read at 660 nm. One unit of the enzyme activity was defined as the amount of the enzyme able to hydrolyze phytate, resulting in 1 µmol of inorganic phosphorus per min under the assay condition, and expressed in international unit (U).
Total protein determination
Protein concentration determination in the sample was carried out according to the method of Bradford (1976).
Purification and characterization of phytase
Ammonium sulfate precipitation
The crude enzyme extract was precipitated using 40–60% ammonium sulfate saturation with gentle and continuous stirring on magnetic stirrer for the complete dissolution of the ammonium sulfate. The precipitate was then centrifuged at 15,000 g using refrigerated centrifuge, and the pellet obtained was dissolved in 10 mL of 20 mM sodium acetate buffer (pH 5.5) and dialyzed against the same buffer for three days with three changes of buffer after 24 h. Phytase activity and protein concentrations were determined according to standard procedure.
Ion exchange chromatography
The dialyzed protein solution was loaded onto DEAE-Sephadex column (2.5 × 35 cm) and equilibrated with 20 mM sodium acetate buffer pH 5.5. After eluting the unbound inactive protein from the column with starting buffer, a linear gradient of 0.5 M NaCl in 20 mM sodium acetate buffer pH 5.5 was applied to elute the bound protein. Absorbance of the fractions was determined, and peak fractions were tested for phytase activity. Fractions exhibiting phytase activity were pooled and concentrated.
Gel filtration chromatography
The concentrated enzyme solution was loaded on Sephadex G-100 column (2.5 × 70 cm; flow rate: 10 mL/h) previously equilibrated with 20 mM sodium acetate buffer at pH 5.5. Absorbance of the fractions collected was determined at 280 nm, and peak fractions were tested for phytase activity, while fractions exhibiting phytase activity were pooled together and concentrated. The concentrated aliquot enzyme was used for SDS-PAGE and characterization.
Estimation of molecular weight
SDS-PAGE was carried out using 10% acrylamide-resolving gel as described by Laemmli (1970) using the Bio-Rad electrophoresis system (Bio-Rad, UK). The electrophoresis pack was run at a voltage of 80 V and 21 mA using the running buffer. After complete electrophoresis movement, the gel was stained with Coomassie brilliant blue for 24 h, after which it was destained using 530 mL of distilled water, 400 mL of methanol, and 70 mL of acetic acid.
Determination pH optimum and stability
The effect of pH on activity of purified phytase was determined using various buffer systems consisting 0.05 M of glycine–HCl (pH 2.0–3.0), sodium acetate (4.0–6.0), and Tris–HCl (pH 7.0–9.0) and incubated at 37 °C for 30 min. Phytase assay was determined according to the method earlier described. The effect of pH on phytase stability was also carried out by incubating the purified enzyme solution between pH 5 and 8 for 2 h. Meanwhile, aliquot enzyme was withdrawn at 0 min, followed by subsequent removal at 20-min interval. Phytase activity was determined according to the standard assay procedure earlier described.
Determination of optimum temperature and thermal stability
The temperature profile of the purified phytase was determined in the temperature range of 30 to 80 °C. The aliquot enzyme in 0.5% sodium phytate containing sodium acetate buffer, 0.2 M, pH 5.5, was incubated in the temperature range 30 to 80 °C. Phytase activity was determined according to standard assay procedure. The phytase thermal stability was also determined by incubating the purified enzyme at 50–80 °C for 2 h. Initial activity was recorded at 0 min, while the subsequent activities were observed at 20-min interval. Phytase activity was determined according to the standard assay procedure.
Effect of cations and EDTA on enzyme activity
Effects of metal ions including Na+, Zn2+, Ca2+, Fe2+, Mg2+, and Hg2+, and inhibitors such as EDTA, were carried out on the purified enzyme at the concentration of 2 mM. Purified phytase solution was incubated in 0.2 M sodium acetate buffer (pH 5.5), containing each of the metal ions and inhibitor solution. Phytase activity was determined according to the standard assay procedure.
Measurement of kinetic parameters
The kinetic constants, Km and Vmax of the purified enzyme were determined by the method of Lineweaver and Burk (1934) using sodium phytate as substrate at various concentrations (0.15–1.0 mM) in 0.2 M sodium acetate buffer (pH 5.5).
Results
Identification and screening of bacteria isolates from African giant snail
The results of bacteria isolated from African giant snails are presented in Table SM1. The features and properties exhibited by each bacterium are presented in the table as identified using different microscopic and biochemical tests. All the isolates exhibited phytate-degrading ability, but with varying degrees shown by the diameter of the zone of inhibition. Bacillus cereus showed widest zone of inhibition followed by Erwinia amylovora, while Corynebacterium xerosid of all isolates exhibited least potential to degrade phytic acid as shown in Table SM2. However, screening of the isolates using phytate screening medium further revealed Bacillus cereus as isolate with maximum phytase-yielding bacterium, as presented in Fig. 1a. Therefore, Bacillus cereus was used further for the study.
