Abstract
Background
Meningitis occurs in 0.8–1.5% of patients undergoing neurosurgery. The aim of the study was to evaluate the characteristics of meningitis after endoscopic endonasal transsphenoidal surgery (EETS) comparing the findings retrieved to those highlighted by literature search.
Materials and methods
Patients treated by EETS during an 18-year period in the Department of Neurosurgery of ‘Federico II’ University of Naples were evaluated and included in the study if they fulfilled criteria for meningitis. Epidemiological, demographic, laboratory, and microbiological findings were evaluated. A literature research according to PRISMA methodology completed the study.
Results
EETS was performed on 1450 patients, 8 of them (0.6%) had meningitis [median age 46 years (range 33–73)]. Endoscopic surgery was performed 1–15 days (median 4 days) before diagnosis. Meningeal signs were always present. CSF examination revealed elevated cells [median 501 cells/μL (range 30–5728)], high protein [median 445 mg/dL (range 230–1210)], and low glucose [median 10 mg/dL (range 1–39)]. CSF culture revealed Gram-negative bacteria in four cases (Klebsiella pneumoniae, Escherichia coli, Alcaligenes spp., and Haemophilus influenzae), Streptococcus pneumoniae in two cases, Aspergillus fumigatus in one case. An abscess occupying the surgical site was observed in two cases. Six cases reported a favorable outcome; two died. Incidence of meningitis approached to 2%, as assessed by the literature search.
Conclusions
Incidence of meningitis after EETS is low despite endoscope goes through non-sterile structures; microorganisms retrieved are those present within sinus microenvironment. Meningitis must be suspected in patients with persistent fever and impaired conscience status after EETS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiology of meningitis is changing due to the introduction of conjugate vaccines against Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis and the increasing number of patients undergoing invasive neurosurgical procedures [1,2,3]. Indeed, these factors determined a decrease of community-acquired cases and, on the other hand, a proportional increase of nosocomial meningitis [4, 5].
Many pathogens, such as Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and staphylococci, reside within the respiratory tract, forming a complex ecosystem where equilibrium is crucial to maintain physiological environment and mucosal integrity. When bacterial overgrowth occurs due to viral infection or other factors including trassphenoidal surgery, bacteria can spread from sinus mucosa to adjacent structures, eventually reaching the meninges and growing within the CSF, where immune response is virtually absent [6,7,8].
In the recent years, neurosurgical techniques have been developed in the attempt of reducing invasiveness, morbidity and mortality, above all the transsphenoidal approach that perfectly fits this conceptual way of thinking [9, 10]. Lesions of the suprasellar region are currently treated also via the endoscopic endonasal approach; this so-called minimally invasive technique has been introduced over the last twenty years to reach initially the sellar area and more recently the supraretrosellar and parasellar areas avoiding morbidity related to brain retraction and manipulation of neurovascular structures [11]. Two main variations of the transsphenoidal approach have been defined: the standard and the extended. Sellar lesions not breaching the diaphragma sellae can be managed and removed through a standard approach, whereas several cases presenting some features such as supradiaphragmatic extension or fibrous or rubbery consistency, cavernous sinus and/or retrosellar invasion require an extended one [12].
On the basis of current literature data, cumulative complication rate is similar in those undergoing endoscopic procedure or in those treated by the microscopic approach, but incidence of meningitis could be higher after an endoscopic approach [13,14,15].
In this retrospective study, we reported the incidence of meningitis in a large cohort of patients, who underwent an endonasal endoscopic procedure (either standard or extended) and detailed clinical presentation, microbiologic and radiologic findings, and the impact of meningitis on the surgical outcome. Additionally, literature review is presented.
Materials and methods
We reviewed the charts of all cases undergoing endoscopic endonasal transsphenoidal surgery (EETS) over a 10-year period and included those reporting meningitis during the post-operative follow-up. The research was conducted in accordance with the Declaration of Helsinki and national and institutional standards and patients gave an informed consent prior to be included in this study.
Surgery
Patients were treated by an endoscopic endonasal approach whose surgical procedure was extensively reviewed elsewhere [16].
Meningitis
On suspicion of meningitis, after a trained infectious diseases specialist evaluation, patients were admitted to the department of Infectious Diseases for a complete clinical and laboratory evaluation and for all therapeutic procedures. Meningitis had to be suspected on the characteristic clinical signs and symptoms (i.e., fever, neck stiffness, and worsened consciousness) and had to be confirmed by an abnormal cerebro-spinal fluid (CSF) examination. The inclusion criteria were: (1) meningitis diagnosis as above established; (2) history of transsphenoidal surgery during the previous 6 months performed at our department of Neurosurgery; (3) post-therapy follow-up of at least 24 months for surviving cases.
