Abstract
Although root canal therapy is the most common and widely used treatment at clinical presentation, there are still some postoperative complications. As cell biology and tissue engineering techniques advance rapidly, the use of biological therapy to regenerate dental pulp has become a new trend; Relevant literatures in recent five years were searched using key words such as “root canal therapy”, “Dental pulp stem cells”, “Dental pulp regeneration”, and “Cell homing” in PubMed, Web of Science, etc; Dental pulp stem cells (DPSCs) have multi-differentiation potential, self-renewal capability, and high proliferative ability. Stem cell-based dental pulp regeneration has emerged as a new research hot spot in clinical therapy. Recently, dental pulp-like structures have been generated by the transplantation of exogenous DPSCs or the induction of homing of endogenous DPSCs. Studies on DPSCs are important and significant for dental pulp regeneration and dental restoration; In this review, the existing clinical treatment methods, dental pulp regeneration, and DPSC research status are revealed, and their application prospects are discussed. The stem cell-based pulp regeneration exerts promising potential in clinical therapy for pulp regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The dental pulp locates in the root canal and communicates with the periapical tissues through a narrow apical foramen, which hinders the self-repairing capability when the dental pulp suffers from inflammation and injury under the influence of bacterial infection or tooth fracture. Currently, root canal therapy (RCT) is the most frequently used clinical manipulation for periapical lesions and dental pulp disorders. In RCT, the dental pulp tissue is extracted utilizing mechanical instruments, and the root canal is firmly cemented using inorganic materials. According to data from the American Dental Association, approximately 22 million RCT cases arise every year throughout the USA, incurring annual expenses of up to 20 ~ 34 billion dollars. Although the success rate of treatment is 78–98% [1, 2], many obstacles remain in RCT.
Traditional RCT cannot promote the regeneration of pulp tissue and the re-development of the young permanent tooth root, making it easy to cause a tooth fracture. Besides, the development of the apical foramen of some young permanent teeth is hindered after treatment. The traditional apical induction method can only promote the formation of the apical mineralized tissue, and cannot produce the normal apical structure containing the pulp tissue, which leads to unsatisfactory long-term effects. After treatment of the affected teeth, the traditional filling material often discolors the crown, thus affecting its appearance [3]. A more ideal treatment to regenerate dental pulp is necessary for dental pulp disease.
The purpose of pulp regeneration is to form a functional dental pulp complex through the regeneration of dentin cells, reconstruction of the pulp blood flow, and innervation. The goal of pulp regeneration in young permanent teeth is to eliminate clinical symptoms, heal periapical lesions, regain pulp vitality, and strengthen the root canal wall.
In recent years, stem cell-based pulp regeneration has been developed as a new technology to treat pulp disease and has the potential to overcome many challenging clinical issues. Two decades ago, Gronthos et al. found that pulp contains stem cells and proposed the concept of dental pulp stem cells (DPSCs). DPSCs show self-renewal, proliferation, and multi-potent differentiation in vitro, and produce a dentin pulp complex after in vivo transplantation [4]. Research has shown that DPSCs possess a latent capacity to differentiate multi-directionally: for instance, differentiation into adipocytes, chondrocytes, osteoblasts, odontoblasts, nerve cells, and endothelial cells [5]. The findings reveal the possibility of extended application of DPSCs in tissue regeneration and cell therapy. Currently, DPSCs are a research focus in terms of tissue regeneration and engineering [6].
2 Pulp regeneration based on stem cell and tissue engineering techniques
The specific process of pulp regeneration based on stem cells and tissue engineering technology is to implant exogenous stem cells into the host root canal system to achieve pulp regeneration. The stem cells derived from allogeneic cells or hosts are isolated and amplified for pulp regeneration [7]. In the process of pulp regeneration, the biological scaffold materials can effectively maintain the survival of stem cells and vascular nerve regeneration. The microenvironment of pulp regeneration is also important for treatment. Stem cells can respond to changes in the microenvironment, undergo differentiation and division, and then repair tissue damage. Therefore, this article is aimed at reviewing the advances in stem cell-based pulp regeneration, summarizing the critical regenerative elements of stem cells, biomaterials, and the regenerative microenvironment, and finally discussing the barriers and prospects of the therapeutic application of stem cell-based pulp regeneration.