Screening of bacteria for phytase activity and effect of incubation time on phytase production. a Screening of bacteria produced from the gastrointestinal tract of African giant snail (Achatina fulica) for phytate-degrading ability; b phytase production, soluble protein production, and growth pattern of Bacillus cereus. The activity is expressed as µmol/min/ml, while the protein concentration of the sample is expressed as mg/ml. The data were collected three times and estimated as mean ± standard deviation
Growth pattern and phytase production of Bacillus cereus
Growth pattern and phytase production of Bacillus cereus is shown in Fig. 1b. Phytase optimum production was obtained at 18 and 24 h incubation with 48 and 49 U/mL, respectively, and optimum growth at 12 h incubation. The maximum protein concentration was achieved after 18 h. The bacterium showed decrease in growth after 12 h of incubation, while it exhibited increased production of phytase between 0 and 24 h, followed by a drastic decrease in both growth and phytase production from 24 to 48 h.
Purification and characterization of phytase
Purification of phytase from Bacillus cereus isolated from African giant snail
The summary of purification profile of phytase from Bacillus cereus is presented in Table 1. The aliquot enzyme after ammonium sulfate precipitation yielded specific gravity of 2.4 U/mg and 1.58 purification fold with 3.0% recovery of its original value. The suspended dialyzed aliquot enzyme on DEAE Sephadex showed single peak phytase activity as shown in Fig. 2. The purification fold of 7.3 with 2.6% recovery was recorded. However, when the enzyme was suspended against Sephadex G-100 of gel-filtration chromatography (Fig. 3), a 1.6% recovery and specific activity of 19.4 U/mg with 12.8 purification fold was achieved.
Molecular weight
Figure 4 shows polyacrylamide gel electrophoresis in the presence of SDS of the purified phytase. The molecular weight of the purified phytase was estimated to be 44.86 kDa.
Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. STD, standard molecular weight; a, phosphorylase b (113,142 Da); b, bovine serum albumin (81,353 Da); c, ovalbumin (47,045 Da); d, carbonic anhydrase (34,173 Da); e, trypsin inhibitor (27,259 Da); f, lysozyme (17,671 Da); PP, purified phytase (44,863 Da); SL, standard lane
Effect of pH on the activity purified phytase from B. cereus
Effect of pH on the activity of the purified phytase is presented in Fig. 5. Optimum activity was obtained at pH 7 (neutral pH). However, the enzyme demonstrated relative activity of 70% at acidic pH 6, and a much higher relative activity of 92% was recorded at alkaline pH 8, while a significant amount of remaining relative activity of 50% was obtained at pH 9.
Effect of pH on the activity of phytase from Bacillus cereus. The effect of pH was investigated between pH 2 and 9 with buffer systems of 0.05 M of glycine–HCl (pH 2.0–3.0), sodium acetate (4.0–6.0), and Tris–HCl (pH 7.0–9.0) and incubated at 37 °C for 30 min while the pH with highest activity was represented as 100% relative to others. The data were collected three times and estimated as mean ± standard deviation
Effect of pH on the stability of the purified phytase
Effect of pH on the stability of the purified phytase is presented in Fig. 6. The enzyme attained its maximum stability at pH 7, with 68% residual activity after 2 h incubation. However, at pH 5, 6, and 8, remaining residual activities of 52, 47, and 44%, respectively, were obtained after a 2 h incubation period.
Effect of pH on the stability of phytase from Bacillus cereus. The effect of pH on phytase stability was investigated between pH 5 and 8 with buffer systems of 0.05 M of sodium acetate (5.0 and 6.0) and Tris–HCl (pH 7.0–8.0) and incubated at 37 °C. There was initial removal of enzyme solution at 0 min, followed by subsequent removal at 20-min interval for 2 h. Phytase activity was determined according to the standard assay procedure. The residual activity was calculated relative to the activity at 0 min as 100%. The data were collected three times and estimated as mean ± standard deviation
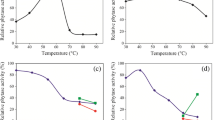
Effect of temperature on activity of purified phytase
Effect of temperature on activity of phytase from B. cereus is presented in Fig. 7. The optimum temperature was obtained at 50 °C. However, very high relative activities of 92 and 75% were obtained at 40 and 60 °C, respectively, followed by drastic decrease in phytase activity and showed severe inhibition at 80 °C with remaining activity of 14% and almost completely inactivated at 90 °C, yielding 3.5% relative activity.
