Abstract
Gemcitabine is used in the treatment of several solid tumors as one of anticancer nucleoside analogues and is necessary to administer high doses to achieve the desired therapeutic response. However, this treatment may be related to severe side effects. For improvement of the encapsulation efficiency of gemcitabine for gemcitabine-loaded nanoparticle composed of biodegradable polymer and reducing the side effects due to the high concentration of gemcitabine, we formulated gemcitabine-loaded PLGA particles using chitosan and different type of surfactant. The gemcitabine-loaded particles were prepared using a double emulsion solvent evaporation technique. The effects of surfactants to modify the size of gemcitabine-loaded particles were different. The mean diameters of gemcitabine-loaded particles ranged from 400.8 nm to 1712.7 nm. The surface charge of gemcitabine particles was − 5.62 mV to 1.46 mV. The encapsulation efficiency of gemcitabine-loaded particles was found to be 29.56–34.38%. Interestingly, the addition of surfactant could be improved an encapsulation efficiency of gemcitabine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gemcitabine (2′,2′-difluoro-2′-deoxycytidine) is an anticancer agent demonstrating to be effective in the treatment of a wide variety of solid malignancies and has been effective for use against pancreatic, non-small cell lung cancer, bladder, and breast cancer (Hertel et al. 1990; Plunkett et al. 1995). It was reported that gemcitabine is rapidly metabolized by cytidine deaminase into the inactive derivative 2′,2′-difluoro-2′-deoxyuridine (Heinemann et al. 1992; Bouffard et al. 1993; Matsuda and Sasaki 2004), which is considered a critical limitation of this anticancer drug (Abbruzzese et al. 1991; Grunewald et al. 1992). Consequently, gemcitabine has a very short half-life in plasma after i.v. administration (8–17 min in human plasma and 9 min in murine plasma) (Storniolo et al. 1997; Reddy and Couvreur 2008), thereby bring about the need for high doses, simultaneously resulting in severe dose-limiting side effects (Bouffard et al. 1993; Matsuda and Sasaki 2004). The synthesis of several gemcitabine derivatives has been attempted to chemically protect the 4-amino group of the drug from metabolic inactivation mechanisms. (Myhren et al. 2002). Some of the derivatives showed high cytotoxic activity, but the chemical derivatives of gemcitabine had decreased solubility and showed inadequate disadvantages for theirs i.v. administration. Oral administration of gemcitabine may be limited because of low bioavailability due to first pass metabolism (Shipley et al. 1992). In addition, oral gemcitabine causes adverse dose-limiting intestinal lesions characterized by moderate-to-severe loss of mucosal epithelium (atrophic enteropathy) throughout the intestinal tract. This phenomenon was reported in mice given a single oral (gavage) gemcitabine hydrochloride dose of 167, 333 or 500 mg/kg (Horton 2004).
Meanwhile, colloidal drug delivery system has received increasing attention as possible means of obtaining higher therapeutic effects, lower toxicity and protection form from in vivo metabolism of incorporated drugs. Poly (D,L-lactide-co-glycolide) (PLGA), a FDA-approved copolymer of lactic and glycolic acid, is one of the most biodegradable and biocompatible used for preparing nanoparticles (Bala et al. 2004). However, negative surface charge of PLGA decreases bioavailability under oral administration (Nafee et al. 2007; Zhang et al. 2012). PLGA is used to particle which prepared using W1/O/W2 method to control the particle size to nano-scale with high drug encapsulation efficiency. However, this method has limitations because it takes a long time to remove the organic solvent used to dissolve PLGA. That is, the long solvent removal time increases the possibility of adhesion droplets prior to particle formation and also increases the chance that the drug in the W1 to diffuse into the W2 phase. For improvement of the encapsulation efficiency of gemcitabine for gemcitabine-loaded nanoparticle composed of biodegradable polymer and reducing the side effects due to the high concentration of gemcitabine, we formulated gemcitabine-loaded PLGA nanoparticle for oral delivery using different combinations of lipid- (Span 80, Span 85 or lecithin) and water-soluble surfactant (Tween 80) were employed.
