Abstract
Cerebrospinal fluid (CSF) leakage is a common but sometimes serious complication after transsphenoidal surgery (TSS). To avoid this postsurgical complication, we usually repair the CSF leaking area using an autologous material, such as fat, fascia, or muscle graft and sometimes nasonasal septal flap. In this report, we propose a technique using a novel autologous material, sphenoid sinus mucosa (SSM), to repair intraoperative CSF leakage or prevent it postoperatively. On 26 February 2007, we introduced the technique of using SSM to repair or prevent CSF leakage in TSS. Until 30th of June 2014, we performed 500 TSSs for patients with pituitary or parasellar lesions. They were 195 men and 305 women with a mean age of 48.5 years (range, 5–85 years). We used SSM for patching or suturing the arachnoid laceration or dural defect, in lieu of fat or fascia harvested from abdomen or thigh, or made pedicle flap of SSM instead of nasonasal septal flap to cover the sellar floor. Comparing the previous 539 cases not using these techniques before 26 February 2007, intraoperative CSF leakage increased from 49 to 69.4 % (p < 0.0001) due to more aggressive surgical technique, mainly related to more extensive approaches and lesion removals, but the rate of using fat was reduced significantly from 35.5 to 19.4 % (p = 0.00021) in small or moderate CSF leaks during TSS without increasing the reoperation rate for postoperative CSF leaks (1.86 vs 1.2 %, p = 0.45). The technique of using SSM to repair intraoperative CSF leaks or prevent them postoperatively in TSS was considered useful, effective, less invasive, easier for graft harvesting (same surgical field), and providing natural anatomical reconstruction, without potential donor site morbidity. We can recommend it as a standard method for CSF leaks repair and prevention in TSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most common complications after transsphenoidal surgery (TSS) is a postoperative cerebrospinal fluid (CSF) leakage resulting often from inadequate repair of an intraoperative CSF leak [1, 3, 4, 7, 8, 20, 21, 27, 28]. Though most of pituitary and parasellar lesions for which TSS is applied are benign tumors, the patient with postsurgical CSF leakage suffers significant disability. CSF leak prevention remains a cardinal issue in TSS. To avoid this complication, we usually use fat, fascia, or muscle graft, and sometimes a nasonasal septal flap as an autologous material for repair in the sella, sphenoidal sinus, or both. In general, these techniques showed good efficacy in postsurgical CSF leakage prevention [14, 32]. However, harvesting those tissue grafts adds time, expense, potential complications, and discomfort to the patient [10, 24]. An alternative procedure for such repair can be the use of sphenoid sinus mucosa (SSM). We considered that SSM could be a good autologous material for repair and used SSM as a free flap or a vascular pedicle flap. This method appeared less invasive and easier to prepare than harvesting fat, fascia, or muscle from the abdomen or the thigh. Yoon et al. previously reported in 2008 that the use of a sphenoid mucosal flap in transsphenoidal surgery decreased the incidence of postoperative cerebrospinal fluid (CSF) leaks and promoted wound healing of the sphenoid sinus.
In this report, we describe the technique of using a free flap of sphenoid sinus mucosa or a vascular pedicle sphenoid mucosal flap to repair intraoperative CSF leakage or prevent postoperative CSF leakage and analyze the outcome of its application in large number of cases.
Methods
On 26 February 2007, we introduced the technique of using sphenoid sinus mucosa (SSM) to repair intraoperative CSF leakage or to prevent postoperative CSF leakage. Until the end of June 2014, we have performed 500 transsphenoidal surgeries at Tokyo Women’s Medical University Hospital for patients with pituitary or parasellar lesions. They were 195 men and 305 women with a mean age of 48.5 years (range, 5–85 years) (Table 1). This is a single surgeon series (Amano K), and closure using this SSM technique in TSS was performed by one surgeon.
We used SSM for patching or suturing lacerations of arachnoid or dural defects as reinforcement, instead of fat or fascia harvested from abdomen or thigh, or made pedicle flap of SSM in lieu of nasonasal septal flap to cover the sellar floor. No patient had external lumbar drainage placement unless postoperative CSF leakage developed.