Effect of temperature on the activity of phytase from Bacillus cereus. The temperature was investigated between 30 and 80 °C at 10-°C interval while the enzyme solution was incubated in 0.5% sodium phytate containing sodium acetate buffer, 0.2 M, pH 5.5. The temperature with highest activity was represented as 100%, and activity of others was calculated relative to the temperature with maximum activity. The data were collected three times and estimated as mean ± standard deviation
Effect of temperature on the stability of purified phytase
Effect of temperature on the stability of phytase is presented in Fig. 8. Phytase from Bacillus cereus yielded 81% residual activity of its original at 50 °C. The enzyme showed rapid instability, with 52, 43, and 25% remaining residual activities recorded at 60, 70, and 80 °C, respectively.
Effect of temperature on the stability of phytase from Bacillus cereus. The temperature was investigated between 50 and 80 °C at 10-°C interval while the enzyme solution was incubated in 0.5% sodium phytate containing sodium acetate buffer, 0.2 M, pH 5.5. There was initial removal of enzyme solution at 0 min, followed by subsequent removal at 20-min interval for 2 h. Phytase activity was determined according to the standard assay procedure. The residual activity was calculated relative to the activity at 0 min as 100%. The data were collected three times and estimated as mean ± standard deviation
Effect of metal ions and inhibitor on the activity of phytase
Effect of metal ions and inhibitors on the activity of phytase is presented in Fig. 9. The enzyme activity was activated in the presence of Mg2+ and Zn2+, with 9 and 6% increase, respectively. However, the activity was moderately inhibited by Na+, and about 50% of the enzymatic activity was inhibited by Ca2+. The phytase activity was greatly inhibited in the presence of EDTA and Fe2+, with over 70% inhibition, and almost totally inactivated by Hg2+, with 4% remaining relative activity.
Effect of metallic ions and EDTA on the activity of phytase from Bacillus cereus. Metal ions such as NaCl, CaCl2, MgCl2, HgCl, FeCl2, ZnCl2, and metal-chelating agent EDTA were incubated with enzyme solution and 0.5% sodium phytate in buffer containing 2 mM sodium acetate (pH 5.5). The activity was determined according to the standard assay procedure. The data were collected three times and estimated as mean ± standard deviation
Kinetic parameters
Lineweaver–Burk plot of [V]−1 against [S]−1 is presented in Fig. 10. Km and Vmax values were estimated to be 0.11 mM and 55.6 μmol/min/mL, respectively.
Double reciprocal curve showing Vmax and Km of phytase of Bacillus cereus. The concentrations of phytase from 0.15 to 1 mM were dissolved, respectively, in 0.2 mM sodium sodium acetate (pH 7). The activity was determined according to the standard assay procedure. Vmax and Km were then extrapolated from Lineweaver–Burk plot
Discussion
Phytase-producing ability has been identified and reported in various microbial strains, mostly in bacteria and fungi (Radcliffe et al. 1998; Vohra and Satanarayana 2003; Vats et al. 2004; Vats and Banerjee 2004; Coban and Demirci 2014; Sanni et al. 2019; Chatterjee et al. 2020; Trivedi et al. 2022). However, Sanni et al. (2019) established mutual relationship between African giant snail and its microbiotas, as most of these microorganisms have exhibited phytate-degrading ability. This consequently tends to result in lessening the anti-nutritional effect of phytate in the feeds of this organism, encourage hydrolysis of this complex compound into absorbable form, and then ensure proper utilization of other macromolecules and minerals by the snail. The result of this research correlates with previous studies where Lactobacillus species (Zotta et al. 2007) and Alcaligene sp. (Vijayaraghavan et al. 2013) were used in the production of phytase with the resultant high yields at different incubation periods.
The screening of bacteria for phytase production revealed Bacillus cereus among nine strains of the bacterial isolates from the gut of African giant snails as highest phytase-producing strain. However, further production of phytase with B. cereus showed the exponential growth rate of the bacterium after 12 h, while the enzyme production was optimum after 24-h incubation period.
Shorter period of bacterial growth and enzyme production observed in this study is consistent with Ogbonna et al. (2017). This short hour of incubation could prevent risk of spoilage according to Romero et al. (1998).
However, decline in the bacterial growth after 12-h incubation and further increase in phytase production infers bacterium’s potential to maximally release the enzyme into the medium at stationary phase despite the depletion of the medium components. This contrasts the report of Sanni et al. (2019) from Aspergillus fumigatus, while it is in consonant with Vijayaraghavan et al. (2013), who also observed optimum enzyme production at the stationary phase of Alkaligenes sp. while the bacterium has already stopped growing.
