Abstract
Purpose
Hepatitis E virus (HEV) is mainly transmitted through contaminated water supplies which make the virus endemic in developing countries including countries of the Middle East and North Africa (MENA) region. Recent reports suggest potential risk of HEV transmission via blood transfusion.
Methods
Related articles on HEV were collected by searching through the 25 countries of the MENA region using Pubmed and Medline within the past 14 years: January 2000–August 2014.
Results
One hundred articles were extracted, of which 25 were not eligible. The articles discussed the seroprevalence of HEV and HEV markers in 12 countries. Eight articles provided data on HEV in blood donors. The seroprevalence of HEV in the general MENA population ranged from 2.0 to 37.5 % and was higher in males than in females. Prevalence increased with age, but exposure seems to be in early life.
Conclusions
In the MENA region, the role of HEV as an infectious threat to blood safety is under-investigated. More data are needed to quantify the risk of transmission and to assess clinical outcomes. This requires, at least, surveillance screening of donors and recipients for HEV markers using sensitive and specific serological tests. At the present time, serious consideration should be given to selective screening for certain groups of patients (e.g., immunocompromised, pregnant women and others) who commonly require blood transfusion and are at high risk of hepatic failure or chronicity from HEV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis E Virus (HEV) is a small (27–34 nm), non-enveloped, icosahedral, single-stranded RNA virus of approximately 7.2 kb in length. Analysis of its RNA helicase and RNA-dependent RNA polymerase region shows that the virus forms a phylogenetically distinct group that was recently placed into a separate genus, Hepevirus in the Hepeviridae family [1, 2]. The family contains mammalian HEV and a more distant avian HEV [3]. Phylogenetic analysis of the mammalian isolates showed that there are at least seven genotypes and four major human genotypes (genotypes 1–4) [4, 5]. The human genotypes can be subdivided in 24 subtypes [6]. Each HEV genotype appears to have a specific geographic distribution: Genotypes HEV-1 and HEV-2 are restricted to humans and are mainly responsible for large water-borne epidemics in Asia and Africa [6], while genotypes HEV-3 and HEV-4 are found in human and animal reservoirs (swine, wild boar, deer, mongooses) and are mainly responsible for sporadic cases of HEV in developed countries [7]. HEV-1, HEV-2 and HEV-4 have a higher pathogenicity and virulence than HEV-3, hence the clinical burden of HEV-3 cannot be compared with that of the other HEV genotypes [8]. The incidental discovery of a novel swine virus closely related to human HEV in 1997 [9, 10] was extremely important for studying the epidemiology of HEV. In recent years, autochthonous (locally acquired) zoonotic hepatitis E has been found in many developed countries in Europe, New Zealand, North America (all HEV-3) [11–13] and Japan (HEV-3 and HEV-4) [14]. It is now believed that HEV in developed countries is zoonotic and food borne, mainly associated with eating uncooked or undercooked meat or viscera of deer, boars and pigs or by exposure to infected animals [15, 16]. This route of transmission in the MENA is unlikely since pig farming is prohibited in the Islamic culture, as is the case in most countries of the region. Furthermore, boars and deer hunting is not commonly practiced in MENA. HEV is currently the focus of greater attention worldwide and the number of HEV-related articles has more than doubled in the last 10 years.
Seroprevalence of HEV
Rates of IgG positivity in a certain area are believed to be a reflection of the frequency of HEV infection in that area. However, estimating the burden of HEV infection in a population is not an easy task. The true figure in many developing countries could be much higher than that reported since earlier studies used assays with poor sensitivity [17]. Higher results are expected when more sensitive assays are used [18]. Sera tested in recent IgG immunoassays based on a variety of HEV antigens gave broadly concordant results suggesting that antibodies detected are truly directed against HEV [19].
In endemic areas, such as India and Southeast Asia, studies on HEV seroprevalence have shown high frequencies in the general population, ranging from 27 to 80 % in the general population. In contrast, in non-endemic regions, seroprevalence varies from 2 to 8 % in Europe, Japan and South America to 18.0 to 21.0 % in the USA, Russia, UK, Southern France, Hong Kong, Korea, and China [20]. In developing countries, water-borne epidemics of HEV mainly affect young adults, the clinical attack rate being highest among 15–35 year olds [21] and men are clinically infected 2–5 times more than women in most outbreaks [22]. Asymptomatic infections have been estimated to exceed the number of symptomatic cases by 2–4 times in water-borne outbreaks [23]. Although routine surveillance and reporting of HEV infection is far from universal and sensitive anti-HEV assays are still lacking, it is clear that the majority of disease burden due to HEV is in the low and medium income countries of Asia and Africa [24].
Mode(s) of transmission and endemicity
One of the earliest documented massive outbreaks of infectious hepatitis took place in New Delhi, India in late 1955 where at least 30,000 clinical cases of jaundice were reported with elevated morbidity and mortality in pregnant women [25]. Outbreaks with similar characteristics to the New Delhi outbreak were also documented in Kashmir Valley in 1978 [26] and in Afghanistan in 1983 [27]. All three outbreaks were associated with fecal contamination of drinking water or flooding. In 1990–1991, the etiological agent of such outbreaks was isolated, partially cloned and was labeled hepatitis E virus [28]. HEV is now considered the leading cause of enterically transmitted non-A hepatitis in low socio-economic communities [29] and is considered endemic in developing countries. In these outbreaks, the severe infection during pregnancy maybe linked to the greater virulence of HEV-1 genotype and the genetic repertoire of Asian populations [8].