Baseline examinations
At admission to the department of infectious diseases, we recorded the demographic data, any previous or underlying disease (focusing the attention on the underlying disease treated with transsphenoidal surgery), presenting signs and symptoms, the results of a complete clinical evaluation, and laboratory tests such as full blood count, erythrocyte sedimentation rate, C-reactive protein, liver enzymes, blood urea and creatinine. In each case, we collected data about pre-operative antibiotic prophylaxis and history of CSF leakage. Results of the microbiological studies (blood or CSF cultures, CSF or urinary antigens) were recorded for all cases.
Microbiological studies
Blood and CSF samples were obtained for the microbiological studies at admission in the department of Infectious Diseases. Blood and CSF samples were inoculated in a BACTEC Ped Plus Aerobic/F medium (Becton–Dickinson) and were incubated in a BACTEC 9240 device (Becton–Dickinson). Positive cultures were inoculated in sheep blood agar and chocolate agar and incubated in 5% CO2. Susceptibility assays were performed using an E-test with the Clinical Laboratory Standard Institute interpretative criteria for minimum inhibitory concentration (MIC) until 2010 when the EUCAST criteria were applied.
Imaging studies
A computed tomographic (CT) scan of the brain was scheduled at admission. A Magnetic Resonance Imaging (MRI) study of the brain was always scheduled immediately before discharge for all cases, regardless of the CT findings at admission.
Treatment
According to our internal protocol for cases with suspected nosocomial meningitis a 20-day course of therapy was scheduled. Patients received drugs active against multi-drug resistant (MDR) bacteria as empiric therapy, before the culture and susceptibility tests were available. After the microbiological results were available, antibiotic treatment was continued on the basis of susceptibility test.
Literature review
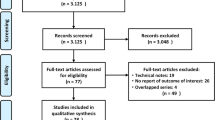
To better evaluate the impact of meningitis on transsphenoidal surgery, we carried out a systematic search of the English language literature using the MEDLINE database with the search string ‘meningitis and endoscopic transsphenoidal surgery’, ‘meningitis and endoscopic transsphenoidal surgery’, ‘meningitis and endoscopic sinus surgery’ for reports published from January 1968 to February 2017 and by checking of the Cochrane Library and manual research of the references from available review reports about meningitis and neurosurgery. This systematic review adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
Two authors independently reviewed the initially selected articles and data were extracted if deemed relevant. Only the studies reporting cases undergoing endoscopic transsphenoidal surgery were considered in the literature analysis. For each case series, we recorded, when available, the cases treated by endoscopic transsphenoidal surgery and those diagnosed with meningitis, the number of cases experiencing post-operative CSF leak, and the relative incidence of meningitis in respect to post-operative CSF leak occurrence. Findings of the clinical presentation and those deriving by blood and CSF examination and by microbiologic investigations completed the report form. The retrieved cases were pooled together with our own cases and the impact of meningitis on transsphenoidal surgery outcome was evaluated.
Statistical analysis
Fisher’s exact test and Chi-squared test were used to compare qualitative variables. p values below 0.05 were considered significant.
Results
EETS was performed to 1450 patients during the study period, 1246 underwent endoscopic transsphenoidal surgery and 204 extended endoscopic endonasal transtuberculum surgery. Eight (0.6%) patients [median age 46 years (range 33–75), 3 males], all presenting intraoperative CSF leakage, were diagnosed as having meningitis after EETS. In these patients, primary surgery was performed because of pituitary macroadenoma or dural fistula, in two cases, and tuberculum sellae meningioma, suprasellar oncocytoma, Rathke’s cleft cyst and intra- and suprasellar craniopharyngioma, respectively, in 1 case. The overall rate of postoperative CSF leakage was found 8.3% (17/204) after extended endoscopic endonasal surgery and 1% (12/1246) after standard endoscopic approaches (17/204 vs 12/1246, p = 0.0001). Meningitis rate was 1.5% in the cases undergoing extended endoscopic endonasal transtuberculum surgery (3/204) and 0.4% (5/1246) in the patients undergoing endoscopic transsphenoidal surgery (3/204 vs 5/1246; p = 0.09).