The three-step purification procedure was efficient considering the fact that a 12.8-fold purification of the enzyme was achieved with a yield of 1.6% and specific activity of 19.4 U/mg, which was similar to the counterpart B. cereus phytase reported by Jain and Chauhan (2014) and Danial and Alkhalf (2016) with purification folds of 10.75 and 12.3, respectively, and to that of Demir et al. (2018) from Lactobacillus cornyformis, where of 9.53-fold purification and recovery of 2.6% were obtained. The result obtained here was also found to be much better compared to Geobacillus sp. Phytase (Dokuzparmak et al. 2017) with 4.60-fold purification. Several authors have also observed different results for bacterial phytase purification. Fasimoye et al. (2014) reported 39-fold purification with 10% recovery by Bacillus licheniformis phytase, El-Toukhy et al. (2013), 57.7% yield, and 3.97-fold purification from Bacillus subtilis MJA and Roy and Ghosh (2014), 102-fold purification fold and 29% yield by Klebsiella sp. phytase and 134-fold purification and 41% recovery by Shiegella sp. phytase. In another report, A. foeditus MTCC 11,682 (Ajith et al. 2019) was reported to have 23.4-fold and a 13% yield, A. ficuum NTG-23 (Zhang et al. 2010) crude extract yielded 71.5 purification fold and 23.8% yield, and Aspergillus fumigatus purified phytase gave 45-fold purification with 15% recovery (Sanni et al. 2019). Among many factors that influence enzyme yield and purification fold are resin type, flow rate, and pH (Sanni et al. 2019; Badejo et al. 2021). Decrease in protein fold and recovery could be a result of several processes the sample was subjected to (Olaniyi et al. 2022), which ensure removal of unwanted protein that may interfere with the purity of the protein (Adeseko et al. 2021a; 2021b).
The molecular weight of ~ 45 kDa obtained in this study is similar to Lactobacillus coryniformis and (43 kDa) (Demir et al. 2018), Shigella spp. (43 kDa), Klebiesella sp. and B. cereus (45 kDa) (Roy and Ghosh 2014; Jain and Chauhan 2014), and Alcaligenes sp. (41 kDa) (Vijayaraghavan et al. 2013), Bacillus subtilis P6 (40 kDa) (Trivedi et al. 2022) and higher than B. subtilis MJA (38 kDa) (El-Toukhy et al. 2013) and Bacillus licheniformis (38 kDa) (Fasimoye et al. 2014). However, bacterial phytases are found to be much smaller than their fungal phytases counterparts, such as Aspergillus foeditus MTCC 11,682 (129.6 kDa) (Ajith et al. 2019) and Aspergillus fumigatus (88 and 118 kDa) (Wang et al. 2007; Sanni et al. 2019).
The optimum pH of the purified phytase from B. cereus is 7.0, and also revealed a very high relative of 92% activity at an alkaline pH of 8.0. The results obtained in this study contrast the reports of its counterpart Bacillus cereus phytase, with pH 5.5 (Danial and Alkhalf 2016) and pH 6.5 (Jain and Chauhan 2014), and Rao et al. (2008), who reported that most microbial phytases are active in the acidic pH range. Some bacterial and fungal phytases have been reported to have a pH optimum of 6.0 to 7.5 (Tran et al. 2010; Fasimoye et al. 2014; Gaind and Singh 2015; Sanni et al. 2019), and Vijayaraghavan et al. (2013) specifically reported Alcaligenes sp. phytase to be optimally active at pH 7 and 8, which is consistent with this study. However, phytases of fungal and bacterial origin have been shown to exhibit optimal activity at lower pH 4.5 to 5.5 (Wang et al. 2007; Dokuzparmak et al. 2017; Demir et al. 2018)). The purified phytase was found to be relatively stable within all the pH investigated, with 70% residual activity recorded at pH 7 and 44% remaining residual activity at pH 8 after 2 h of incubation. This result is similar to Sung et al. (2011), who reported Bacillus subtilis CF92 phytase to be relatively stable over a pH range of 4.0 to 8.0. Sanni et al. (2019), however, observed residual activity of 83% after 6 h within pH 4–7 for A. fumigatus phytase. B. cereus phytase optimum activity and stability at neutral and alkaline pH shows suitability for dietary supplements in aquaculture species with digestive system at neutral pH and food and feed processing industries (Yu and Chen 2013).