Materials and methods
Materials
Gemcitabine hydrochloride was purchased from Dalian Wista Pharma Co. Ltd. (Beijing, China). Chitosan (M.W; above 10 kDa) was supplied byChitolife Co. Ltd. (Pyungtaek, Korea). Span 80, Span 85, Tween 80, lecithin, mannitol and polyvinyl alcohol (PVA) (M.W; 8000–9000) were purchased from Sigma–Aldrich Korea (Yongin, Korea). Sodium chloride was purchased from Samchun chemical Co. Ltd (Pyungtaek, Korea). All other chemicals were commercially available for analytical or reagent grad and were used without further purification.
Preparation of gemcitabine-loaded particles
Gemcitabine-loaded particles were prepared using a double-emulsion method; the first emulsification was conducted to prepare the w/o primary emulsion and the second emulsification was carried out to complete the w/o/w multiple emulsion. Briefly, the gemcitabine and chitosan (the weight ratio of chitosan to gemcitabine was 0.2) previously dissolved in 0.5 mL of distilled water (W1). PLGA was dissolved in 2 mL of methylene chloride containing 1% (w/v) surfactant (O). The W1 and O phase were emulsified using homogenizer at 12,000 rpm for 10 min to form the primary W1/O emulsion. The prepared emulsion was mixed with the W2 phase containing 1% (w/v) PVA and 0.9% (w/v) NaCl and then, homogenized at 8,000 rpm for 10 min. After evaporation of the methylene chloride, the obtained solution was centrifugation at 8,000 rpm for 20 min to recover the prepared gemcitabine-loaded PLGA particles. The isolated gemcitabine-loaded PLGA particles were suspendedin a mannitol solution and lyophilized (Tables 1).
Characterization of the prepared gemcitabine-loaded particles
Drug loading and encapsulation efficiency
Lyophilized particles (10 mg) were dissolved with 5 mL dichloromethane to decompose PLGA layer. Then, dichloromethane was evaporated for overnight and 20 mL sodium phosphate buffer : methanol (90:10, v/v) was added. These were sonicated for 10 min and centrifugation at 3000 rpm for 10 min. The supernatant (1 mL) was filtered through a 0.2 µm polyvinylidene difluoride membrane and analyzed using HPLC (Agilent 1100 Series, Agilent Technologies Inc., USA) in which a Zorbax SB-C18 column (5 µm particle size, 4.6 × 250 mm) was used. Gradient elution of the analyses was performed using mono sodium phosphate buffer (A) and methanol (B). The initial condition of mobile phase was A-B (97:3, v/v) for 8 min, linearly changed to A-B (50:50, v/v) to 13 min. The total run time was 15 min. The column temperature was maintained at 35 °C and the flow rate was 1.2 mL/min. The UV wavelength of gemcitabine was measured at 275 nm. And the yield, drug loading and encapsulation efficiency were calculated as follows:
Surface morphology
The morphology and surface characteristics of gemcitabine-loaded PLGA particles were confirmed by scanning electron microscopy (SEM) (JEOL JSM7500 Field Emission Scanning Electron Microscope, Thermo, USA) at an acceleration voltage of 20 kV. The samples were mounted on a metal stubs using a double-sided adhesive tape yo which the samples were applied. The stubs were sputter-coated with gold particles in a sputter coater for 50 s.
Particle size, zeta-potential and interfacial tension
Mean particle size and zeta-potential were detected through electrophoretic light scattering (ELS-8000 Particle size analyzer, Otsuka Electronics, Japan). About 20 mg of lyophilized particles was dispersed in 4 mL of diluted water. Interfacial tension was measured using tensiometer (Sigma 702 Force tensiometer, KSV Instruments, Finland).
Determination of physicochemical properties of gemcitabine-loaded particles
The physicochemical properties of gemcitabine-loaded particles at the solid state were observed using Fourier transform infra-red (NICOLET 380 FT-IR, Thermo, USA). Gemcitabine-loaded particles (5 mg) were attached into ATR prism and scanned in the IR range from 400 to 4000 cm− 1. In order to assess the changes of solid state, differential scanning calorimetry (DSC; DSC 1, Mettler-Toledo Inc., USA) analysis was performed on gemcitabine-loaded particles. Samples accurately weighed about 2.5 mg were placed in an aluminum pan and analyzed by DSC. The DSC analyzes were performed in the range of 20 to 400 °C and back at a rate of 20℃/min. The crystalline changes were identified by X-ray powder diffraction (XRD; D/MAX-220 Ultima, Rigaku Inc., Japan). The data were collected in angular ranges between 5° and 70° with a step size of 0.01°. As well as, gemcitabine-loaded particles after storage at room temperature for 2 wk were analyzed by DSC and XRD.