2.1 Dental pulp stem cells
Stem cells are partially differentiated cells that possess latent capacities to differentiate multi-directionally and to self-renew. In 2000, Gronthos et al. demonstrated the self-renewal ability DPSCs, which could be induced to differentiate into dentin-like cells from the third molar pulp group for the first time. This confirmed the existence of a stem cell population in dental pulp tissue [4]. DPSCs are undifferentiated mesenchymal cells with the ability of cell cloning and proliferation in pulp tissue. DPSCs have multiple differentiation potential and can differentiate into neuroectoderm-cells, adipocytes, odontoblasts, osteoblasts, chondrocytes, myoblasts, and other mesoderm-derived cells [8, 9]. DPSCs have a similar immunophenotype to bone marrow mesenchymal stem cells (BMSCs). Considering their plasticity, DPSCs have been used as seed cells in tissue engineering and regeneration in recent years [10, 11].
2.1.1 Proliferation and self-renewal ability
DPSCs have high proliferative ability and can be isolated and amplified from pulp tissue fragments attached to plastic Petri dishes and then transferred to other Petri dishes without interrupting cell proliferation. Stem cell characteristics are maintained in vitro for more than 20 generations. After adding bromodeoxyuridine to pulp tissue cultured in vitro, Gronthos found that DPSCs proliferated with higher hyperplasia than BMSCs[4]. A clone formation assay confirmed that DPSCs possessed strong self-renewal ability.
Some studies have used the gold standard, continuous in vivo transplantation, to validate DPSCs [12]. Human DPSCs in hydroxyapatite-tricalcium phosphate (HA/TCP) ceramic particles were implanted subcutaneously into nude mice for 6 weeks and formed dentin pulp-like tissue expressing the dentin-specific protein, dentin sialophosphoprotein, and dentine sialophosphate protein (DSPP). These mesenchymal cells in the transplants were then harvested and amplified in vitro and transplanted into nude mice again. These cells also produced human Alu positive dentin cells and dentin pulp complexes, indicating the ability of DPSCs to renew themselves in vivo.
2.1.2 Multidirectional differentiation potential
DPSCs have multiple differentiation potential under different induction conditions. A study of osteogenic differentiation showed that the DPSCs could differentiate into osteoblasts in vitro and in vivo under specific mineralization conditions. After subcutaneous transplantation into immunocompromised mice for 4 weeks, DPSCs generated well-developed plate bone with bone cells in the bone lacunae. After lipogenic induction for 5 weeks, DPSCs generated lipid droplets and expressed two specific transcription factors in adipocytes [13]. DPSCs have the potential to differentiate into nerve cells. Researchers determined that DPSCs express glial fibrillary acidic protein and nestin, which provide nutritional support for dopamine-powered neurons. Importantly, after in vivo transplantation, DPSCs could generate odontoblast-like cells and pulp-like tissue, suggesting their potency for dental pulp regeneration [14]. DPSCs derived from deciduous teeth also possess robust regeneration capacities. Several studies found that stem cells from underage individuals have a stronger regeneration ability than adult MSCs, suggesting an alternative seed cell source in dental pulp regeneration [15].
2.2 Selection of biomaterials for dental pulp regeneration
The selection of a suitable scaffold material is essential for the success of pulp regeneration. The scaffolds commonly used in dental pulp tissue engineering can be divided into two categories: Natural scaffolds and artificial polymers.
The natural scaffolds used for pulp regeneration mainly include recombinant collagen and silk fibroin. Prescott et al. inoculated DPSCs onto a dentin matrix protein 1 (DMP1)-collagen scaffold and placed it into a dental implant in mice [16]. The formation of pulp-like tissue was observed six weeks later. Recently, platelet-rich plasma (PRP), platelet-rich fibrin, and blood clots have been used as scaffolds for pulp regeneration [17,18,19].
Artificial polymer scaffolds for dental pulp regeneration are mainly polyglycolic acid (PGA), polylactic acid (PLA), and poly lactic-co-glycolic (PLAGA). The selection of artificial polymerized porous scaffolds for stem cell survival and vascular and nerve regeneration is crucial for pulp regeneration. To reconstruct the pulp tissue, the scaffold material needs to have excellent biodegradability, such that the newly formed tissue can replace the original degraded scaffold to form a regenerative structure. Scaffold materials should also have appropriate pore size to facilitate the entrance of cell nutrients and oxygen [20]. The scaffold can also carry growth factors that are beneficial for stem cell proliferation, differentiation, and adhesion [21].
2.3 The micro-environment of pulp regeneration
The survival, proliferation, and differentiation of DPSCs in vivo depends on the appropriate microenvironment. Establishing a microenvironment conducive to regenerative process is the key to pulp regeneration.
2.3.1 Cytokines and signaling molecules
Cytokines and signaling molecules in the stem cell niche are essential for the proliferation, differentiation, and migration of DPSCs [22]. Suitable signaling molecules play a critical role in the regeneration of dental pulp. Several cytokines and signaling molecules have been applied in dental-pulp regeneration. A pulp cavity implanted with growth factors or chemokines guides homing cells more easily and promotes the regeneration of pulp tissue [7]. Furthermore, cytokines that induce pulp angiogenesis are also helpful for the survival of stem cell transplants [23, 24]. Some common cytokines and signaling molecules are described below (Table 1).