Antibiotic prophylaxis was administered during the 24 h preceding surgery; it was based on third generation cephalosporin in two cases, clarithromycin in three cases and vancomycin in three cases. Surgical procedure was uneventful until meningitis diagnosis in all cases, except for the occurrence of post-operative CSF leakage in seven of these cases.
The onset of general clinical symptoms varied between 1 and 15 days (median 4 days) before diagnosis of meningitis. Presenting symptoms and laboratory findings are reported in Table 1. Aspecific symptoms, such as new onset or worsening fever and headache, and a worsening conscience status were reported in all but one case presenting with multiple abscesses occupying the surgical site. Meningeal signs (nuchal rigidity, Kerning’s and Brudzinski sign) were reported in seven cases at the time of meningitis diagnosis. Two cases reported left VI cranial nerve palsy. One case reported visual disturbance.
Cerebrospinal fluid (CSF) examination revealed elevated CSF cells [median 501 cells/μL (range 30–5728)], high CSF protein [(median 445 mg/dl (range 230–1210)], and low glucose [median 10 mg/dl (range 1–39)].
Culture attempted from CSF revealed Gram-negative bacteria in four cases (Klebsiella pneumonia, Escherichia coli Alcaligenes spp., and Haemophilus influenzae), Streptococcus pneumoniae in two cases and Aspergillus fumigatus in one case, and no microbiologic evidence in one case. No multi-drug resistant microorganism was cultured. Blood cultures were negative in all cases.
Empiric therapy was based on protocols containing meropenem in all cases, vancomycin was administered in four cases and amikacin in 1 case. After microbiologic evidence was obtained, therapy was modified on the basis of susceptibility test. Liposomal amphotericin B was administered to the case with meningitis sustained by Aspergillus.
An intracranial abscess occupying the surgical site was diagnosed in two cases; in one case it was associated to VI cranial nerve palsy and visual disturbance. Both cases had undergone extended transsphenoidal endoscopic surgery and were cured after a prolonged course of antibiotic therapy without further surgery.
Outcome was favorable in six cases. One patient died because had meningitis-related complication and one patient died because of pneumonia 120 days after surgery.
Literature review
A total of 234 studies were extracted by the Literature search, 167 were excluded from the analysis. Main reasons for the exclusion were: non-English language [31], meta-analysis or review on the argument [17], endoscopic treatment for sinus diseases [14], non-endoscopic neurosurgery [9], endoscopic non-transsphenoidal surgery [9], description of surgical technics [7]. Finally, 67 studies were included in the literature analysis, all were retrospective analysis.
Overall, 7027 cases undergoing EETS (508 extended approach, 5249 standard approach and 1270 mixed population undergoing standard or extended surgery) were considered in the studies selected (see supplemental material). Only 17 studies reported more than 100 cases, and pituitary adenoma was the main reason for the EETS. Cumulative number of the cases was 137 (6 were case report). Cumulative incidence of meningitis approached to 2%. Meningitis after an extended approach was more frequent than after a standard approach (37/508 vs 73/5249, p = 0.0001).
Two studies investigating a total of 611 cases undergoing endoscopic surgery compared the incidence of meningitis after endoscopic or microscopic surgery. Meningitis was reported in 9/283 cases undergoing endoscopic surgery and in 2/317 cases undergoing microscopic surgery (p = 0.03) Thirty-three studies investigated the link between post-operative leak and meningitis. Meningitis was reported in 49/213 cases with post-operative leak and in 15/3638 without clinical evidence of post-operative leak (p < 0.0001).
Microbiologic investigations were reported in 28 cases, being bacteria colonizing upper respiratory tract those more frequently cultured. Three cases deriving by the same study were sustained by polymicrobial flora. Bacteria identified were: Streptococcus pneumoniae (six cases), Staphylococcus epidermidis (three cases), Haemophilus influenzae (two cases), Corynebacterium spp. (two cases), and Streptococcus viridans, Serratia spp., Enterococcus spp., Klebsiella pneumoniae, Stenotrophomonas maltophilia, Acinetobacter baumannii, Candida albicans (one case).
Very little information was available on the clinical findings of meningitis after EETS, and no study compared the outcome of those with or without post-operative meningitis. Time lasting from endoscopic surgery to meningitis evidence ranged between 2 days and 12 months (median 14 days), as assessed in 15 patients for whom this information was available. Clinical evidence of meningeal irritation was reported in 9 of 11 cases and alteration of conscience status was reported in 4 of 10 cases. Meningitis-related death was reported in 3 (6%) of 50 cases whose outcome was clearly stated. Table 2 summarizes the findings of the cases retrieved by literature search and of those reported in our case series.