Sphenoid sinus mucosa patching to the laceration
Harvested mucosa was immersed in saline with antibiotics such as gentamicin before using it for repairing CSF leaks. The laceration or hole of the arachnoid was well defined in size and location under endoscopic view, and then the harvested mucosa was cut to a size adequate to cover the arachnoid laceration. The mucosa dipped with fibrin glue component A (fibrinogen solution) was placed as a patch on the laceration and, immediately after gelatin sponge with fibrin glue B component (thrombin solution), was placed over the first one, compressed by cottonoid pledget using the suction tip (Fig. 1).
Sphenoid sinus mucosa suturing to the laceration
In cases of relatively large arachnoid lacerations or space between dura and remaining normal pituitary gland, SSM was sutured to the laceration edges by 6-0 nylon (Fig. 2).
Intraoperative photographs of sphenoid sinus mucosa suturing to the laceration. a Arrow indicating CSF leakage from a laceration between the arachnoid and normal gland. b Mucosa (asterisk) was patched and sutured using 6-0 nylon. c Arrow pointing at the laceration of arachnoid under endoscopic view. d A mucosa patch (asterisk) was sutured to the laceration using 6-0 nylon
Sphenoid sinus mucosa suturing to the dural defect
In TSS, we routinely sutured the dura using 6-0 nylon, pulling it toward the center of the defect, except in cases when it was invaded by the tumor or coagulated for stopping venous bleeding from the inter-cavernous sinus. In some cases, the dura was sutured to SSM to cover the relatively large dural defect. We used for suturing a needle-holder particularly designed for TSS and a small needle (5 mm in diameter, 1/2 Circle) with 6-0 monofilament nylon.
Though it could have been ideal that SSM edge was sutured to the dura in a watertight fashion, if there was even one loose suture, postoperative CSF leak occurred [38]. For those reasons, SSM was placed under the dural edge, expecting when CSF pressure would increase later, align tighter SSM to the dura, and close more reliably the fistula [30] (Fig. 3).
However, it is not easy to suture SSM in this deep and narrow area especially to the arachnoid which is usually at the deeper side of tumor bed, and we passed the stitch in two separate steps: through mucosa in advance at outside of nasal cavity and then the deeper edge.
Sphenoid sinus mucosal flap
Prior to opening the sellar floor, its mucosa was stripped off as a flap and reflected to one side, above, or below, covered with oxidized absorbable cellulose (Surgicel®) for hemostasis and kept moist with a wet cotton pledget. After the CSF fistula repair and sellar floor reconstruction, the SSM flap was repositioned on the surface of the sellar floor (Fig. 4a–h). When a larger flap was needed to cover the opening area, additional SSM was taken from the superior, anterior, or lateral walls of sphenoid sinus and trans-positioned (Fig. 4i–l). After patching the SSM flap, gelatin sponge was overlaid and fibrin glue was sprayed for its fixation. The blood “oozing” from bone was stopped using Surgicel® and fibrin glue.
Illustrative images obtained in a 35-year-old man with a nonfunctioning pituitary adenoma. a Preoperative sagittal magnetic resonance image (MRI), T1-weighed image with gadolinium enhancement (T1WIGd). b Endoscopic view of the sellar floor. Black triangle indicating the opened window of the sellar floor. A mucosal flap (asterisk) was reflected to the sellar floor inferiorly. c The sphenoid sinus mucosal flap (asterisk) is covering the sellar floor. d Postoperative MRI, sagittal T1WIGd. Illustrative images obtained in a 51-year-old man with a GH-producing pituitary adenoma. e Preoperative sagittal MRI, T1WIGd. f Endoscopic view of the sellar floor. After a T-shaped incision, mucosa was peeled off and put aside as mucosal flap (asterisk). Black triangle points at the opened window of the sellar floor. g Three sphenoid sinus mucosal flaps (asterisk) covering the sellar floor. h Postoperative sagittal MRI, T1WIGd. Illustrative images obtained in a 41-year-old woman with a nonfunctioning pituitary adenoma. i Preoperative sagittal MRI, T1WIGd. j Endoscopic view of the opened window on the sellar floor (white triangle). k Endoscopic view of a trans-positioned sphenoid sinus mucosal flap (asterisk) covering the sellar floor. l Postoperative sagittal MRI, T1WIGd
After introducing these techniques, we compared the rates of postoperative CSF leakage occurrence and the fat usage between the groups before and after the introduction of the technique and applied statistical analyses to estimate significance level using the Pearson’s chi-square test. The p values less than 0.05 were considered statistically significant, and all of the analyses were conducted by using the JMP® 9 (SAS Institute Inc., Cary, NC, USA).