Recent reports showed higher HEV seroprevalence in specific groups as paid blood donors and in repeatedly transmitted hemodialysis patients [30, 31]. Furthermore, the recent reporting of transmission of HEV through blood transfusion from Saudi Arabia [32], Japan [33] and the UK [34] has led to the suggestion that the parenteral route could be an important route in the transmission of HEV. As early as 1996 we speculated on the possible transmission of HEV by blood transfusion in Saudi Arabia [35]. Blood safety came into the light recently when Hewitt and her colleagues in England [36] showed that blood components (platelets preparations, red cells granulocyte pool and fresh frozen plasma) were associated with HEV-3 transmission while in Japan, the most prevalent genotypes were HEV-3 and HEV-4 [8]. Since HEV is endemic in developing countries, including countries of the MENA region, the awareness of an infectious threat to blood safety is legitimate. The purpose of this manuscript, therefore, is to review the status of HEV in the MENA region countries and to see whether the transfusion–transmission route has been critically evaluated.
Methods
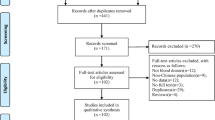
Related articles were collected, by searching through the countries of the MENA region. Over the past 14 years (from January 2000 to August 2014), literature was reviewed using countries of the MENA region Pubmed and Medline. The search was conducted using predefined combination of keywords: “Hepatitis E Virus”, “Hepatitis E”, “Hepatitis E Infection”, “blood donors” in combination with all the names of the countries of the MENA region. The MENA region includes 25 countries (Algeria, Bahrain, Cyprus, Djibouti, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Libya, Mauritania, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, Turkey, UAE and Yemen) and covers a population of over 380 million people. One hundred articles were reviewed, of which 25 articles were excluded; the excluded papers were written in non-English Language or had uninterpretable data (did not have seroprevalence data).
Results
Hepatitis E virus seroprevalence (anti-HEV IgG and IgM) and HEV-RNA across age groups and in different categories of populations in various countries of the MENA region during the past 14 years are shown in Table 1.
There were eight articles published on HEV in blood donors (Table 2) of which only two studies performed HEV-RNA testing to see whether or not the donor was viremic at the time of donation. Only one study from Saudi Arabia was a prospective study [32].
Anti-HEV in Egypt exceeds 12.5 % in children and can reach 84.0 % in adults. In Iran, anti-HEV varies from region to another (2–14 %), but it does not exceed 14 %. Similarly anti-HEV among Saudis varied from 4.0 to 8.0 % in the general population and in one study a seroprevalence of 19 % was reported among blood donors [107]. Regional variation in anti-HEV seroprevalence was also reported in the Turkish population (2––13 %) and anti-HEV was as high as 35 % among agriculture workers. Anti-HEV variation (18–22 %) was reported among Iraqis and a seroprevalence of 11 % was reported in Yemen. Molecular studies were not attempted in most of the studies reported. High HEV-RNA positivity was reported in pediatric patients with acute hepatitis in Egypt (23 %), in hepatitis outbreaks in Sudan (20–27 %) in patients with chronic hepatitis C in Turkey (55 %) and in pregnant women in UAE (30 %). Table 2 summarizes the seroprevalence of HEV markers among blood donors in the MENA countries.
Discussion
In recent years, studies from developed countries have shown asymptomatic viremia (HEV-RNA) in blood donors which is suggestive of ongoing subclinical infection [36, 109–112]. The presence of HEV-RNA is in the serum of healthy donors indicates that there is a potential risk of transmission of HEV through blood and indeed transmission of HEV by transfusion has been reported on several occasions from Europe and Japan [34, 113–116]. The first molecularly confirmed case of transfusion-transmitted HEV-4 was reported in 2004 from Japan [114]. Other cases have been confirmed in Sweden, Germany and the United States [109, 110], in the United Kingdom [34] and in France [113]; to mention a few. Recent articles have already reviewed the risk of HEV infection by blood donation from developed countries. The incidence of HEV in the blood donor population in developed countries, however, is unclear and is likely underestimated and this could be possibly due to several factors including: (1) the lack of well-validated assays; (2) the asymptomatic nature of HEV infection among most of the adults; (3) the lack of testing; and (4) finally, the underreporting of HEV disease in all countries.
On the other hand, in endemic countries including countries of the MENA region, the impact of HEV transmission through blood transfusion has rarely been evaluated. The possibility of transmission through blood transfusion was based mainly on retrospective evaluation in transfusion recipients [113, 117] and that multi-transfused had significantly higher prevalence of markers for acute HEV (anti-IgM and HEV-RNA) as compared to controls [31, 118, 119]. In the MENA region, out of the 75 published articles that showed interpretable data during the past 14 years, only 8 articles provided data on HEV in blood donors: seven of the articles reported on the seroprevalence of HEV in blood donors and only one study was prospective. Anti-HEV (IgG) in blood donors ranged from 5.4 % among Tunisians to over 50 % among Egyptians. In one study from Saudi Arabia, anti-HEV (IgG) in blood donors was as high as 19.0 % [107]. It is interesting to note that the blood donors in this Saudi study were from one location (Makkah) where the majority of the people drank well water [107]. Moreover, 4.3 % of the blood donors tested were anti-HEV (IgM) positive implicating HEV as a potential transfusion risk. In the prospective study from Saudi Arabia [32], HEV disease developed in 3 of 22 susceptible patients following blood transfusion. The infections were traced to infected donor samples (HEV-RNA positive) and occurred within the incubation period of HEV infection. Thus, asymptomatic viremia may occur in healthy adults in endemic areas and viremia and fecal shedding have been reported in symptom-free carriers. The available but scanty data, therefore, show that the disease burden of HEV infection in blood donors in countries with experience of outbreaks of HEV infection is grossly under-investigated. This fact, in addition to the high endemicity of HEV in developing countries, and the risk of transmission of HEV by transfusion documented in some developed countries lead to the emerging awareness of threat of HEV infection to blood safety in developing countries.