Discussion
Incidence rates of meningitis occurring after neurosurgical procedures are estimated to range between 0.8 and 1.5% with the highest risk reported after the open procedures and in the cases reporting CSF leakage during the surgery [5, 18]. In this study, we demonstrated that meningitis is an important complication of EETS, above all in the extended approach for the management of suprasellar supradiaphragmatic lesions. On the basis of the available data, the risk of meningitis after microscopic is lower than after endoscopic transsphenoidal neurosurgery, but the low number of the cases reported in the studies analyzed does not permit to draw definitive conclusions on the argument [13, 14]. Moreover, the risk of developing postsurgical meningitis after endonasal procedure is comparable to the risk of patients undergoing other neurosurgical procedures, although the surgical route is burdened by a higher risk of infection as related to the nasal corridor, where physiological bacterial flora of the respiratory tract is present [19, 20].
The majority of patients presented with nuchal rigidity and fever, while a compromised conscience status was reported in 11/18 cases, considering both the cases observed in our institution and those retrieved by Literature analysis. Even though the clinical findings suggest meningitis, a correct diagnosis could be difficult to establish early in the course of meningitis occurring after transsphenoidal surgery. This is because fever and headache, the main prodromal symptoms of meningitis, are frequently reported in patients undergoing neurosurgery because of the bleeding and the release of pyrogenic substance due to tissue violation. Furthermore, in these patients, nuchal rigidity and an impaired conscience status, the main signs reported in patients with meningitis, have low accuracy because these could be related to the surgical procedure itself that involving dura and to the CSF contamination by blood during the surgical procedure. Moreover, an impaired conscience status can be observed after surgery due to the sedation induced by the anesthesiologic treatment and procedure complications [21,22,23,24,25,26,27].
In the present series, we reported eight cases complaining of postsurgical meningitis, four of them occurring within post-operative day 4. Data from the literature highlight an onset ranging between 2 days and 12 months (median 14 days) after surgery. Incidence of meningitis after EETS peaks within 2–3 weeks after surgery and is higher in those reporting post-operative leakage, as assessed by our case series and literature analysis. These observations have an important impact because they suggest, on clinical grounds, that CSF leakage should increase the suspect of meningitis, also in those presenting with aspecific symptoms and warrant, on surgical grounds, that leakage repair should be performed at an early stage to reduce the risk of meningitis [28, 29].
It should be said that neurosurgical procedures carry the risk of infections sustained by MDR bacteria and staphylococci [5, 30]. MDR hospital-acquired bacteria were not cultured among the cases diagnosed in our institution, where agents constitutively present in the sinus micro-environment were found to be the causative agents cultured in the majority of cases. Microbiological data derived from literature analysis only in part confirmed our observation, demonstrating that hospital-acquired bacteria could be reported and that bacteria colonizing the upper respiratory tract were frequently involved [5]. Of note, polymicrobial infection and a high number of MDR bacteria were reported in a single study, but both were not demonstrated to be so frequent on the basis of the observations deriving from our case series and remaining literature analysis (see supplemental material).
The information about microbiological characteristics of meningitis following EETS have a practical impact on the choices in term of pre-operative prophylaxis that should be tailored on the microorganisms colonizing the upper respiratory tract, including Gram-negative bacteria constitutively present within the sinus micro-environment. As cumulative data demonstrated a low incidence of fungal infections, we believe that prophylaxis against fungi eventually colonizing the upper respiratory tract such as Aspergillus should be considered only in the cases reporting specific risk (i.e., cases with asthma allergic treated with inhaled steroids or severely immunocompromised patients). Moreover, no association between the findings of inner and outer nose microenvironment was reported by literature analysis, suggesting that a preliminary microbiologic investigation of nasal microenvironment could be ineffective in suggesting prophylaxis protocol [31, 32].
An unfavorable outcome related to meningitis was reported in one patient dying because of meningitis complications and septic shock 8 weeks after the endoscopic procedure. One patient died because of pneumonia 16 weeks after the surgical procedure, when he was in a rehabilitative center. Cumulative mortality of meningitis after EETS reported by literature analysis was 6%, lower than reported in the adult population of patients with community-acquired bacterial meningitis. This was probably due to the low prevalence of S. pneumoniae that is associated with the highest meningitis-related mortality [5, 33,34,35,36].