Results
Of the total of 500 TSSs between 26th of February 2007 and 30th of June 2014, SSM techniques were used in 295 operations. SSM patching was applied in 82 cases of which 38 were with suturing, SSM flap in 239, and a combination of them in 24. The patients characteristics before (group A, n = 539) and after (group B, n = 500) using SSM methods are shown in Table 1.
In cases where mucosa was very thin, invaded by the tumor, or became infected, this method was difficult to use. Sixty-seven out of 500 patients had previous surgery, and these cases had difficult to harvest or preserve mucosa in general. SSM was found fragile, and as a result, only 18/67 (26.9 %) cases were repaired with SSM.
The incidence of CSF leakage and the rate of fat packing are shown in Table 2. Reoperation for postsurgical CSF leakage was performed in 16 cases, and there were other 17 cases where a CSF leak was suspected. In 9 of the 16 cases were placed lumbar drainage before reoperation. After introducing the SSM technique, we experienced six reoperations in intraoperatively confirmed and three in suspected postsurgical CSF leakage cases. Five out of these six cases underwent lumbar drainage before reoperation, four had past history of previous surgery or radiotherapy, and in two was used SSM technique. At the revision surgery, packing with fat was used in all cases without a nasoseptal flap. The three suspected for CSF leakage cases remained unconfirmed, as CSF leaks and symptoms disappeared after lumbar drainage in one case and lumbar puncture in two cases.
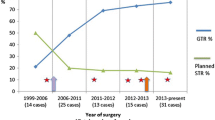
Comparing both groups for the rate of intraoperative CSF leakage, after adopting the intraoperative CSF leaks grading system of Esposito [15], it showed an increase from 49 to 69.4 % (p < 0.0001). However, the usage rate of fat or fascia during TSS showed reduction from 50 to 43 % (p = 0.141) for group B. Especially in cases of minor or moderate CSF leaks during TSS (grades 1 and 2), the fat usage rate dropped from 35.5 to 19.9 % (p = 0.00021). The rate of reoperation for confirmed postsurgical CSF leaks did not increase for group B (1.86 vs 1.2 %, p = 0.45) (Table 2). After the surgery, none of these patients experienced a mucocele formation, meningitis, or other complications related to the use of these methods.
Discussion
Postoperative CSF leaks are the most common complication after TSS [1, 3, 4, 7, 8, 20, 21, 27, 28]. For its prevention, many kinds of grafts and devices have been tried. Different types of autologous materials have been used for reconstruction of the sellar floor [14, 32]. Though fat and fascia from the abdomen or thigh are the most popular autologous graft, harvesting them adds to the surgical trauma and invasiveness, resulting in additional scarring with sometimes dimple or keloid formation or postoperative hematoma [10, 24]. Five patients in our series developed such subcutaneous abdominal hematomas after harvesting fat and fascial graft due to inadequate hemostasis and suture. All these patients required surgical reexploration, and two of them needed blood transfusion. The patients, in whom fascia was obtained from the thigh, frequently complained of unpleasant sensation or pain. On the other hand, harvesting mucosa from the sphenoid sinus did not have any of these inconveniences.