2.3.2 Inflammation
Inflammation is considered an important factor to induce pulp regeneration [25]. During the recovery process, inflammation stimulates DPSCs hidden in the blood vessels to migrate to the injured location, proliferate, and eventually differentiate into endothelial cells, which can form neovascularization [26]. Inflammation also promotes the regeneration course of dental pulp vessels by inducing the expression of basic fibroblast growth factor, vascular endothelial growth factor (VEGF), and transforming growth factor β (TGF-β) in the damaged pulp cells and endothelial cells [27,28,29]. Pulp cells isolated from dental caries strongly expressed vascular factors and can be used as seed cells for pulp vascular regeneration, suggesting that pulp cells in the inflammatory environment can guide vascular regeneration [30]. These studies implied that inflammation facilitates stem cell migration and differentiation, and enhances the expression of angiogenic factors, which helps to regenerate blood vessels.
2.3.3 Oxygen supply
DPSCs showed faster proliferation, a higher vascular formation ability, and a stronger vascular factor secretion ability when cultured under hypoxic conditions. Cultivated DPSCs and periodontal ligament stem cells in a hypoxic environment possess robust proliferation and differentiation abilities [31]. Hypoxia-inducible factor 1 (HIF-1) is expressed rapidly in DPSCs cultured in a hypoxic environment and regulates the expression of various vascular factor-related genes, including placental growth factor (PIGF), angiogenin, platelet-derived growth factor (PDGF), and vascular endothelial growth factors (VEGF). Meanwhile, HIF-1 also regulates stromal cell-derived factors, sphingosine phosphate, and their receptors [32]. When new angiogenesis and oxygen levels reverse to customary, HIF-1 expression declines, resulting in decreased expression of a series of vascular factors [24, 33]. The experiments above showed that DPSCs have an enhanced vascular formation ability in a hypoxic environment, demonstrating that a hypoxic environment might be one of the most crucial conditions for pulp vascular regeneration.
2.3.4 Dental pulp revascularization
Blood transport reconstruction and vascular regeneration are essential for pulp regeneration. Dental pulp revascularization refers to the reintroduction of blood vessels in the root canal system. Pulp blood circulation regeneration promotes the regeneration of active pulp-like tissue in the root canal and assists the root to develop. The core of pulp revascularization is to provide a suitable matrix (such as an autologous blood clot or platelet-rich plasma) and concentrated growth factors to facilitate vascular regeneration [34].
2.3.5 Nerve regeneration
Dental pulp nerve regeneration can make the teeth respond protectively when they are stimulated by mechanical, temperature, or chemical elements, and thus can maintain the long-term survival of teeth. DPSCs also possess the potential to express nestin, a neuroepithelial stem cell surface marker, and to differentiate neuronally, which allows the generation of glial cells and neurons. Woods et al. confirmed that DPSCs have a special effect on the treatment of adult brain injury [35]. Studies have shown that the utilization of certain neurotrophic drugs and electrical stimulation positively increase the ability of nerve regeneration. Thus, the application of nerve induction conditions in stem cell masses and local injection of nerve regeneration drugs might represent a novel research direction in pulp regeneration.
3 Basic research and the clinical application of dental pulp regeneration
Currently, mesenchymal stem cells that are known to have differentiation potential include DPSCs, dental papilla stem cells, dental sac stem cells, bone marrow stromal stem cells, exfoliated deciduous tooth pulp stem cells, and other dentin-like cells. Among these stem cells that can be exploited for the regeneration of dental pulp, dentin, and periodontal ligament, DPSCs are discussed most frequently in related research. As eminently self-renewable, multiphase differentiating, and proliferative cells, DPSCs occupy an essential position in the regenerating and healing process of the dental pulp. We discuss the application of DPSCs in dental pulp regeneration from two perspectives, cell homing and stem cell transplantation, in the following section.
3.1 Basic research on pulp regeneration
3.1.1 Stem cell transplantation
Research has shown that dental pulp or other stem cell transplantation can produce ectopic pulp-like tissue [36,37,38,39,40]. Moreover, the number of transplanted cells can be controlled and the cell subspecies with the best potential efficacy for pulp regeneration can be selected. DPSCs and stem cells from human exfoliated deciduous teeth (SHED) are the most widely used mesenchymal stem cell (MSC) seed cells. There are four main applications of exogenous MSC transplantation for pulp regeneration: (1) Simple MSC transplantation and organic or synthetic scaffolds; (2) MSC co-transplantation with microvascular endothelial cells to provide additional vascularization; (3) MSC pretreatment or transplantation in combination with growth factors; and (4) Transplantation with self-organized MSC aggregates or granules [39, 41].