The optimum activity was observed at 50 °C for B. cereus phytase isolated from African giant snails. Bacterial phytases that have shown optimum activity between 55 and 60 °C were B. subtilis (Farhat et al. 2008), Bacillus licheniformis (Fasimoye et al. 2014), Klebiesilla sp. (Roy and Ghosh 2014), (55 °C) Sighella sp. (Roy and Ghosh 2014), and B. cereus 10,072 (60 °C). Gulati et al. (2007) and Dokuzparmak et al. (2017) reported phytase from Bacillus laevolacticus and Geobacillus sp. to have higher optimum temperatures of 70 and 85 °C, respectively. Remarkably, Thermo-stability of phytase from B. cereus with maximum residual activity at 50 °C and significant remaining activity of 44 and 47% between 60 and 70 °C after 2 h incubation period is comparable with B. subtilis CH92 phytase (Sung et al. 2011), which was stable up to 70 °C with 40% remaining activity after 30 min. B. cereus EME 48 phytase (Danial and Alkhalf 2016) was reported to have maximum thermal stability at 75 °C with 80% residual activity. However, Fasimoye et al. (2014) reported B. licheniformis phytase to retain residual activity of 55% at 80 and 90 °C. Similarly, Roy and Ghosh (2014) reported Shigella spp. phytase to be stable at 80 °C with 80% of its initial activity recorded. However, A. fumigatus phytase reported by Sanni et al. (2019) showed comparatively lower residual activity of 48% at 50 °C and was thereafter inhibited by further increase in temperature. Thermal stability of B. cereus phytase could be exploited in animal feed preparation as the process requires high temperatures (Dokuzparmak et al. 2017).
Effects of some metal ions and EDTA on the activity of phytase from Bacillus cereus revealed that only Mg2+ and Zn2+ had an activating effect at 2 mM, while Fe2+ and Ca2+ showed inhibitory effects, but Hg2+ nearly inactivated enzymatic activity. The report of Yu and Chen (2013) that the activity of purified phytase from Bacillus nealsonii Zj0702 was inhibited by Ca2+, Cu2+, Co2+, Zn2+, Mn2+, Ba2+, and Ni2+ ions was comparable to the results obtained in this study, while it contrasts Casey and Walsh (2003), who reported that A. niger ATCC 9142 phytase to be activated in the presence of Cd2+, Cu2+, Hg2+, Mg2+, Mn2+, and Zn2+ ions, but showed inhibitory effect towards Ca2+ and was mildly affected by Fe2+ and Fe3+. Chelating effect of EDTA on the enzymatic activity of the phytase such that it resulted in over 80% inhibition was in agreement with the report of Sadaf et al. (2022) and depicted requirement of metal ions by the enzyme. Phytase has been observed to be Ca2+-dependent and was found to lose its stability in the absence of the metal ion (Jain and Chauhan 2014). A. fumigatus phytase was activated by EDTA and showed enhancement towards some metal ions investigated (Sanni et al. 2019). EDTA has been known to be a chelating agent and for its ability to chelate important modulators such as metal ions at the enzyme active site and thereby hinder enzyme functionality (Olaniyi et al. 2023).
The Km and Vmax of 0.11 mM and 55.6 μmol/min/mL, respectively, obtained in this study are similar to B. cereus EME 48 (Danial and Alkhalf 2016), Lactobacillus coryniformis (Demir et al. 2018), and Bacillus subtilis MJA (El-Toukhy et al. 2013) phytases, with Km of 0.23 mM, 0.32 mM, and 0.48 mM, respectively. The Km observed in this study was found to be smaller and therefore have more substrate affinity compared to Geobacillus sp. (Dokuzparmak et al. 2017) and Aspergillus fumigatus (Sanni et al. 2019) with Km of 1.31 and 7.2 mM, respectively. High catalytic efficiency and substrate affinity of Bacillus cereus phytase isolated from African giant snails have shown it to be an efficient phytate hydrolyzer which could be applied in industrial and commercial processes.
Conclusion
The study has revealed inherent phytate-degrading ability of bacteria in the gut of African giant snails and exceptional physicochemical properties of phytase produced from Bacillus cereus (a bacterium exhibiting maximum phytate-degrading ability), which could enhance hydrolysis of phytate and alleviate the burden it poses on the environment and health of animals and human. The inhibition of the B. cereus purified phytase by EDTA and enzymatic activity enhancement by divalent ions showed that it is a metalloprotein. The thermo-tolerant and thermostability of the purified phytase, including its pH stability, could enhance processes such as feed pelleting, food, and other biotechnological processes.
Data availability
Not applicable.