There was no postoperative meningitis after the introduction of SSM methods. We immersed the harvested mucosa in saline with antibiotics (such as gentamicin) before repairing the CSF leaks and irrigated copiously the sphenoid sinus before opening dura, using pressure-controlled dual irrigation-suction system with saline containing antibiotics (200–300 cc) [40]. These procedures might have reduced the rate of postsurgical meningitis.
Another technical variation of the SSM, the pedicle sphenoid sinus mucosal flap, had some advantages as a material for successful reconstruction of the sellar floor. It is easy to obtain, as it is in the same surgical field, avoiding side effects or complications. Additionally, it is soft and elastic but sufficiently firm and with easily adjustable size. The SSM flap is tailored before stripping from the sellar floor, then reflected away from the area of intended sellar opening, kept moist for the time of intrasellar work, and finally positioned over the sellar opening at the end of intrasellar work. Another effect of the SSM flap application is support for repair of the sphenoid sinus. Within the concept of this method is included the idea of sphenoid sinus restoration to its previous original condition. As Fig. 5 shows, the SSM flap was successful in grafting the defect, and the sellar floor was completely covered with vascularized normal mucosa 3 months after the first TSS. If the mucosa on the sellar floor was coagulated or removed at the initial approach, regeneration would have taken longer time, usually more than 1 month. We think partial cover of sellar floor is also useful for promoting the regeneration of mucosa. It has been reported that patients who have undergone prior surgery or radiation therapy are at greater risk for postoperative CSF rhinorrhea [37]. Ogawa et al. [39] also mentioned that postoperative irradiation disrupts the degenerated mucosa of the sphenoid sinus and it can be a possible cause of CSF leakage. We also used artificial materials, such as fibrin glue, oxidized cellulose, and gelatin foam to repair the CSF fistulas [5, 16, 29, 35, 36, 43]. They were effective only temporarily until biological membrane formation was complete in the healing stage. From practical point of view, fibrin glue was almost absorbed within 1 or 2 weeks, oxidized absorbable cellulose (Surgicel®) in about 2 weeks, and gelatin foam (Gelfoam®) within a month. In order to create the best conditions for self-regeneration and repair process before these materials are absorbed, the sphenoid sinus and sellar floor should be reconstructed and repaired to their natural anatomical condition. We experienced a case presenting with delayed postoperative CSF leakage 31 days after TSS. In this case, a very small defect of sphenoid mucosa was found at reoperation for CSF leak, and we supposed that insufficient mucosa regeneration could have been the cause of such a delayed CSF leak. Initially, we used to coagulate or remove the SSM while preparing to open the sellar floor, but have not done that since we started to repair the sellar floor and the sphenoid sinus as close as to their preexisting anatomical structure.
Illustrative images obtained in a 44-year-old woman with a Rathke’s cleft cyst. a Preoperative sagittal MRI, T1WI. b Endoscopic view of the sellar floor. A mucosal flap (asterisk) was reflected. Black triangle indicating the opened window on the sellar floor. c A sphenoid sinus mucosal flap (asterisk) covering the sellar floor. d MRI T1-weighed image, 3 months after the first operation demonstrated recurrence of the cyst. e The sellar floor was completely covered with mucosa 3 months after the first operation (observation at a reoperation). f Endoscopic close-up view of the sellar floor demonstrated vascularized mucosa
The SSM flap with pedicular shape has better vascularity than a free mucosal graft. Yoon et al. reported that to avoid potential wound complications with TSS, the sphenoid sinus should be covered with vascularized pedicle mucosa as much as possible [46]. When we attempted to cover the sellar floor completely, the SSM flap was obtained from the lateral, superior, and/or inferior walls of the sphenoid sinus, which were able to provide sufficient length and width for such total covering without tension. In case there was a septum in the sphenoid sinus, the mucosal surface area of the sphenoid sinus became larger, allowing an SSM flap of greater surface. Though it is ideal that the SSM flap covers the sellar floor completely, as one of the purposes of the SSM flap is to support faster reproliferation of mucosa and prevent delayed CSF leaks, partial covering of sellar floor can be also justified and appropriate to contribute the regeneration of the mucosa, and we performed that in cases of no or a little intraoperative CSF leakage. If we could make SSM flap, it had better to be used in all cases except for the case whose SSM was too thin or fragile, because SSM flap does not increase invasiveness, provides natural anatomical reconstruction, and promotes the regeneration of mucosa. With routine use, we could make SSM flap in 157/195 cases (80.1 %), and the breakdown of reasons why it was difficult or impossible to harvest adequate SSM was the following: reoperation 18 (with radiation 9), very thin mucosa 10, tumor invasion 9 (adenoma 5, chordoma 3, metastatic tumor 1), and infection 2 (sinusitis 1, fungal granuloma 1).