Conclusions
Our study demonstrated that the incidence of meningitis after EETS surgery is relatively low and comparable to that reported after other neurosurgical procedures. Meningitis must be suspected in the cases with persistent fever and impaired conscience status after surgery, when a careful evaluation of the signs of meningeal irritation may favor an early diagnosis. Prophylaxis has to consider broad-spectrum antibiotics covering all the microorganisms colonizing the sinus including Gram-negative bacteria, considering specific anti-fungal agents only if there is a specific risk of Aspergillus colonization.
References
McIntyre PB, O’Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012;380:1703–11. doi:10.1016/S0140-6736(12)61187-8.
Castelblanco RL, Lee M, Hasbun R. Epidemiology of bacterial meningitis in the USA from 1997 to 2010: a population-based observational study. Lancet Infect Dis. 2014;14:813–9. doi:10.1016/S1473-3099(14)70805-9.
McClelland S 3rd, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55–9. doi:10.1086/518580.
Pellegrino P, Carnovale C, Perrone V, Salvati D, Gentile M, Brusadelli T, et al. Epidemiological analysis on two decades of hospitalisations for meningitis in the United States. Eur J Clin Microbiol Infect Dis. 2014;33:1519–24. doi:10.1007/s10096-014-2102-2.
van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146–54. doi:10.1056/NEJMra0804573.
Chochua S, D’Acremont V, Hanke C, Alfa D, Shak J, Kilowoko M, et al. Increased nasopharyngeal density and concurrent carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are associated with pneumonia in febrile children. PLoS One. 2016;11:e0167725. doi:10.1371/journal.pone.0167725.
Revai K, Mamidi D, Chonmaitree T. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis. 2008;46:e34–7. doi:10.1086/525856.
Wolter N, Tempia S, Cohen C, Madhi SA, Venter M, Moyes J, et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis. 2014;210:1649–57. doi:10.1093/infdis/jiu326.
Cappabianca P, Cavallo LM, de Divitiis E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery. 2004;55:933–40.
de Divitiis E, Cappabianca P, Cavallo LM. Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery. 2002;51:699–705.
Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A, de Divitiis E. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. In: Pickard JD, Akalan N, Di Rocco C, Dolenc VV, Lobo Antunes J, Mooij JJA, Schramm J, Sindou M, editors. Advances and technical standards in neurosurgery. Wien New York: Springer; 2008. p. 152–99.
Catapano G, de Notaris M, Di Maria D, Fernandez LA, Di Nuzzo G, Seneca V, et al. The use of a three-dimensional endoscope for different skull base tumors: results of a preliminary extended endonasal surgical series. Acta Neurochir. 2016;158:1605–16. doi:10.1007/s00701-016-2847-8.
Halvorsen H, Ramm-Pettersen J, Josefsen R, Rønning P, Reinlie S, Meling T, et al. Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: a consecutive series of 506 procedures. Acta Neurochir. 2014;156:441–9. doi:10.1007/s00701-013-1959-7.
Guvenc G, Kizmazoglu C, Pinar E, Abdülkadir I, Kaya I, Bezircioglu H, et al. Outcomes and complications of endoscopic versus microscopic transsphenoidal surgery in pituitary adenoma. J Craniofac Surg. 2016;27:1015–20. doi:10.1097/SCS.0000000000002684.
Somma T, Maraolo AE, Esposito F, Cavallo LM, Tosone G, Orlando R, et al. Efficacy of ultra-short single agent regimen antibiotic chemo-prophylaxis in reducing the risk of meningitis in patients undergoing endoscopic endonasal transsphenoidal surgery. Clin Neurol Neurosurg. 2015;139:206–9. doi:10.1016/j.clineuro.2015.10.007.
Cavallo LM, Messina A, Cappabianca P, Esposito F, de Divitiis E, Gardner P, Tschabitscher M. Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations. Neurosurg Focus. 2005;19:E2.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi:10.1136/bmj.i4086.
Tian R, Hao S, Hou Z, Gao Z, Liu B. The characteristics of post-neurosurgical bacterial meningitis in elective neurosurgery in 2012: a single institute study. Clin Neurol Neurosurg. 2015;139:41–5. doi:10.1016/j.clineuro.2015.09.002.
Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2006;59:126–33. doi:10.1227/01.neu.0000243291.61566.21.
Korinek AM, Golmard JL, Elcheick A, Bismuth R, van Effenterre R, Coriat P, Puybasset L. Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4578 patients. Br J Neurosurg. 2005;19:155–62. doi:10.1080/02688690500145639.