In vitro release study
The release profile of gemcitabine from nanoparticles was assessed by the dialysis method. Briefly, a weighed amount of the gemcitabine-loaded particles (20 mg) were dispersed in 4 mL of phosphate-buffered saline (PBS) solution. This dispersion was placed inside a dialysis membrane (MWCO 10,000 Da) and dialyzed against 20 mL of PBS. At predetermined time intervals, 500 µL of solution was collected from the released media and replaced with fresh PBS. The collected samples were with a 0.2 µm filter and analyzed for drug content by HPLC.
Cytotoxicity assay
Caco-2 cells were grown in DMEM supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 units/mL penicillin and 100 µg/mL streptomycin in 5% CO2 atmosphere at 37 °C. Briefly, after incubation of cells with gemcitabine-loaded particles for 72 h, MTT (3-(4,5-dimethylthoazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide) was dispensed into each well and incubated for 2 h at 37 °C. The crystals of viable cells were solubilized in isopropanol. Absorbance was measured at 570 nm in a microplate reader (Sunrise, Tecan, Austria). Cell viability was expressed as a percentage of the absorbance versus absorbance measured for cells that were not exposed to any material.
Results and Discussion
Yield and drug loading
Gemcitabine is readily soluble in water and therefore difficult to encapsulate into particles in aqueous media. In order to increase encapsulation efficiency, gemcitabine needs to be paired with a counter ion. In this study, we conducted with chitosan have positive charge for ion pairing with gemcitabine which have negative charge (-30.00 ± 5.83 mV). Among ion paired complex, gemcitabine-chitosan complex showed the lowest zeta potential when the weight ratio of chitosan to gemcitabine was 0.2 (data not shown). Finally, this ion paired complex was used for gemcitabine-loaded particles. We proposed that the mixture of two different surfactants allows more efficient packing at the water–oil interface. Thus, we used Span 80, lecithin separately and mixture of Span 85 and Tween 80 as w/o surfactant. Double emulsions stabilized with Span 80, lecithin, or mixture of Span 85 and Tween 80 as surfactants were used for entrapping hydrophilic chitosan-gemcitabine complex (Table 2).
Surface morphology
Figure 1 shows SEM micrographs of gemcitabine-loaded particles prepared with different type of PLGA and surfactants. Gemcitabine-loaded particles were appeared a spherical and smooth morphology. The sorts of surfactants and the type of PLGA did not affect the morphology of gemcitabine-loaded particles.
Encapsulation efficiency, particle size, zeta-potential and interfacial tension
We used Span 80, lecithin or mixture of Span 85 and Tween 80 as surfactants to identify its effect on particle size and interfacial tension of gemcitabine-loaded particles. Figure 2 showed the encapsulation efficiency, particle size and interfacial tension of gemcitabine-loaded particles and founded that there was a close correlation between particle size and interfacial tension. The encapsulation efficiency of each formulation were 29.56 ~ 34.38% (Fig. 2(a)). When the surfactant for gemcitabine-loaded particles was used, the encapsulation efficiency was not significantly different among the formulations, suggesting that surfactant don’t have a significant role on the encapsulation efficiency. It is interesting that gemcitabine-loaded particles in this study showed relatively higher encapsulation efficiency when compared that gemcitabine exhibited extremely low encapsulation (< 2%), which may be attributed to the hydrophilic nature of gemcitabine (Jaidev et al. 2015). On the other hand, the particle size of gemcitabine-loaded particles was increased with the increase in interfacial tension. Surfactants are compounds that lower the interface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid. Therefore, formulation (F3 to F8) containing surfactants had lower particle size and interfacial tension than formulation (F1, F2) without surfactants.
Other studies have shown that particles below 10 µm can be delivered through a group of small intestinal endothelial cells called Peyer’s patches. (van der Lubben et al. 2001). The main mechanism for the absorption of particles in the gastrointestinal tract has been thought to be an endocytosis process by M cells in the Peyer’s patches (Jang et al. 2007). Thus, the ideal oral drug delivery vehicle should be small size enough to pass through the gastrointestinal barrier (M cells). The type of internal surfactants was reported to be main factor affecting particle size (Khoee and Yaghoobian 2009).