The use or not of fat in grades 1 and 2 intraoperatively is a difficult decision. The reason of fat packing in those 45 cases was either objective or surgeon’s subjective conclusion. Eleven cases had poor SSM (including 8 reoperation cases), 10 cases had very large dural defect due to tumor invasion, 8 cases had the arachnoid or normal gland unable to descend and had large tumor bed, and other 16 cases needed additional measures to prevent postsurgical CSF leak (11 cases were far distant resident patients; 5 cases included pediatric and elder patients).
There are reports on the effectiveness of a nasonasal septal mucosal flap, applied to some extent with the same indications [2, 11, 18, 22, 23, 25, 26, 31, 34, 42, 45]. It is the ultimate method to repair or prevent CSF leakage and enable the extended TSS for the anterior skull base [6, 9, 12, 13, 17, 19]. In general, nasoseptal flap was tougher, thicker, and available to cover larger area than SSM flap. However, the most important difference between nasospetal and SSM flaps is the affection or not of normal anatomical structure. This technique is relatively more complicated and alters the natural anatomical condition of the nasal passages and the sphenoid sinus [7, 18, 33, 41, 44]. It can bring synechiae, septal perforations, crusting, and epistaxis [26]. As a result, nasal discomfort, hyposmia, or anosmia may occur to some patients. Therefore, considering invasiveness, time and effort, and anatomical change of nasal airways by the nasoseptal flap, this method should not be applied indiscriminately to all the patients with intraoperative CSF leakage, but restricted clearly indicated cases when the specific conditions exist. While this method is definitely effective and gives a durable solution for preventing CSF leakage, we applied it exclusively in the cases of extended TSS, recurrent surgery, or after radiotherapy. In contrast to nasoseptal flap, advantages of SSM flap are its noninvasiveness, easy preparation, and reconstruction of original nasal cavity, so we can use this technique without any hesitation and consider it the preferable one.
Esposito et al. [15] reported an intraoperative CSF leaks grading system as follows: grade 0, no leak observed; grade 1, small leak without obvious diaphragmatic defect; grade 2, moderate leak; or grade 3, larger diaphragmatic/dural defect. Under this classification, Table 2 demonstrates the incidence of CSF leakage and the rate of surgeries with fat packing use in both of our groups. Since we adopted this SSM technique, the rate of fat packing was reduced from 50 to 43.8 %, but there was no significant statistical difference between the groups (p = 0.141). However, in cases of small and moderate CSF leaks (grades 1 and 2), the rate of fat packing significantly declined from 35.5 to 19.4 % (p = 0.00021) without increasing the rate of reoperation for postoperative CSF leaks (1.86 vs 1.2 %, p = 0.45). On the other hand, comparing with the previous period before February 2007, our procedure of tumor removal in TSS has recently become more aggressive. The surgeon’ s learning curve, development of surgical devices, evolution of CSF repair techniques, and the introduction of extended TSS enabled more aggressive dissection of tumors. Correspondingly, the incidence of intraoperative CSF leakage increased from 49 to 69.4 % (p < 0.0001) with similar increase of large diaphragmatic/dural defects, grade 3, from 11.3 to 23 % (p < 0.0001).