As for the seroprevalence of HEV in the MENA region, published data during the past 14 years confirm the endemicity of HEV in all 12 MENA countries studied, but data on the remaining 13 countries are still lacking. Except for Egypt where anti-HEV reaches 100 % in certain populations, the seroprevalence of HEV in the general population ranges from 2.3 to 37.5 % and is higher in males than in females. Prevalence increased with age, but exposure seems to be in early life in Egypt as high prevalence was detected in young children [120]. This increase in percentage of anti-HEV with age could be consistent with cumulative exposure to infection over time [121].
It must be emphasized, however, that our study suffers serious limitations. The major limitation is in the methodology of the reported studies where the selection of patients and the assays used were very different. Moreover, earlier assays used were less sensitive than the recent ones [122] which cast some doubt on the frequencies reported as underestimated and hence not representative. In addition, data on HEV on the remaining 13 countries of the MENA region are still missing. Furthermore, only three studies reported on HEV genotypes. In Egypt, HEV-1 (2 cases) [43] and HEV-3 (1 case) [41] were reported while in Sudan, HEV-1 (23 cases) was detected during an outbreak in Darfur [79]. Based on these few cases, it can be concluded that HEV-1 is the prevalent genotype in the MENA region which concurs with the global distribution of HEV genotypes [8]. However, more research is needed to identify the predominant genotype(s) in various countries and even in various populations of the MENA region.
It can be concluded that more extensive investigations are required to determine the risk of HEV transmission by blood donations in developing countries [112]. This will include exploring epidemiological, virological and cultural data in evaluating the risk of HEV exposure via blood transfusion in these countries. National surveillance screening of donors and recipients for markers of recent HEV infection is of utmost importance. The reliability of the data depends on the reliability of the tests employed. IgM antibodies to HEV are present for several weeks following acute HEV infection [123] indicating that perhaps screening for HEV IgM class antibodies could be a marker for detecting active HEV infection. No infectivity data, however, are available for anti-HEV IgM-positive donations in the MENA region. HEV-RNA on the other hand does not persist for long (1–2 months) and in some patients may persist for a longer period [8] where in immunocompromised patients the persistence extends to several years [124].
Although the risk of transmission for HEV at present is higher than other viruses such as HIV, screening for HEV in blood donors is still not a universal requirement [112]. However, selective screening is to be recommended in certain circumstances. Recent evidence shows that HEV infection can take a severe or even fatal course resulting in liver failure in immunocompromised, pregnant women or patients with chronic liver disease [123, 125–129]. Since these patients often require blood transfusion, it is prudent that screening for HEV-RNA or at least for anti-HEV IgM in donated blood for these patients should be implemented as soon as possible.
References
Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE. Sequence data suggests big liver and spleen disease virus (blsv) is genetically related to hepatitis E virus. Vet Microbiol. 1999;68:119–25.
Chandra V, Kar-Roy A, Kumari S, Mayor S, Jameel S. The hepatitis E virus ORF3 protein modulates epidermal growth factor receptor trafficking, STAT3 translocation, and the acute-phase response. J Virol. 2008;82:7100–10.
Huang FF, Haqshenas G, Shivaprasad HL, Guenette DK, Woolcock PR, Larsen CT, Pierson FW, Elvinger F, Toth TE, Meng XJ. Heterogeneity and seroprevalence of a newly identified avian hepatitis E virus from chickens in the United States. J Clin Microbiol. 2002;40:4197–202.
Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, Tang Y, Sivakumar S, Xie J, Bai R, Wernery R, Wernery U, Yuen KY. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis. 2014;20:1044–8.
Johne R, Dremsek P, Reetz J, Heckel G, Hess M, Ulrich RG. Hepeviridae: an expanding family of vertebrate viruses. Infect Genet Evol. 2014;27:212–29.
Kim JH, Nelson KE, Panzner U, Kasture Y, Labrique AB, Wierzba TF. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect Dis. 2014;14:308.
Dalton HR, Hunter JG, Bendall RP. Hepatitits E. Curr Opin Infect Dis. 2013;26:471–8.
Krain LJ, Nelson KE, Labrique AB. Host immune status and response to hepatitis E virus infection. Clin Microbiol Rev. 2014;27:139–65.
Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci. 1997;94:860–9865.
Meng XJ, Dea S, Engle RE, Friendship R, Lyoo YS, Sirinarumitr T, Urairong K, Wang D, Wong D, Yoo D, Zhang Y, Purcell RH, Emerson SU. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J Med Virol. 1999;59:297–302.
Wichmann O, Schimanski S, Koch J, Kohler M, Rothe C, Plentz A, Jilg W, Stark K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198:1732–41.
