Abstract
Introduction
Staphylococcus aureus frequently causes infections in outpatient and hospital settings and can present as a highly variable entity. Typical manifestations are endocarditis, osteoarticular infections or infection of implanted prostheses, intravascular devices or foreign bodies. A thorough diagnostic evaluation with early focus identification is mandatory to improve patient outcome.
Case report
We report a case of a 68-year old patient with a history of double allogeneic stem cell transplant for acute myeloid leukemia who developed a S. aureus bacteremia with dissemination, severe sepsis and lethal outcome due to nasal handkerchief packing after nose bleeding.
Conclusion
A thorough medical examination with further diagnostic work-up is most important in S. aureus blood stream infection to identify and eradicate the portal(s) of entry, to rule out endocarditis, to search for spinal abscesses, osteomyelitis or spondylodiscitis. Adherence to management guides for clinicians must be of major importance to achieve optimal quality of clinical care, and thus improve patient outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is a human and animal commensal and frequently identified as pathogen causing infections [1, 2]. Staphylococcus aureus colonizes the skin, mucosae and typically the anterior nares of the nose [2]. Staphylococcus aureus nasal carriage varies between different ethnic groups with higher rates in Caucasians [3], men [4, 5], and shows different rates due to age (declining colonization in infants with increasing age) [6, 7]. Staphylococcus aureus carriage is co-determined by host characteristics (e.g. inherent resistance to colonization or occupied rhinal niche by other bacteria) [8] and hands are the major vector for the transmission of S. aureus to the nose [9].
Staphylococcus aureus bacteremia is one of the most frequent bacterial infection throughout the world and its clinical presentation can be highly variable. Typical manifestations are endocarditis, osteoarticular infections (spondylodiscitis, osteomyelitis, or joint infections), infection of implanted prostheses, intravascular devices/foreign bodies, septic thrombophlebitis, and or visceral abscesses [10].
For patients with S. aureus bacteremia mortality of 20–40% are reported in the literature [11, 12]. In patients, were the focus cannot be established, a substantially higher day 90-mortality is reported in comparison to patients with identified focus [13]. Staphylococcus aureus exotoxins, especially the toxic shock syndrome toxin-1 (TSST-1) lead to shock, erythroderma and multiorgan failure due to their ability of simultaneously activating a large number of T cells (superantigen) leading to massive cytokine release.
Here, we describe the case of a 68-year-old man who presented to our department with a livid discoloration of his nose, an increasing dyspnea and malaise due to a severe disseminated S. aureus bacteremia with consecutive sepsis triggered by nasal handkerchief packing with lethal outcome.
Case presentation
A 68-year old man presented to the emergency department because of increasing dyspnea and malaise, decreased vigilance and a livid discoloration of his nose with partly necrotizing tissue and swelling of his face, which had started the week before his presentation (Fig. 1). The patient reported a history of two allogeneic stem cell transplantations (aSCT) for acute myeloid leukemia (AML) (Cytogenetics: 46 XY, complex aberrant karyotype, TP53-deletion, NF1-deletion; molecular characterization: high WT1 expression, NPM1/MLL/FLT3/IDH1: negative) He had received the first aSCT at the age of 65 (MUD 10/10). He had undergone a second aSCT 1 year prior to admission (MUD 10/10) because of relapse of AML 30 months after the first aSCT. Prior to the second aSCT, an acute kidney disease was diagnosed due to compression of the ureters by granulocytic sarcomas (so called chloromas). The last change of double J stents was performed 7 days prior to admission to the clinic.
Ten days prior to his hospitalization the patient presented to the outpatient clinic of the bone marrow transplant unit due to increasing polyserositis and oral steroids with 100 mg daily were administered for 4 days. Polyserositis was leading symptom of his chronic graft-versus-host disease (GvHD) with recurring pleural effusion and ascites, but he was otherwise in good general condition and capable of all self-care with assistance of his wife. Due to recurrent thrombocytopenia after aSCT the patient received repetitive thrombocyte transfusions and his wife described long lasting nose bleeds in the last days prior to admission to the hospital.
On examination, the patient appeared in bad general condition. The temperature was 38.7 °C, the heartrate 120 beats per minute, the blood pressure 110/63 mmHg, the respiratory rate 43 breaths per minute, and the oxygen saturation 96% while breathing 4 L oxygen (85% on ambient air).
Examination of the legs showed bilateral edema of the lower extremities, ascites and bilateral pleural effusions. Examination of the skin revealed a livid discoloration of his nose, open-grained and in parts with necrotizing tissue (Fig. 1a).
Laboratory examination revealed high C-reactive protein levels of 308.7 mg/L (2940.06 nmol/L), procalcitonin levels of 5.1 µg/L and white blood cell count of 2.17 × 1E9/L (2170/µL). At presentation in the emergency room an empiric antibiotic therapy was initiated intravenously with ceftriaxone (2 g every day) and clarithromycin (500 mg, twice a day) after collection of blood cultures, which then was empirically switched to piperacillin/tazobactam (4,5 g, three times a day) at admission to the intensive care unit (ICU) after 1 day due to initially suspected community-acquired pneumonia in a highly immunosuppressed patient.