Wang KW, Chang WN, Huang CR, Tsai NW, Tsui HW, Wang HC, Su TM, Rau CS, Cheng BC, Chang CS, Chuang YC, Liliang PC, Tsai YD, Lu CH. Post-neurosurgical nosocomial bacterial meningitis in adults: microbiology, clinical features, and outcomes. J Clin Neurosci. 2005;12:647–50. doi:10.1016/j.jocn.2004.09.017.
van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, ESCMID, et al. Study Group for Infections of the Brain (ESGIB). ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22:S37–62. doi:10.1016/j.cmi.2016.01.007.
Zarrouk V, Vassor I, Bert F, Bouccara D, Kalamarides M, Bendersky N, et al. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007;44:1555–9.
Somma T, Solari D, Beer-Furlan A, Guida L, Otto B, Prevedello D, et al. Endoscopic endonasal management of rare sellar lesions: clinical and surgical experience on 78 cases and review of the literature. World Neurosurg. 2017;100:369–80. doi:10.1016/j.wneu.2016.11.057.
Chong MY, Quak SM, Chong CT. Cerebral ischaemia in pituitary disorders—more common than previously thought: two case reports and literature review. Pituitary. 2014;17:171–9. doi:10.1007/s11102-013-0485-1.
Sylvester PT, Moran CJ, Derdeyn CP, Cross DT, Dacey RG, Zipfel GJ, et al. Endovascular management of internal carotid artery injuries secondary to endonasal surgery: case series and review of the literature. J Neurosurg. 2016;125:1256–76. doi:10.3171/2015.6.JNS142483.
Takeuchi K, Watanabe T, Nagatani T, Nagata Y, Chu J, Wakabayashi T. Incidence and risk factors of subdural hematoma after intraoperative cerebrospinal fluid leakage during the transsphenoidal approach. Pituitary. 2016;19:565–72. doi:10.1007/s11102-016-0746-x.
Amano K, Hori T, Kawamata T, Okada Y. Repair and prevention of cerebrospinal fluid leakage in transsphenoidal surgery: a sphenoid sinus mucosa technique. Neurosurg Rev. 2016;39:123–31. doi:10.1007/s10143-015-0667-6.
Bhatki AM, Pant H, Snyderman CH, Carrau RL, Kassam AB, Prevedello DM, et al. Reconstruction of the cranial base after endonasal skull base surgery: local tissue flaps. Oper Techn Otolaryngol. 2010;21:74–82. doi:10.1016/j.otot.2009.10.003.
Beer R, Pfausler B, Schmutzhard E. Infectious intracranial complications in the neuro-ICU patient population. Curr Opin Crit Care. 2010;16:117–22. doi:10.1097/MCC.0b013e328338cb5f.
Ivan ME, Iorgulescu JB, El-Sayed I, McDervott MW, Parsa AT, Pletcher SD, et al. Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci. 2015;22:48–54. doi:10.1016/j.jocn.2014.08.009.
Schenck LP, Surette MG, Bowdish DME. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590:3705–20. doi:10.1002/1873-3468.12455.
Vickery TW, Ramakrishnan VR. Bacterial Pathogens and the Microbiome. Otolaryngol Clin North Am. 2017;50:29–47. doi:10.1016/j.otc.2016.08.004.
Pagliano P, Ascione T, Boccia G, De Caro F, Esposito S. Listeria monocytogenes meningitis in the elderly: epidemiological, clinical and therapeutic findings. Infez Med. 2016;24:105–11 PMID: 27367319.
Pagliano P, Attanasio V, Rossi M, Ascione T, Fraganza F, Di Sarno R, et al. Pneumococcal meningitis in cirrhotics: distinctive findings of presentation and outcome. J Infect. 2012;65:577–9. doi:10.1016/j.jinf.2012.08.019.
Pagliano P, Attanasio V, Rossi M, Carleo MA, Carannante N, Ascione T, et al. Listeria monocytogenes meningitis in the elderly: distinctive characteristics of the clinical and laboratory presentation. J Infect. 2015;71:134–6. doi:10.1016/j.jinf.2015.02.003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Internal review board judged the project of the study and the final title of the paper compliant with ethical standards.
Informed consent
Each patient signed an informed consent before surgical procedure and when he was admitted to the Unit of Infectious Diseases.
Funding
No funding was received for this study.
Transparency declarations
None to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pagliano, P., Caggiano, C., Ascione, T. et al. Characteristics of meningitis following transsphenoidal endoscopic surgery: a case series and a systematic literature review. Infection 45, 841–848 (2017). https://doi.org/10.1007/s15010-017-1056-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-017-1056-6