References
Adeseko CJ, Sanni DM, Salawu SO, Kade IJ, Bamidele SO, Lawal OT (2021) Purification and biochemical characterization of polyphenol oxidase of African bush mango (Irvingia gabonensis) fruit peel. Biocatal Agric Biotechnol 36:102119
Adeseko CJ, Sanni DM, Lawal OT (2021) Biochemical studies of enzyme-induced browning of African bush mango (Irvingia gabonensis) fruit pulp. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2021.1998113
Ajith S, Ghosh J, Shet DS, ShreeVidhya S, Punith BD, Elangovan AV (2019) Partial purification and characterization of phytase from Aspergillus foetidus MTCC 11682. AMB Express 9:1–11
Badejo OO, Olaniyi OO, Ayodeji AO, Tosin-Lawal O (2021) Biochemical properties of partially purified surfactant-tolerant alkalophilic endo beta-1,4 xylanase and acidophilic beta-mannanase from bacteria resident in ruminants’ guts. Biocatal Agric Biotechnol 34:101982
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caliskan-Ozdemir S, Onal S, Uzel A (2021) Partial purification and characterization of a thermostable phytase produced by thermotolerant Aspergillus tubingensis TEM 37 Isolated from Hot Spring Soil in Gediz Geothermal Field. Turkey. Geomicrobiol J 38(10):895–904
Casey A, Walsh G (2003) Purification and characterization of extracellular phytase from Aspergillus niger ATCC 9142. Biores Technol 86:183–188
Chatterjee A, Mathew A, George AO, Sengupta P, Pundir P, Jobi F, Xavier Venkatanagaraju E (2020) Purification strategies for microbial phytase. Int J Pharm Sci Res 11:25–34
Cho J, Lee C, Kang S, Lee J, Lee H, Bok J, Woo J, Moon Y (2005) Molecular cloning of a phytase gene (phy M) from Pseudomonas syringae MOK1. Curr Microbiol 51:11–15
Choi WC, Oh BC, Kim HK, Kang SC, Oh TK (2002) Characterization and cloning of a phytase from Escherichia coli WC7. J Microbiol Biotechnol 30:1
Coban HB, Demirci A (2014) Screening of phytase producers and optimization of culture conditions for submerged fermentation. Bioproc Biosyst Engine 37:609–616
Craxton A, Caffrey JJ, Burkhart W, Safrany ST, Shears SB (1997) Molecular cloning and expression of a rat hepatic multiple inositol polyphosphate phosphatase. Biochem J 328:75–81
Dahiya S, Singh N (2016) Effect of varying doses of phytase enzyme from a novel strain of Bacillus cereus MTCC 10072 in animal feed. Europ J Biotechnol Biosci 4:11–14
Danial EN, Alkhalf MI (2016) Purification and characterization of phytase from novel slated Bacillus cereus EME 48 and study its kinetic properties. J Pure Appl Microbiol 10:2521–2529
Demir Y, Dikbaş N, Beydemir S (2018) Purification and biochemical characterization of phytase enzyme from Lactobacillus coryniformis (MH121153). Mol Biotechnol 60(11):783–790. https://doi.org/10.1007/s12033-018-0116-1
Dokuzparmak E, Sirin Y, Cakmak U, Ertunga NS (2017) Purification and characterization of a novel thermostable phytasefrom the thermophilic Geobacillus sp. TF16. Int J Food Prop 20:1104–1116
El-Toukhy NM, Youssef AS, Mikhail MG (2013) Isolation, purification and characterization of phytase from Bacillus subtilis MJA. Afr J Biotechnol 12(20):2957–2967
Engelen AJ, Van der HFC, Randsdorp PHG, Somer WAC, Schaefer J, Van der Vat BJC (2001) Determination of phytase activity in feed by a colorimeteric enzymatic method: collaborative interlaboratory study. J AOAC Int 84:629–633
Farhat A, Chouayekh H, Ben FM, Bouchaala K, Bejar S (2008) Gene cloning and characterization of a thermostable phytase from Bacillus subtilis US417 and assessment of its potential as a feed additive in comparison with a commercial enzyme. Mole Biotechnol 40:127–135
Fasimoye OF, Olajuyigbe FM, Sanni DM (2014) Purification and characterization of a thermostable extracellular phytase from bacillus licheniformis Pfbl-03. J Prep Biochem Biotechnol 44:193–205
Gaind S, Singh S (2015) Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC 6720. Int Biodeterior Biodegrad 99:15–22
Greiner R, Haller E, Konietzny U, Jany KD (1997) Purification and characterization of a phytase from Klebsiella terrigena. Archi Biochem Biophys 341:201–206
Gulati HK, Chadha BS, Saini HS (2007) Production and characterization of thermostable alkaline phytase from Bacillus laevolacticus isolated from rhizosphere soil. J Ind Microbiol Biotechnol 34:91–98
Haros M, Bielecka M, Honke J, Sanz Y (2007) Myo-inositol hexakisphosphate degradation by Bifidobacterium infantis ATCC 15697. Int J Food Microbiol 117:76–84