Most gemcitabine-loaded particles had negative charge, except particles prepared with mixture of Span 85 and Tween 80. And negative charge of F1-6 was attributable to the presence of polymeric carboxylic groups on the surface of PLGA particles. It appears that the surface charges of gemcitabine-loaded particles are not affected by using of surfactants, because the zeta-potential changes were negligible, which were − 5.62 to 1.46 mV.
Determination of physicochemical properties of gemcitabine-loaded particles
The FT-IR spectra of gemcitabine-loaded particles are shown in Fig. 3. The major peak of gemcitabine was around 1640 cm− 1 (amide). The major peak of gemcitabine was disappeared and the broad peak of gemcitabine at around 3200–3400 cm− 1 (OH stretching) exhibited to each samples in gemcitabine-loaded particles. It indicated that there exists a chemical interaction between gemcitabine and PLGA. It was reported that the presence of the drug in the PLGA matrix is confirmed by comparing the FTIR spectra of blank PLGA nanoparticles and the drug-loaded PLGA nanoparticles. Drug molecules generally tend to be located between the polymer chains of the nanoparticles forming the matrix system. They found that the hydrophilic drug was trapped between the polymer chains of nanoparticles (Jaidev et al. 2015).
The thermal curve of gemcitabine is typical of crystalline with endothermic peak at about 280.69 °C (Fig. 4). The gemcitabine-loaded particles did not exhibit a crystalline with endothermic peaks or broad and very weak endothermic peaks at differential temperature, suggesting that gemcitabine-loaded particles might be exist in the amorphous form. And, gemcitabine-loaded particles which kept at room temperature for 2 wk might be exist in the amorphous form. So, it was concluded that gemcitabine-loaded particles are stable.
DSC and XRD analysis results. (a), DSC thermograms of gemcitabine, gemcitabine loaded particle (F8), and gemcitabine loaded particle which kept at room temperature for 2 wk after preparation (F8′). The DSC runs were conducted from 150 to 350℃at a rate of 20℃/min. (b), XRD diagram of gemcitabine, gemcitabine loaded particle (F8) and gemcitabine loaded particle which kept at room temperature for 2 wk after preparation (F8′)
XRD pattern of gemcitabine-loaded particles did not show any characteristic peak, indicating that gemcitabine-loaded particles might be exist in the amorphous form (Fig. 4). This phenomenon was maintained during 2-wk storage at room temperature.
In vitro release study
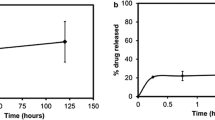
Figure 5 shows the release profile of gemcitabine from gemcitabine-loaded particles (F8) into PBS (pH 7.4). The drug release follows a two-phase kinetics. In the first phase, 20% of gemcitabine was rapidly released in 2 day. The second phase corresponds to a steady state with a constant and much slower release rate. The first phase can be interpreted as a feature of gemcitabine diffusion through the surface layer of the polymer particles. The second phase, which is a much slower stage of release, can be described as the slow release of gemcitabine through diffusion from the inner polymer matrix(Tewes et al. 2007). At pH7.4 medium, a two-phase drug release profile was observed and about 40% of gemcitabine in the PLGA nanoparticles showed a rapid initial release in the first week and an additional sustained release of 20% gemcitabine in the next 3 weeks. The specific release profile of gemcitabine from nanoparticles suggests that the loaded drug is localized in two regions within the polymer nanoparticles (Jaidev et al. 2015).
Cytotoxicity test
Cytotoxicity studies of gemcitabine and gemcitabine-loaded nanoparticles on Caco-2 Cells were performed to determine IC50 values before cell uptake studies of gemcitabine-loaded particles proceeded.
Figure 6 shows that IC50 of gemcitabine-loaded particle against Caco-2 cells was determined to be 86.04 mM. Interestingly, gemcitabine-loaded particles showed less cytotoxicity compared to gemcitabine solution only. Based on these results, uptake and in vivo test will be conducted.
Conclusion
The gemcitabine-loaded particles could be successfully prepared by double emulsification method using biodegradable polymer, PLGA. When the mixture of Span 85 and Tween80 for gemcitabine-loaded particles was used, the encapsulation efficiency of gemcitabine was 34%. Gemcitabine-loaded particles maintained the amorphous status for 2 weeks.