Dalton HR, Fellows HJ, Gane EJ, Wong P, Gerred S, Schroeder B, Croxson MC, Garkavenko O. Hepatitits E in New Zealand. J Gastroenterol Hepatol. 2007;22:1236–40.
Tsang TH, Denison EK, Williams HV, Venczel LV, Ginsberg MM, Vugia DJ. Acute hepatitis E infection acquired in California. Clin Infect Dis. 2000;30:618–9.
Mitsui T, Tsukamoto Y, Hirose A, Suzuki S, Yamazaki C, Masuko K, Tsuda F, Endo K, Takahashi M, Okamoto H. Distinct changing profiles of hepatitis A and E virus infection among patients with acute hepatitis, patients on maintenance hemodialysis and healthy individuals in Japan. J Med Virol. 2006;78:1015–24.
Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol. 2005;76:341–9.
Aggarwal R, Naik S. Epidemiology of hepatitis E: current status. J Gastroenterol Hepatol. 2009;24:1484–93.
Mansuy JM, Legrand-Abravanel F, Calot JP, Peron JM, Alric L, Agudo S, Rech H, Destruel F, Izopet J. High prevalence of anti-hepatitis E virus antibodies in blood donors from South West France. J Med Virol. 2008;80:289–93.
Wenzel JJ, Preiss J, Schemmerer M, Huber B, Jilg W. Test performance characteristics of anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J Infect Dis. 2013;207:497–500.
Bendall R, Ellis V, Ijaz S, Thurairajah P, Dalton HR. Serological response to hepatitis E virus genotype 3 infection: IgG quantitation, avidity and IgM response. J Med Virol. 2008;80:95–101.
Zhu G, Qu Y, Jin N, Sun Z, Liu T, Lee H, Tian M, Wang T. Seroepidemiology and molecular characterization of hepatitis E virus in Jilin, China. Infection. 2008;36:140–6.
Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503.
Aggarwal R, Kumar R, Pal R, Naik S, Semwal SN, Naik SR. Role of travel as a risk factor for hepatitis E virus infection in a disease-endemic area. Indian J Gastroenterol. 2002;21:14–8.
Guthmann JP, Klovstad H, Boccia D, Hamid N, Pinoges L, Nizou JY, Tatay M, Diaz F, Moren A, Grais RF, Ciglenecki I, Nicand E, Guerin PJ. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin Infect Dis. 2006;42:1685–91.
Kmush B, Wierzba T, Krain L, Nelson K, Labrique AB. Epidemiology of hepatitis E in low-and Middle-income countries of Asia and Africa. Semin Liver Dis. 2013;33:15–29.
Viswanathan R. Epidemiology. Indian J Med Res. 1957;45:1–29.
Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818–24.
Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31.
Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–31.
Rapicetta M, Monarca R, Kondili LA, Chionne P, Madonna E, Madeddu G, Soddu A, Candido A, Carbonara S, Mura MS, Starnini G, Babudieri S. Hepatitis E virus and hepatitis A virus exposures in an apparently healthy high-risk population in Italy. Infection. 2013;41:69–76.
Arankalle VA, Chobe LP. Hepatitis E virus: can it be transmitted parenterally? J Viral Hepat. 1999;6:161–4.
Mitsui T, Tsukamoto Y, Yamazaki C, Masuko K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: evidence for infection with a genotype 3 HEV by blood transfusion. J Med Virol. 2004;74:563–72.
Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778–84.
Matsubayashi K, Nagaoka Y, Sakata H, Sato S, Fukai K, Kato T, Takahashi K, Mishiro S, Imai M, Takeda N, Ikeda H. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934–40.
Boxall E, Herborn A, Kochethu G, Pratt G, Adams D, Ijaz S, Teo CG. Transfusion-transmitted hepatitis E in a “nonhyperendemic” country. Transfus Med. 2006;16:79–83.
Al-Fawaz I, Al-Rasheed S, Al-Mugeiren M, Al-Salloum A, Al-Sohaibani M, Ramia S. Hepatitis E virus infection in patients from Saudi Arabia with sickle cell anaemia and beta-thalassemia major: possible transmission by blood transfusion. J Viral Hepat. 1996;3:203–5.
Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, Russell K, Tettmar KI, Tossell J, Ushiro-Lumb I, Tedder RS. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–73.
Shata MT, Daef EA, Zaki ME, Abdelwahab SF, Marzuuk NM, Sobhy M, Rafaat M, Abdelbaki L, Nafeh MA, Hashem M, El-Kamary SS, Shardell MD, Mikhail NN, Strickland GT, Sherman KE. Protective role of humoral immune responses during an outbreak of hepatitis E in Egypt. Trans R Soc Trop Med Hyg. 2012;106:613–8.
El-Tras WF, Tayel AA, El-Kady NN. Seroprevalence of hepatitis E virus in humans and geographically matched food animals in Egypt. Zoonoses Publ Health. 2013;60:244–51.
Gad YZ, Mousa N, Shams M, Elewa A. Seroprevalence of subclinical HEV infection in asymptomatic, apparently healthy, pregnant women in Dakahlya Governorate, Egypt. Asian J Transfus Sci. 2011;5:136–9.
El Sayed Zaki M, Othman W. Role of hepatitis E infection in acute on chronic liver failure in Egyptian patients. Liver Int. 2011;31:1001–5.