Holt JG, Krieg NR, Sneath PHA, Stanley JT, Williams ST (1994) Bergey’s manual determ bacteriol, 9th edn. Williams and Willkins, Baltimore, p 783
Idris EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, Richter T, Borriss R (2002) Extracellular phytase activity of Bacillusamyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiol 148:2097–2109
Jain U, Chauhan N (2014) Bacillus cereus 10072 phytase - detection, purification, characterization and physiological role. Inter J Sci Res Dev 2:1–6
Jatuwong K, Suwannarach N, KumLa J, Penkhrue W, Kakumyan P, Lumyong S (2020) Bioprocess for production, characteristics, and biotechnological applications of fungal phytases. Front Microbiol 11:188
LaemmLi UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lan GQ, Abdullah N, Jalaludin S, Ho Y (2002) Optimization of carbon and nitrogen sources for phytase production by Mitsuokella jalaludinii, a new rumen bacterial species. Letter Appl Microbiol 35:157–161
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–660
Mehta BD, Jog SP, Johnson SC, Murthy PPN (2006) Lily pollen alkaline phytase is a histidine phosphatase similar to mammalian multiple inositol polyphosphate phosphatase (MINPP). Phytochemistry 67:1874–1886
Nissar J, Ahad T, Naik HR, Hussain SZ (2017) A review phytic acid: as antinutrient or nutraceutical. J Pharm Phytochem 6:1554–1560
Ogbonna FO, Milala MA, Abubakar M, Burah B (2017) Isolation and optimization of phytase from Pseudomonas aeruginosa and Aspergillus niger isolated from poultry faeces. Inter J Curr Microbiol Appl Sci 6:3666–3673
Olaniyi OO, Damilare AO, Lawal OT, Igbe FO (2022) Properties of a neutral, thermally stable and surfactant-tolerant pullulanase from worker termite gut-dwelling Bacillus safensis as potential for industrial applications. Heliyon. https://doi.org/10.1016/j.heliyon.2022.e10617
Olaniyi OO, Ajulo AS, Lawal OT, Olatunji VK (2023) Engineered Alcaligenes sp. by chemical mutagen produces thermostable and acido-alkalophilic endo-1,4-β-mannanases for improved industrial biocatalyst. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2023.2172038
Olopoda IA, Lawal OT, Omotoyinbo OV, Kolawole AN (2022) Biochemical characterization of a thermally stable, acidophilic and surfactant-tolerant xylanase from Aspergillus awamori AFE1 and hydrolytic efficiency of its immobilized form. Process Biochem 121:45–55
Radcliffe JS, Zhang Z, Kornegay ET (1998) The effect of microbial phytase, citric acid and their interaction in a corn soybean meal based diet for weaning pigs. J Animal Sci 76:1880–1886
Rao DE, Rao KV, Reddy VD (2008) Cloning and expression of Bacillus phytase gene (phy) in Escherichia coli and recovery of active enzyme from the inclusion bodies. J Appl Microbiol 105:1128–1137
Romero F, García L, Díaz M (1998) Protease production from whey at high concentrations by Serratia marcescens. Resour Environmenral Biotechnol 2:93–115
Roopesh KS, Ramachandran KM, Nampoothiri GS, Pandey A (2006) Comparison of phytase production on wheat bran and oilcakes in solid-state fermentation by Mucor racemosus. Bioresour Technol 97:506–511
Roy MP, Ghosh S (2014) Purification and characterization of phytase from two enteric bacteria isolated from cow dung. In: Proceedings of 5th Inter Confer Environ Aspects Bangladesh [ICEAB 2014]. ICEAB
Sadaf N, Haider MZ, Iqbal N, Abualreesh MH, Alatawi A (2022) Harnessing the phytase production potential of soil-borne fungi from wastewater irrigated fields based on eco-cultural optimization under shake flask method. Agriculture 12:103
Sanni DM, Jimoh MB, Fatoki TH, Ekundayo FO, Omotoyinbo VO (2017) Isolation of pyhtate degrading activity from African giant land snail (Archachatina marginata). J Agric Food Technol 7:1–9
Sanni DM, Lawal OT, Enujiugha VN (2019) Purification and characterization of phytase from Aspergillus fumigatus isolated from African giant snail (Achatina fulica). Biocat Agric Biotechnol 17:225–232
Sanni DM, Lawal OT, Enujiugha VN (2021) Purification and characterization of phytase from Aspergillus fumigatus isolated from African giant snail (Achatina fulica). Biocatal Agric Biotechnol 17:235–232
Sariyska MV, Gargova SA, Koleva LA, Angelov AI (2005) Aspergillus niger phytase: purification and characterization. Biotechnol Biotechnol Equip 19:98–105