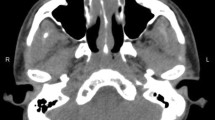
Reasons for admission to ICU were septic shock and decompensated heart and renal failure with edema, pleural effusion and ascites. Diuretics and vasopressor support were initiated and computed tomography of the cranium, chest and abdomen was performed. It showed destruction and swelling of the rhinal cartilages at the distal end and at the alae of the nose without signs of bone affection (Fig. 2). Furthermore, an increasing facial soft tissue swelling without signs of sinusitis was found. Due to the livid discoloration of the nose with necrotizing tissue, increasing dyspnea and septic shock we immediately performed a nasal endoscopy to take biopsies for microbiological and histopathological work-up. During endoscopy, small pieces of a handkerchief were extracted from the nasal cavity. Upon questioning, the patient`s wife confirmed that a handkerchief was placed by the patient about 10 days prior to admission for nasal packing to reduce the nose bleeding. Upon cessation of nose bleeding, the patient had forgotten the handkerchief, resulting in a foreign body in the nasal cavity.
Microbiological and histopathological diagnostics showed signs of deep tissue infiltration of bacteria with necrotizing tissue adjacent without signs of tumor or fungal hyphae (Figure S1). Herpes simplex infection was ruled out by negative PCRs from full blood and negative local swab from the necrotizing tissue at the nose. Culture of the tissue biopsy revealed methicillin-susceptible Staphylococcus aureus (MSSA). Cultured urine, blood and ascites grew methicillin-susceptible S. aureus repeatedly.
With the result of MSSA growth in culture flucloxacillin (2 g, three times a day, dose adjusted because of renal failure) was added on day 2 and indwelling intravascular catheters and ureteral double J stents were changed. A transesophageal echocardiogram did not show any sign of endocarditis. With progressive hypotension despite fluid therapy and encephalopathy, with disorientation, confusion, and reduced vigilance a lumbar puncture was performed to rule out MSSA meningitis. During the antibiotic therapy with flucloxacillin blood cultures did not reveal growth of S. aureus again, however, vancomycin-resistant Enterococcus faecium on day 5 (VRE), linezolid was added and indwelling catheters were changed again. Due to suspected calcineurin inihibtor toxicity indicated by an increase of schistocytes to 10‰ tacrolimus was stopped and Prednisolone (2 mg/kg body weight) was administered as bridge immunosuppression therapy. At day 12 tracheal intubation was needed due to further respiratory deterioration. Due to sustaining anuria a slow low-efficient daily dialysis (SLEDD) was started. At day 17 blast cells were detected in ascites and peripheral blood with increasing expression of the Wilms tumor 1 gene (WT1). Chimerism analysis showed complete engraftment of the bone marrow transplant consistent with extramedullary relapse of AML. Due to hematemesis and melena an upper endoscopy showed a necrotizing esophagitis with gastral ulcers and a diffuse oozing hemorrhage. By means of proton pump inhibitors and platelet transfusions the hemorrhage remained stable. Full blood testing showed a reactivation of Cytomegalovirus (CMV), so that a therapy with ganciclovir was initiated. With recovery of the neurological state the patient was extubated on day 21. 5 days later the general state of health deteriorated rapidly. The patient remained febrile, inflammation parameters increased without relapse of S. aureus bacteremia. The patient developed respiratory insufficiency, hypotension and reduced vigilance. In light of the long medical history with progressive leukemia, we discussed the situation in-depth with the patient`s family and agreed to change the main goal of treatment to best supportive care. The patient died of progressive leukemia after severe disseminated S. aureus bacteremia on day 27.
Discussion
We report an unusual case of a patient with severe disseminated S. aureus bacteremia associated with nasal handkerchief packing. Our patient developed septic shock with fever, rash, hypotension, multisystem involvement combined with disseminated cultural evidence of S. aureus in blood cultures, ascites, urine, and in the nasal biopsy.
Staphylococcus aureus bacteremia must always be considered clinically relevant, carrying increasing risk of dissemination with metastatic seeding and complications [14]. Once diagnosis of S. aureus bacteremia is established, repeated blood cultures for the documentation of clearance should be drawn, as length of bacteremia and fever is critical for further diagnostic work-up, for the duration of antibiotic therapy and prognosis. Key predictors for complicated S. aureus bloodstream infection are community acquired infection, skin examination findings suggesting acute systemic infection (petechiae, vasculitis, infarcts, echymoses or pustules), persistent fever at 72 h, and positive follow-up blood culture results at 48–96 h [15].