Kamel AH, Ali MA, El-Nady HG, Deraz A, Aho S, Pothier P, Belliot G. Presence of enteric hepatitis viruses in the sewage and population of Greater Cairo. Clin Microbiol Infect. 2011;17:1182–5.
Eldin SS, Seddik I, Daef EA, Shata MT, Raafat M. Abdel Baky L, Nafeh MA. Risk factors and immune response to hepatitis E viral infection among acute hepatitis patients in Assiut, Egypt. Egypt J Immunol. 2010;17:73–86.
Blackard JT, Rouster SD, Nady S, Galal G, Marzuuk N, Rafaat MM, Daef E, El Din SS, Purcell RH, Emerson SU, Sherman KE, Shata MT. Genotypic characterization of symptomatic hepatitis E virus (HEV) infections in Egypt. J Clin Virol. 2009;46:140–4.
Youssef A, Yano Y, Utsumi T, Abd El-alah EM, Abd El-Hameed Ael E, Serwah Ael H, Hayashi Y. Molecular epidemiological study of hepatitis viruses in Ismailia, Egypt. Intervirology. 2009;52:123–31.
Zaki Mel S, Salama OS, Mansour FA, Hossein S. Hepatitis E virus coinfection with hepatotropic viruses in Egyptian children. J Microbiol Immunol Infect. 2008;41:254–8.
Zakaria S, Fouad R, Shaker O, Zaki S, Hashem A, El-Kamary SS, Esmat G, Zakaria S. Changing patterns of acute viral hepatitis at a Major Urban Referral Center in Egypt. Clin Infect Dis. 2007;44:e30–6.
El-Sayed Zaki M, El-Deen Zaghloul MH, El Sayed O. Acute sporadic hepatitis E in children: diagnostic relevance of specific immunoglobulin M and immunoglobulin G compared with nested reverse transcriptase PCR. FEMS Immunol Med Microbiol. 2006;48:16–20.
Meky FA, Stoszek SK, Abdel-Hamid M, Selim S, Abdel-Wahab A, Mikhail N, El-Kafrawy S, El-Daly M, Abdel-Aziz F, Sharaf S, Mohamed MK, Engle RE, Emerson SU, Purcell RH, Fix AD, Strickland GT. Active surveillance for acute viral hepatitis in rural villages in the Nile Delta. Clin Infect Dis. 2006;2:628–33.
Darwish MA, Faris R, Darwish N, Shouman A, Gadallah M, El-Sharkawy MS, Edelman R, Grumbach K, Rao MR, Clemens JD. Hepatitis c and cirrhotic liver disease in the Nile delta of Egypt: a community-based study. Am J Trop Med Hyg. 2001;64:147–53.
El Sayed Zaki M, El Aal AA, Badawy A, El-Deeb DR, El-Kheir NY. Clinicolaboratory study of mother-to-neonate transmission of hepatitis E virus in Egypt. Am J Clin Pathol. 2013;140:721–6.
Albatanony MA, El-Shafie MK. Work-related health effects among wastewater treatment plants workers. Int J Occup Environ Med. 2011;2:237–44.
El-Esnawy NA. Examination for hepatitis E virus in wastewater treatment plants and workers by nested RT-PCR and ELISA. J Egypt Publ Health Assoc. 2000;75:219–31.
El-Esnawy NA, Ali MA, Bayoumi FS, Abo-El-Khir A, Abdel-Wahab KS. Waterborne viruses associated with repeated abortion. J Egypt Publ Health Assoc. 2001;76:487–503.
Stoszek SK, Engle RE, Abdel-Hamid M, Mikhail N, Abdel-Aziz F, Medhat A, Fix AD, Emerson SU, Purcell RH, Strickland GT. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg. 2006;100:89–94.
Stoszek SK, Abdel-Hamid M, Saleh DA, El Kafrawy S, Narooz S, Hawash Y, Shebl FM, El Daly M, Said A, Kassem E, Mikhail N, Engle RE, Sayed M, Sharaf S, Fix AD, Emerson SU, Purcell RH, Strickland GT. High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg. 2006;100:95–101.
Fix AD, Abdel-Hamid M, Purcell RH, Shehata MH, Abdel-Aziz F, Mikhail N, el Sebai H, Nafeh M, Habib M, Arthur RR, Emerson SU, Strickland GT. Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am J Trop Med Hyg. 2000;62:519–23.
Zaki Mel S, Foud MF, Mohamed AF. Value of hepatitis E virus detection by cell culture compared with nested PCR and serological studies by IgM and IgG. FEMS Immunol Med Microbiol. 2009;56:73–9.
Zekavat OR, Makarem A, Karami MY, Amanat A, Mohandes M, Habibagahi M. Serological investigation for hepatitis E virus infection in the patients with chronic maintenance hemodialysis from southwest of Iran. Asian J Transfus Sci. 2013;7:21–5.
Khameneh ZR, Sepehrvand N. Author’s reply: hepatitis E virus infection in iranian kidney-transplant patients. Hepat Mon. 2011;11:929–30.
Taremi M, Khoshbaten M, Gachkar L, EhsaniArdakani M, Zali M. Hepatitis E virus infection in hemodialysis patients: a seroepidemiological survey in Iran. BMC Infect Dis. 2005;5:36.