When the arachnoid was widely opened or gapped widely after tumor removal, in grade 3, we routinely repaired the CSF leakage with fat packing. Even if there was no CSF leakage observed during surgery, grade 0, we started recently to apply an SSM flap too. We also applied the technique for those few patients, who demonstrated pneumocephalus on the postoperative CT and in whom an intraoperative CSF leak was not detected. Based on the above-mentioned advantages of this method and no disadvantages, together with providing natural anatomical reconstruction, we currently perform the SSM flap technique routinely to promote the regeneration of mucosa and prevent postoperative CSF leak even without detection of intraoperative CSF leak. We currently believe that if the SSM flap is available, it is better to be used in all cases except in those with too thin or fragile SSM.
The use of SSM can be an alternative procedure for repairing and preventing CSF leaks in TSS. The rationale for attempting the repair with SSM is fivefold. First, its harvesting is less invasive than harvesting fat, fascia, or muscle from the abdomen or the thigh. Second, it avoids an esthetic damage or nasal complications. Third, it is easy to prepare and does not take long time because of operating in the same surgical field. Fourth, mucosa does not induce a foreign body reaction or a chronic inflammatory response. Fifth, SSM flap provides natural anatomical reconstruction and promotes the regeneration of mucosa.
Conclusions
The technique of using SSM to repair intraoperative CSF leaks or prevent postoperative CSF leakage in TSS was considered useful, effective, less invasive, easier for graft harvesting (in the same field of surgery), and providing natural anatomical reconstruction, without potential donor site morbidity. Though the SSM technique is not the only available method and a preferable choice of repairing intraoperative CSF leaks, the surgeons should consider it among the other available for repairing CSF leakage. Our results encourage its further application and evaluation of indications and long-term effect.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- SSM:
-
Sphenoid sinus mucosa
- TSS:
-
Transsphenoidal surgery
- MRI:
-
Magnetic resonance image
- T1WIGd:
-
T1-weighed image with gadolinium enhancement
- CT:
-
Computed tomography
References
Berker M, Hazer DB, Yücel T, Gürlek A, Cila A, Aldur M, Onerci M (2012) Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 15(3):288–300
Bhatki AM, Pant H, Snyderman CH, Carrau RL, Kassam AB, Prevedello D, Gardner P (2010) Reconstruction of the cranial base after endonasal skull base surgery: local tissue flaps. Oper Techn Otolaryngol 21:74–82
Cappabianca P, Cavallo LM, Colao A, de Divitiis E (2002) Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 97:293–298
Cappabianca P, Cavallo LM, Esposito F, Valente V, De Divitiis E (2002) Sellar repair in endoscopic endonasal transsphenoidal surgery: results of 170 cases. Neurosurgery 51:1365–1372
Cappabianca P, Cavallo LM, Valente V, Romano I, D’Enza AI, Esposito F, de Divitiis E (2004) Sellar repair with fibrin sealant and collagen fleece after endoscopic endonasal transsphenoidal surgery. Surg Neurol 62:227–233
Cavallo LM, Messina A, Esposito F, Divitiis E, Fabbro MD, Cappabianca P (2007) Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg 107:713–720
Charalampaki P, Ayyad A, Kockro RA, Perneczky A (2009) Surgical complications after endoscopic transsphenoidal pituitary surgery. J Clin Neuroscience 16(6):786–9
Ciric I, Ragin A, Baumgartner C, Pierce D (1997) Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery 40:225–237
Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T (2004) Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches:Surgical experience in 105 cases. Neurosurgery 55:539–550
Couldwell WT, Kan P, Weiss MH (2006) Simple closure following transsphenoidal surgery. Technical note. Neurosurg Focus 20:E11
Dehdashti AR, Ganna A, Witterick I, Gentili F (2009) Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery 64(4):677–879
Divitiis E, Cavallo LM, Esposito F, Stella L, Messina A (2007) Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neurosurgery 61(5 Suppl 2):229–38
Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, Martin NA (2005) The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg 102:832–841