In our patient, we initially established a sufficient antibiotic therapy empirically with piperacillin/tazobactam due to the suspicion of community acquired pneumoniae in a immunosuppressed patient and added flucloxacillin with the cultural proof of S. aureus bacteremia. Following blood cultures after the extraction of the handkerchief, changing of indwelling catheters and double J stents during antibiotic therapy remained negative for S. aureus, after a 27 day course of therapy. We did not add clindamycin (only in vitro data for TSST-1 synthesis suppression), intravenous immune globulin (IVIG) or corticosteroids initially due to limited clinical data. An acceptable and well-tolerated alternative for treatment of methicillin-susceptible S. aureus is cefazolin (2 g iv every 8 h) [16,17,18].
Asking for and understanding the patient’s medical history and a thorough medical examination with further diagnostic work-up is most important to identify and eradicate the portal(s) of entry, to rule out endocarditis, to search for spinal abscesses, osteomyelitis or spondylodiscitis. Repetitive bedside examinations can reveal the development of complications that may evolve in the course of infection (e.g. metastatic seeding). Infectious disease consulting at the bedside is a major component of management of patients with S. aureus sepsis and should be asked for whenever feasible [19].
Due to rising age of population, increasing case severity, and growing number of patients with immunosuppression S. aureus associated skin and soft tissue infections with consecutive blood stream infection put patients at a high risk and adherence to management guides for clinicians must be of major importance to achieve optimal quality of clinical care, and thus improve patient outcome [10, 20].
References
Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–32.
Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71.
Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, et al. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol. 2001;8:1064–9.
Eriksen NH, Espersen F, Rosdahl VT, Jensen K. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol Infect. 1995;115:51–60.
Herwaldt LA, Cullen JJ, French P, Hu J, Pfaller MA, Wenzel RP, et al. Preoperative risk factors for nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 2004;25:481–4.
Armstrong-Esther CA. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann Hum Biol. 1976;3:221–7.
Peacock SJ, Justice A, Griffiths D, de Silva GD, Kantzanou MN, Crook D, et al. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol. 2003;41:5718–25.
Nouwen J, Boelens H, van Belkum A, Verbrugh H. Human factor in Staphylococcus aureus nasal carriage. Infect Immun. 2004;72(11):6685–8.
Wertheim HF, van Kleef M, Vos MC, Ott A, Verbrugh HA, Fokkens W. Nose picking and nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 2006;27:863–7.
Jung N, Rieg S. Essentials in the management of S. aureus bloodstream infection. Infection. 2018;46(4):441–2. https://doi.org/10.1007/s15010-018-1130-8.
Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis. 1987;9:891–907.
Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:273–9.
Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG Jr, Hellmich M, Hopkins S, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect. 2014;68:242–51.
Lehmann C, Berner R, Bogner JR, Cornely OA, de With K, Herold S, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45:263–8.
Fowler VG Jr, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066–72.
Bai AD, Showler A, Burry L, Steinberg M, Ricciuto DR, Fernandes T, et al. Comparative effectiveness of cefazolin versus cloxacillin as definitive antibiotic therapy for MSSA bacteraemia: results from a large multicentre cohort study. J Antimicrob Chemother. 2015;70:1539–46.
Lee S, Choe PG, Song KH, Park SW, Kim HB, Kim NJ, et al. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother. 2011;55:5122–6.
Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS. 2nd. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58:5117–24.
Forsblom E, Ruotsalainen E, Ollgren J, Jarvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56:527–35.
Yahav D, Shaked H, Goldberg E, Yassin S, Eliakim-Raz N, Paul M, et al. Time trends in Staphylococcus aureus bacteremia, 1988–2010, in a tertiary center with high methicillin resistance rates. Infection. 2017;45:51–7.
Roberts EA, Troiano C, Spiegel JH. Standardization of guidelines for patient photograph deidentification. Ann Plast Surg. 2016;76:611–4.
Acknowledgements
For this publication written approval was obtained from the patient’s wife and attorney regarding his case report to appear in publications in any media worldwide. We thank the patient and his family for providing private photographs during the development of the disease at the outpatient setting and for allowing us to publish the case for educational purposes. Facial photo deidentification was performed by the treating physician according to Roberts’ and colleagues’ Standardization of Guidelines for Patient Photograph Deidentification to ensure the patient’s anonymity [21]. Laboratory results in full text were converted by use of the SI Conversion Calculator by the American Medical Association http://www.amamanualofstyle.com/page/si-conversion-calculator. Accessed April 5th, 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PK reports non-financial support from Merck/MSD, non-financial support from MedImmune, and lecture honoraria from Astellas outside the submitted work. NJ received lecture fees from Labor Stein, Novartis, Gilead, MSD, Infectopharm and travel grants from Novartis, Pfizer, Astellas, Basilea, Gilead. MK declares no potential conflict of interest in regard to this work. PL declares no potential conflict of interest in regard to this work. ASV declares no potential conflict of interest in regard to this work. BB declares no potential conflict of interest in regard to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koehler, P., Jung, N., Kochanek, M. et al. ‘Lost in Nasal Space’: Staphylococcus aureus sepsis associated with Nasal Handkerchief Packing. Infection 47, 307–311 (2019). https://doi.org/10.1007/s15010-018-1221-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-018-1221-6