Sato VS, Jorge JA, Guimarães LHS (2016) Characterization of a thermotolerant hytase produced by Rhizopus microsporus var. microsporus biofilm on an inert support using sugarcane bagasse as carbon source. App Biochem Biotech 179:610–624. https://doi.org/10.1007/s12010-016-2018-7
Shah V, Parekh LJ (1990) Phytase from Klebsiella sp. purification and properties. Indian J Biochem Biophys 27:98–102
Shimizu M (1992) Purification and characterization of phytase from Bacillus subtilis (natto) N-77. Biosci Biotechnol Biochem 56:1266–1269
Singh B, Satyanarayana T (2008) Phytase production by a thermophilic mould Sporotrichum thermophilein solid-state fermentation and its potential applications. Biores Technol 99:2824–2830
Soman S, Kumarasamy S, Narayanan M, Ranganathan M (2020) Biocatalysis phytase production in solid state fermentation by OVAT strategy. Biointer Res Appl Chem 5:6119–6127
Song H-Y, El Sheikha AF, Hu DM (2019) The positive impacts of microbial phytase on its nutritional applications. Trends Food Sci Technol. https://doi.org/10.1016/j.tifs.2018.12.001
Sung WH, In HC, Kun SC (2011) Purification and biochemical characterization of thermostable phytase from newly isolated Bacillus subtilis CF92. J Korean Soc Appl Biol Chem 54:89–94
Tran TT, Mamo G, Mattiasson B, Hatti-Kaul R (2010) A thermostable phytase from Bacillus sp. MD2: cloning, expression and high-level production in Escherichia coli. J Ind Microbiol Biotechnol 37:279–287
Trivedi S, Hussain I, Sharma A (2022) Purification and characterization of phytase from Bacillus subtilis P6: evaluation for probiotic potential for possible application in animal feed. Food Front 3:194–205
Ullah AHJ (1988) Production, rapid purification and catalytic characterization of extracellular phytase from Aspergillus ficuum. Prep Biochem Biotechnol 18:443–458
Vats P, Banerjee UC (2004) Production studies and catalytic properties of phytases (myoinositolhexakisphosphate phosphohydrolases): an overview. Enz Microb Technol 35:3–14
Vats P, Sahoo DK, Banerjee UC (2004) Production of phytase (myo-Ino63sitol hexakisphosphate phosphohydrolase) by Aspergillus nigervanTeighem in laboratory-scale fermenter. Biotechnol Prog 20:737–743
Vijayaraghavan PR, Primiya R, Vincent SGP (2013) Thermostable alkaline phytase from Alcaligenes sp. in improving bioavailability of phosphorus in animal feed: in vitro analysis. ISRN Biotechnol. https://doi.org/10.5402/2013/394305
Vohra A, Satanarayana T (2003) Phytases: microbial sources, production, purification, and potential biotechnological applications. Critical Rev Biotechnol 23:29–60
Wang Y, Gao X, Su Q, Wu W, An L (2007) Expression of a heat stable phytase from Aspergillus fumigatus in tobacco (Nicotiana tabacum L. cv. NC89). Indian J Biochem Biophy 44:26–30
Yang WJ, Matsuda Y, Sano S, Masutani H, Nakagawa Y (1991) Purification and characterization of phytase from rat intestinal mucosa. Biochim Biophys Acta 1075:75–82
Yu P, Chen Y (2013) Purification and characterization of a novelneutral and heat-tolerant phytase from a newly isolated strain Bacillus nealsonii ZJ0702. BMC Biotechnol 13:1–7
Zhang G, Dong X, Wang Z, Zhang Q, Wang H, Tong J (2010) Purification, characterization, and cloning of a novel phytase with low pH optimum and strong proteolysis resistance from Aspergillus ficuum NTG-23. Biores Technol 101:4125–4131
Zotta T, Ricciardi A, Parenta E (2007) Enzymatic activities of lactic acid bacteria isolated from Cornetto di Matera sourdoughs. Inter J Food Microbiol 115:165–172
Author information
Authors and Affiliations
Contributions
David Sanni conceived the ideas, supervised the research, and read the manuscript; Monsurat Jimoh carried out the research and wrote the manuscript; Olusola Lawal wrote and edited the manuscript; Samuel Bamidele co-supervised the research and analyzed the research data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethic approval and consent to participate
This research study has no connection to human participants or animals by any of the authors.
Consent for publication
All authors read and agreed to the submission of this research work to this journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanni, D.M., Jimoh, M.B., Lawal, O.T. et al. Purification and biochemical characterization of phytase from Bacillus cereus isolated from gastrointestinal tract of African giant snail (Achatina fulica). Int Microbiol 26, 961–972 (2023). https://doi.org/10.1007/s10123-023-00350-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00350-4