El-Banhawy OA, Halaka AN, Altuwaijri MA, Ayad H, El-Sharnoby MM (2008) Long-term outcome of endonasal endoscopic skull base reconstruction with nasal turbinate graft. Skull base 18(5):297–308
Esposito F, Dusick JR, Fatemi N, Kelly DF (2007) Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery 60(4 Suppl 2):295–304
Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF (2008) The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery 63(4 Suppl 2):244–56
Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciarretta V, Farneti G, Calbucci F (2006) The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery 59(1 Suppl 1):ONS75–83
Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, Stefko S (2008) Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg 109(1):6–16
Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM (2008) Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery 63(1):36–54
Gardner P, Zanation A, Duz B, Stefko ST, Byers K, Horowitz MB (2011) Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg 114(6):1544–68
Gondim JA, Almeida JP, Albuquerque LA, Schops M, Gomes E, Ferraz T, Sobreira W, Kretzmann MT (2011) Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary 14(2):174–83
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A (2011) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116:1882–6
Horiguchi K, Murai H, Hasegawa Y, Hanazawa T, Yamakami I, Saeki N (2010) Endoscopic endonasal skull base reconstruction using a nasal septal flap: surgical results and comparison with previous reconstructions. Neurosurg Rev 33(2):235–41
Kaptain GJ, Kanter AS, Hamilton DK, Laws ER (2011) Management and implications of intraoperative cerebrospinal fluid leak in transnasoseptal transsphenoidal microsurgery. Neurosurgery 68(1 Suppl Operative):144–51
Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A (2005) Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 19(1):E8
Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, Mintz A, Gardner P (2008) Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 63(1 Suppl 1):ONS44–53
Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, Zanation A, Duz B, Stefko ST, Byers K, Horowitz MB (2011) Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg 114(6):1544–68
Kawamata T, Iseki H, Ishizaki R, Hori T (2002) Minimally invasive endoscope-assisted endonasal trans-sphenoidal microsurgery for pituitary tumors: experience with 215 cases comparing with sublabial trans-sphenoidal approach. Neurol Res 24(3):259–65
Kelly DF, Oskouian RJ, Fineman I (2001) Collagen sponge repair of small cerebrospinal fluid leaks obviates tissue grafts and cerebrospinal fluid diversion after pituitary surgery. Neurosurgery 49:885–890
Kitano M, Taneda M (2004) Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery 54:653–661
Liu JK, Schmidt RF, Choudhry OJ, Shukla PA, Eloy JA (2012) Surgical nuances for nasoseptal flap reconstruction of cranial base defects with high-flow cerebrospinal fluid leaks after endoscopic skull base surgery. Neurosurg Focus 32(6):E7
Locatelli D, Vitali M, Custodi VM, Scagnelli P, Castelnuovo P, Canevari FR (2009) Endonasal approaches to the sellar and parasellar regions: closure techniques using biomaterials. Acta Neurochir (Wien) 151(11):1431–7
McCoul ED, Anand VK, Schwartz TH (2012) Improvements in site-specific quality of life 6 months after endoscopic anterior skull base surgery: a prospective study Clinical article. J Neurosurg 117(3):498–506
Nakagawa T, Asada M, Takashima T, Tomiyama K (2001) Sellar reconstruction after endoscopic transnasal hypophysectomy. Laryngoscope 111(11Pt 1):2077–81
Narotam PK, Van Dellen JR, Bhoola KD, Raidoo D (1993) Experimental evaluation of collagen sponge as a dural graft. Br J Neurosurg 7:635–641
Narotam PK, Van Dellen JR, Bhoola KD (1995) A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurg 82:406–412
Nishioka H, Haraoka J, Ikeda Y (2005) Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery. Acta Neurochir (Wien) 147(11):1163–6
Nishioka H, Izawa H, Ikeda Y, Namatame H, Fukami S, Haraoka J (2009) Dural suturing for repair of cerebrospinal fluid leak in transnasal transsphenoidal surgery. Acta Neurochir (Wien) 151(11):1427–30
Ogawa Y, Tominaga T (2007) Delayed cerebrospinal fluid leakage 10 years after transsphenoidal surgery and gamma knife surgery - case report -. Neurol Med Chir (Tokyo) 47(10):483–5