Rostamzadeh Khameneh Z, Sepehrvand N, Khalkhali HR. Seroprevalence of hepatitis E among pregnant women in Urmia, Iran. Hepat Mon. 2013;13:e10931.
Ahmadi Ghezeldasht S, Miri R, Hedayatimoghadam M, Shamsian A, Bidkhori H, Fathimoghadam F, Rezaee SA. Population movement and virus spreading: HEV spreading in a Pilgrimage City, Mashhad in Northeast Iran: an example. Hepat Mon. 2013;13:e10255.
Ramezani A, Velayati AA, Khorami-Sarvestani S, Eslamifar A, Mohraz M, Banifazl M, Bidari-Zerehpoosh F, Yaghmaei F, McFarland W, Foroughi M, Keyvani H, Mostafavi E, Aghakhani A. Hepatitis E virus infection in patients infected with human immunodeficiency virus in an endemic area in Iran. Int J STD AIDS. 2013;24:769–74.
Mohebbi SR, Rostami Nejad M, Tahaei SM, Pourhoseingholi MA, Habibi M, Azimzadeh P, Naghoosi H, Karayiannis P, Zali MR. Seroepidemiology of hepatitis A and E virus infections in Tehran, Iran: a population based study. Trans R Soc Trop Med Hyg. 2012;106:528–31.
Raoofi R, Nazer MR, Pournia Y. Seroepidemiology of hepatitis E virus in Western Iran. Braz J Infect Dis. 2012;16:302–3.
Saffar MJ, Farhadi R, Ajami A, Khalilian AR, Babamahmodi F, Saffar H. Seroepidemiology of hepatitis E virus infection in 2–25-year-olds in Sari district, Islamic Republic of Iran. East Mediterr Health J. 2009;15:136–42.
Ataei B, Nokhodian Z, Javadi AA, Kassaian N, Shoaei P, Farajzadegan Z, Adibi P. Hepatitis E virus in Isfahan Province: a population-based study. Int J Infect Dis. 2009;13:67–71.
Taremi M, Mohammad Alizadeh AH, Ardalan A, Ansari S, Zali MR. Seroprevalence of hepatitis E in Nahavand, Islamic Republic of Iran: a population-based study. East Mediterr Health J. 2008;14:157–62.
Utba NM. The prevalence of hepatitis E virus in Al-Sadr City—Baghdad. Clin Lab. 2013;59:115–20.
Al-Nasrawi KK, Al Diwan JK, Al-Hadithi TS, Saleh AM. Viral hepatitis E outbreak in Al-Sadr city, Baghdad, Iraq. East Mediterr Health J. 2010;16:1128–32.
Al-Naaimi AS, Turky AM, Khaleel HA, Jalil RW, Mekhlef OA, Kareem SA, Hasan NY, Dhadain AA. Predicting acute viral hepatitis serum markers (A and E) in patients with suspected acute viral hepatitis attending primary health care centers in Baghdad: a one year cross-sectional study. Glob J Health Sci. 2012;4:172–83.
Myint KS, Duripunt P, Mammen MP Jr, Sirisopana N, Rodkvamtook W, Gibbons RV. Hepatitis E virus infection in Thai troops deployed with U.N. peacekeeping forces. Mil Med. 2007;172:1217–9.
Chironna M, Germinario C, Lopalco PL, Carrozzini F, Barbuti S, Quarto M. Prevalence rates of viral hepatitis infections in Refugee Kurds from Iraq and Turkey. Infection. 2003;31:70–4.
Lachish T, Tandlich M, Schwartz E. Acute hepatitis in Israeli travelers. J Travel Med. 2013;20:232–6.
Potasman I, Koren L, Peterman M, Srugo I. Lack of hepatitis E infection among backpackers to tropical countries. J Travel Med. 2000;7:208–10.
Ayoola A, Aderoju A, Gadour MO, Al-Hazmi M, Hamza MK, Ene D, Hafeez M, Anderson D, Riddell M. Serological profile of sporadic acute viral hepatitis in an area of hyper-endemic hepatitis B virus infection. Saudi J Gastroenterol. 2001;7:95–102.
Ayoola EA, Want MA, Gadour MO, Al-Hazmi MH, Hamza MK. Hepatitis E virus infection in haemodialysis patients: a case–control study in Saudi Arabia. J Med Virol. 2002;66:329–34.
Boccia D, Guthmann JP, Klovstad H, Hamid N, Tatay M, Ciglenecki I, Nizou JY, Nicand E, Guerin PJ. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis. 2006;42:1679–84.
Nicand E, Armstrong GL, Enouf V, Guthmann JP, Guerin JP, Caron M, Nizou JY, Andraghetti R. Genetic heterogeneity of hepatitis E virus in Darfur, Sudan, and Neighboring Chad. J Med Virol. 2005;77:519–21.
Ahmed RE, Karsany MS, Adam I. Brief report: acute viral hepatitis and poor maternal and perinatal outcomes in pregnant sudanese women. J Med Virol. 2008;80:1747–8.
Mudawi HM, Yousif BA. Fulminant hepatic failure in an African setting: etiology, clinical course, and predictors of mortality. Dig Dis Sci. 2007;52:3266–9.
Hannachi N, Boughammoura L, Marzouk M, Tfifha M, Khlif A, Soussi S, Skouri H, Boukadida J. Viral infection risk in polytransfused adults: seroprevalence of seven viruses in central Tunisia. Bull Soc Pathol Exot. 2011;104:220–5.