References
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, Plunkett W (1991) A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 9:491–498
Bala I, Hariharan S, Kumar MN (2004) PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst 21:387–422
Bouffard DY, Lalibert´e J, Momparler RL (1993) Kinetic studies on 2′,2′-difluorodeoxycytidine (gemcitabine) with purified human deoxycytidine kinase and cytidine deaminase. Biochem Pharmacol 45:1857–1861
Grunewald R, Kantarjian H, Du M, Faucher K, Tarassoff P, Plunkett W (1992) Gemcitabine in leukemia: a phase 1, clinical, plasma, and cellular pharmacology study. J Clin Oncol 10:406–413
Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, Plunkett W (1992) Cellular elimination of 2′,2′-difluorodeoxycytidine 5′-triphosphate: a mechanism of self-potentiation. Cancer Res 52:533–539
Hertel LW, Border GB, Kroin JS, Rinzel SM, Poore GA, Todd GC, Grindey GB (1990) Evaluation of the antitumor activity of gemcitabine(2′,2′-difluoro-2′-deoxycytidine). Cancer Res 50:4417–4422
Horton ND (2004) Toxicity of single-dose oral gemcitabine in mice. Cancer Res 4:27–31
Jaidev LR, Krishnan UM, Sethuraman S (2015) Gemcitabine loaded biodegradable PLGA nanospheres for in vitro pancreatic cancer therapy. Mater Sci Eng C 47:40–47
Jang JY, Kwon BS, Lee HE, Kim DH, Kang HK, Kang JS, Lee SK, Choi GJ (2007) Preparation of biodegradable PLGA nanospheres employing a fast solvent evaporation method. J Ind Eng Chem 13:1043–1046
Khoee S, Yaghoobian M (2009) An investigation into the role of surfactants in controlling particle size of polymeric nanocapsules containing penicillin-G in double emulsion. Eur J Med Chem 44:2392–2399
Matsuda A, Sasaki T (2004) Antitumor activity of sugar-modified cytosine nucleosides. Cancer Sci 95:105–111
Myhren F, Borretzen B, Dalen A, Sandvold ML (2002) Gemcitabine derivatives. US Patent US2,002,042,391
Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr CM (2007) Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine: nanotechnology biology medicine 3:173–183
Plunkett W, Huang P, Gandhi V (1995) Preclinical characteristics of gemcitabine. Anticancer drugs 6:7–13
Reddy LH, Couvreur P (2008) Novel approaches to deliver gemcitabine to cancers. Curr Pharm Design 14:1124–1137
Shipley LA, Brown TJ, Cornpropst JD, Hamiton M, Daniels WD, Culp HW (1992) Metabolism and disposition of gemcitabine, and oncolytic deoxycytidine analog, in mice, rats, and dogs. Drug Metab Dispos 20:849–855
Storniolo AM, Allerheiligen SRB, Pearce HL (1997) Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol 24:S7-2–S7-7
Tewes F, Munnier E, Antoon B, Ngaboni Okassa L, Cohen-Jonathan S, Marchais H, Douziech-Eyrolles L, Soucé M, Dubois P, Chourpa I (2007) Comparative study of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm 66:488–492
van der Lubben IM, Verhoef JC, van Aelst AC, Borchard G, Junginger HE (2001) Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials 22:687–694
Zhang X, Sun M, Zheng A, Cao D, Bi Y, Sun J (2012) Preparation and characterization of insulin-loaded bioadhesive PLGA nanoparticles for oral administration. Eur J Pharm Biopharm 45:632–638
Acknowledgements
This article does not contain any studies with human and animal subjects performed by any of the authors. All authors (J.H. Lim, Y.G. Na, H.K. Lee, K.H. Bang, S.J. Kim, H.J. Lee, M. Wang, Y.C. Pyo, H.W. Huh, C.W. Cho) declare that they have no conflict of interest. This work was supported by the research fund of Chungnam National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, JH., Na, YG., Lee, HK. et al. Effect of surfactant on the preparation and characterization of gemcitabine-loaded particles. J. Pharm. Investig. 49, 271–278 (2019). https://doi.org/10.1007/s40005-018-0402-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-018-0402-8