Okada Y, Kawamata T, Kawashima A, Yamaguchi K, Hori T (2009) Pressure-controlled dual irrigation-suction system for microneurosurgery: technical note. Neurosurgery 65(3):E625
Pant H, Bhatki AM, Snyderman CH, Vescan AD, Carrau RL, Gardner P, Prevedello D, Kassam AB (2010) Quality of Life Following Endonasal Skull Base Surgery. Skull Base 20(1):035–040
Saeki N, Horiguchi K, Murai H, Hasegawa Y, Hanazawa T, Okamoto Y (2010) Endoscopic endonasal pituitary and skull base surgery. Neurol Med Chir (Tokyo) 50(9):756–64
Seiler RW, Mariani L (2000) Sellar reconstruction with resorbable vicryl patches, gelatin foam, and fibrin glue in transsphenoidal surgery: a 10-year experience with 376 patients. J Neurosurg 93(5):762–5
Vaezeafshar R, Hwang PH, Harsh G, Turner JH (2012) Mucocele formation under pedicled nasoseptal flap. Am J Otolaryngol 33(5):634–6
Yano S, Kawano T, Kudo M, Makino K, Nakamura H, Kai Y, Morioka M, Kuratsu J (2009) Endoscopic endonasal transsphenoidal approach through the bilateral nostrils for pituitary adenomas. Neurol Med Chir (Tokyo) 49(1):1–7
Yoon TM, Lim SC, Jung S (2008) Utility of sphenoid mucosal flaps in transnasal transsphenoidal surgery. Acta Otolaryngol 128(7):785–9
Acknowledgments
We would like to thank Dr. Kostadin Karagiozov for his review of this manuscript and Dr. Atsushi Watanabe for providing statistical analyses.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods in this study or the findings specified in this paper.
Consent
Written informed consent was obtained from the patient for the publication of this report and any accompanying images.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Dorian Chauvet, Paris, France
I congratulate the authors for this well-illustrated article, describing their experience of sphenoid sinus mucosa (SSM) graft, in order to repair CSF leaks. The statistics do not seem dramatic, but the concept is quite innovative and totally included in a mini invasive perspective. Two main techniques are nicely described: free flap SSM (patching or suturing) and pedicle flap SSM patching, which appears particularly relevant to me. Indeed, the SMM should be more often considered for sellar reconstruction and/or dural repair, as it is easily harvested in the same surgical field, without any clinical consequence for the patient (on the contrary of nasoseptal flap). However, one must notice that this study is a single surgeon work that mucosa cannot be always used because of its fragility and that wide laceration cannot be strongly repaired by SSM. Moreover, suturing techniques in this deep-seated area, with a very thin flap, can present many difficulties. To conclude with a touch of provocation, SSM techniques presented by Amano et al. are very encouraging, just because it would be a pity not to use this autologous material.
Juan Antonio Ponce-Gómez and Luis Alberto Ortega-Porcayo, Mexico City, Mexico
This is an interesting paper, for which the authors presented their single center experience using either a free flap of sphenoid sinus mucosa or a vascular pedicle sphenoid mucosal flap to prevent CSF leakage after transsphenoidal surgery.
During the last years, multiple reconstruction techniques with autologous and synthetic materials using vascularized or free flaps have been used with promising results. Even though the results are getting better, most of these techniques added an extra morbidity obtaining the fat and fascia and postoperative nasal complications. This well-described technique is a promising option for sellar floor reconstruction. They showed an impressive CSF leak rate of 1.2 % (6/500 cases), decreasing grafts from the abdomen or thigh, nasoseptal flap dissection, and avoiding prophylactic lumbar drain postoperatively. Reproducibility of the same technique in different centers around the world with the same results will give this technique the proper place in neurosurgery.
Rights and permissions
About this article
Cite this article
Amano, K., Hori, T., Kawamata, T. et al. Repair and prevention of cerebrospinal fluid leakage in transsphenoidal surgery: a sphenoid sinus mucosa technique. Neurosurg Rev 39, 123–131 (2016). https://doi.org/10.1007/s10143-015-0667-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-015-0667-6