Hannachi N, Hidar S, Harrabi I, Mhalla S, Marzouk M, Ghzel H, Ghannem H, Khairi H, Boukadida J. Seroprevalence and risk factors of hepatitis E among pregnant women in central Tunisia. Pathol Biol (Paris). 2011;59:e115–8.
Rezig D, Ouneissa R, Mhiri L, Mejri S, Haddad-Boubaker S, Ben Alaya N, Triki H. Seroprevalences of hepatitis A and E infections in Tunisia. Pathol Biol (Paris). 2008;56:148–53.
Bayram A, Eksi F, Mehli M, Sözen E. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology. 2007;50:281–6.
Uçar E, Cetin M, Kuvandik C, Helvaci MR, Güllü M, Hüzmeli C. Hepatitis E virus seropositivity in hemodialysis patients in Hatay province, Turkey. Mikrobiyol Bul. 2009;43:299–302.
Cevahir N, Demir M, Bozkurt AI, Ergin A, Kaleli I. Seroprevalence of hepatitis E virus among primary school children. Pak J Med Sci. 2013;29:629–32.
Maral I, Budakoglu II, Ceyhan MN, Atak A, Bumin MA. Hepatitis E virus seroepidemiology and its change during 1 year in primary school students in Ankara, Turkey. Clin Microbiol Infect. 2010;16:831–5.
Eker A, Tansel O, Kunduracilar H, Tokuç B, Yuluğkural Z, Yüksel P. Hepatitis E virus epidemiology in adult population in Edirne province, Turkey. Mikrobiyol Bul. 2009;43:251–8.
Kaya AD, Ozturk CE, Yavuz T, Ozaydin C, Bahcebasi T. Changing patterns of hepatitis A and E sero-prevalences in children after the 1999 earthquakes in Duzce, Turkey. J Paediatr Child Health. 2008;44:205–7.
Oncu S, Oncu S, Okyay P, Ertug S, Sakarya S. Prevalence and risk factors for HEV infection in pregnant women. Med Sci Monit 2006;12:CR36-39.
Sencan I, Sahin I, Kaya D, Oksuz S, Yildirim M. Assessment of HAV and HEV seroprevalence in children living in post-earthquake camps from Diizce. Turkey. Eur J Epidemiol. 2004;19:461–5.
Atabek ME, Fýndýk D, Gulyuz A, Erkul I. Prevalence of anti-HAV and anti-HEV antibodies in Konya, Turkey. Health Policy. 2004;67:265–9.
Cevrioglu AS, Altindis M, Tanir HM, Aksoy F. Investigation of the incidence of hepatitis E virus among pregnant women in Turkey. J Obstet Gynaecol Res. 2004;30:48–52.
Ceylan A, Ertem M, Ilcin E, Ozekinci T. A special risk group for hepatitis E infection: Turkish agricultural workers who use untreated waste water for irrigation. Epidemiol Infect. 2003;131:753–6.
Yayli G, Kiliç S, Ormeci AR. Hepatitis agents with enteric transmission—an epidemiological analysis. Infection. 2002;30:334–7.
Cesur S, Akin K, Doğaroğlu I, Birengel S, Balik I. Hepatitis A and hepatitis E seroprevalence in adults in the Ankara area. Mikrobiyol Bul. 2002;36:79–83.
Colak D, Ogunc D, Gunseren F, Velipasaoglu S, Aktekin MR, Gültekin M. Seroprevalence of antibodies to hepatitis A and E viruses in pediatric age groups in Turkey. Acta Microbiol Immunol Hung. 2002;49:93–7.
Sidal M, Unüvar E, Oğuz F, Cihan C, Onel D, Badur S. Age-specific seroepidemiology of hepatitis A, B, and E infections among children in Istanbul, Turkey. Eur J Epidemiol. 2001;17:141–4.
Abro AH, Abdou AM, Saleh AA, Ustadi AM, Hussaini HS. Hepatitis E: a common cause of acute viral hepatitis. J Pak Med Assoc. 2009;59:92–4.
Kumar RM, Uduman S, Rana S, Kochiyil JK, Usmani A, Thomas L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol. 2001;100:9–15.
Bawazir AA, Hart CA, Sallam TA, Parry CM, Beeching NJ, Cuevas LE. Seroepidemiology of hepatitis A and hepatitis E viruses in Aden, Yemen. Trans R Soc Trop Med Hyg. 2010;104:801–5.
Ibrahim EH, Abdelwahab SF, Nady S, Hashem M, Galal G, Sobhy M, Saleh AS, Shata MT. Prevalence of Anti-HEV IgM among blood donors in Egypt. Egypt J Immunol. 2011;18:47–58.
Ehteram H, Ramezani A, Eslamifar A, Sofian M, Banifazl M, Ghassemi S, Aghakhani A, Mashayekhi P. Seroprevalence of Hepatitis E Virus infection among volunteer blood donors in central province of Iran in 2012. Iran J Microbiol. 2013;5:172–6.
Assarehzadegan MA, Shakerinejad G, Amini A, Rezaee SA. Seroprevalence of hepatitis E virus in blood donors in Khuzestan Province, Southwest Iran. Int J Infect Dis. 2008;12:387–90.
Taremi M, Gachkar L, MahmoudArabi S, Kheradpezhouh M, Khoshbaten M. Prevalence of antibodies to hepatitis E virus among male blood donors in Tabriz, Islamic Republic of Iran. East Mediterr Health J. 2007;13:98–102.
Johargy AK, Mahomed MF, Khan MM, Kabrah S. Anti-Hepatitis E virus seropositivity in a group of male blood donors in Makkah, Saudi Arabia. J Pak Med Assoc. 2013;63:185–9.
Houcine N, Jacques R, Salma F, Anne-Gaëlle D, Amin S, Mohsen H, Hamadi B, Christophe R, Patrice A, Mahjoub A, Caroline S. Seroprevalence of hepatitis E virus infection in rural and urban populations, Tunisia. Clin Microbiol Infect. 2012;18:E119-2.
Vollmer T, Diekmann J, Johne R, Eberhardt M, Knabbe C, Dreier J. Novel approach for detection of hepatitis E virus infection in German blood donors. J Clin Microbiol. 2012;50:2708–13.
Baylis SA, Gärtner T, Nick S, Ovemyr J, Blümel J. Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sang. 2012;103:89–90.
Dreier J, Juhl D. Autochthonous hepatitis E virus infections: a new transfusion-associated risk? Transfus Med Hemother. 2014;41:29–39.
Pawlotsky JM. Hepatitis E screening for blood donations: an urgent need? Lancet. 2014;384:1729–30.
Colson P, Coze C, Gallian P, Henry M, De Micco P, Tamalet C. Transfusion-associated hepatitis E, France. Emerg Infect Dis. 2007;13:648–9.
Matsubayashi K, Kang JH, Sakata H, Takahashi K, Shindo M, Kato M, Sato S, Kato T, Nishimori H, Tsuji K, Maguchi H, Yoshida J, Maekubo H, Mishiro S, Ikeda H. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368–75.
Tamura A, Shimizu YK, Tanaka T, Kuroda K, Arakawa Y, Takahashi K, Mishiro S, Shimizu K, Moriyama M. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol Res. 2007;37:113–20.
Matsubayashi K, Sakata H, Ikeda H. Hepatitis E virus infection and blood transfusion in Japan. ISBT Sci Ser. 2011;6:344–9.
Arankalle VA, Chobe LP. Retrospective analysis of blood transfusion recipients: evidence for post-transfusion hepatitis E. Vox Sang. 2000;79:72–4.
Toyoda H, Honda T, Hayashi K, Katano Y, Goto H, Kumada T, Takahashi K, Abe N, Mishiro S, Takamatsu J. Prevalence of hepatitis E virus IgG antibody in Japanese patients with hemophilia. Intervirology. 2008;51:21–5.
Irshad M, Peter S. Spectrum of viral hepatitis in thalassemic children receiving multiple blood transfusions. Indian J Gastroenterol. 2002;21:183–4.
Verghese VP, Robinson JL. A systematic review of hepatitis E virus infection in children. Clin Infect Dis. 2014;59:689–97.
Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, Farrington L, Hamad N, Sieberhagen C, Ellis V, Mitchell J, Hussaini SH, Banks M, Ijaz S, Bendall RP. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–90.
Mansuy JM, Bendall R, Legrand-Abravanel F, Sauné K, Miédouge M, Ellis V, Rech H, Destruel F, Kamar N, Dalton HR, Izopet J. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–12.
Clayson ET, Myint KS, Snitbhan R, Vaughn DW, Innis BL, Chan L, Cheung P, Shrestha MP. Viremia, fecal shedding and IgM and IgG responses in patients with hepatitis E. J Infect Dis. 1995;172:927–33.
Passos-Castilho AM, Porta G, Miura IK, Pugliese RP, Danesi VL, Porta A, Guimarães T, Seda J, Antunes E, Granato CF. Chronic hepatitis E virus infection in a pediatric female liver transplant recipient. J Clin Microbiol. 2014;52:4425–7.
Nelson KE, Kmush B, Labrique AB. The epidemiology of hepatitis E virus infections in developed countries and among immunocompromised patients. Exp Rev Anti Infect Ther. 2011;9:1133–48.
Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–9.
Pfefferle S, Frickmann H, Gabriel M, Schmitz N, Günther S, Schmidt-Chanasit J. Fatal course of an autochthonous hepatitis E virus infection in a patient with leukemia in Germany. Infection. 2012;40:451–4.
Neukam K, Barreiro P, Macías J, Avellón A, Cifuentes C, Martín-Carbonero L, Echevarría JM, Vargas J, Soriano V, Pineda JA. Chronic hepatitis E in HIV patients: rapid progression to cirrhosis and response to oral ribavirin. Clin Infect Dis. 2013;57:465–8.
Te HS, Drobeniuc J, Kamili S, Dong C, Hart J, Sharapov UM. Hepatitis E virus infection in a liver transplant recipient in the United States: a case report. Transplant Proc. 2013;45:810–3.
Acknowledgments
The authors would like to acknowledge Ms. Yasmine Abou Taha for English language editing, Ms. Maha Abul Naja for her typing, and Ms. Rana Charide and Ms. Nour Rahhal for their assistance.
Conflict of interest
No conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yazbek, S., Kreidieh, K. & Ramia, S. Hepatitis E virus in the countries of the Middle East and North Africa region: an awareness of an infectious threat to blood safety. Infection 44, 11–22 (2016). https://doi.org/10.1007/s15010-015-0807-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-015-0807-5