Preliminary studies by Kuo et al. and Iohara et al. showed that implantation of DPSCs with bone morphogenetic protein 2 (BMP2) into incisors of canine teeth achieved histologically effective pulp regeneration [40, 42]. A follow-up study conducted by the same team in 2009 tested pulp regeneration using CD31-, CD146- subtypes of SP cells after complete pulp cutting. The pulp regeneration potential of piglet autogenous DPSCs in the jaw micro-environment was tested in Kodonas et al. in 2012 [43], which confirmed that DPSCs can successfully regenerate pulp-like tissue with characteristics similar to normal pulp morphology. Iohara et al. showed that the cell layer had the typical columnar morphology of polarized dentin cells and extended to the dentine tubules to the cementum–enamel junction [44].
3.1.2 Cell homing
Cell homing represents an alternative option for dental pulp regeneration. Stem cell homing can supply host endogenous cells to the injured tissue [45]. Cell homing in pulp regeneration refers to the recruitment of host endogenous stem cells into tooth root canals by the induction of signal molecules to form pulp-tooth-like tissues. Cell homing avoids the cell processing steps needed for cell transplantation and makes full use of the patient's stem cells, which to some extent reduces the difficulty and risk of operation [46].
In recent studies, stem cell homing was induced through apical hemorrhage. For immature dead pulp teeth, inducing bleeding can cause a large number of undifferentiated mesenchymal stem cells to pour into the root canal [47]. The new tissue that grows into the root canal is periodontal ligament-like, odontoid, or bone-like, which may be related to stem cells derived from residual living pulp and the apical papilla [48].
Other cell-independent methods applied growth factors and cytokines to induce the migration of endogenous stem cells into the root canal. For example, granulocyte colony-stimulating factor (G-CSF) was used to stimulate the mobilization of DPSCs to promote DPSCs-based pulp regeneration [15], suggesting that autologous cell therapy is feasible. Although the therapeutic effect still needs to be confirmed in further studies, its feasibility was proven by the recent proof-of-concept research, indicating a broad prospect of clinical application [49].
3.2 Clinical application of pulp regeneration
Some progress has been made in animal experiments, after which several pioneer studies have been performed to explore the possibility of stem cell-based dental pulp regeneration in the clinic. In 2017, Nakashima et al. performed in situ transplantation of DPSCs in five patients with irreversible pulpitis and monitored them for up to 24 weeks [50]. Using cone-beam computed tomography and magnetic resonance imaging, experts confirmed that three of the five patients showed complete pulp regeneration and dentin formation. Besides, four of the five patients were positive for electric pulp testing (EPT) at 24 weeks after follow-up, indicating that the regenerated pulp restored nerve activity.
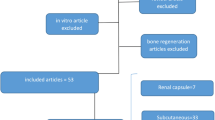
One randomized clinical trial using SHED aggregates for functional pulp regeneration in population cavities in 2018 provided stronger evidence (Fig. 1) [51]. In that study, children aged 7 to 12 years old with traumatic teeth received transplantation of the aggregates of autologous SHEDs. The trial included 36 patients with pulp necrosis, including 26 in the treatment group and 10 in the control group. All patients were followed up for 12 months. Xuan et al. found that SHED transplantation regenerated the entire pulp tissue with blood vessels (as demonstrated using laser Doppler flowmeter) and nerves (as shown by radiological and histological examination) [51]. A more important clinical finding was that the root length of immature permanent teeth and the width of the apical foramen decreased after SHED implantation, which indicated that the regenerated pulp has the normal function of maintaining the continuous development of the root. Studies by Nakashima and Xuan et al. reported that pulp stem cell application has no adverse effect or toxicity, which confirms their clinical safety [51, 52]. These results provide the first clinical evidence that pulp stem cell transplantation can regenerate complete pulp tissue with physiological and neurovascular functions.
4 Key steps of pulp stem cell transplantation
To achieve the ultimate goal of pulp regeneration, many possible factors should be taken into consideration besides the difficulty of reconstructing dental pulp. Considering the possible effects of mechanical instruments and chemical disinfection preparations on subsequent pulp regeneration, the preparation of the pulp cavity and procedure of stem cell transplantation are also important. The process of pulp stem cell transplantation includes opening pulp to expose the root canal and remove the infected pulp. Followed by selecting the reagent for washing and the sealing agent, washing and disinfecting the root canal, and seal to ensure that the pulp cavity cannot be infected. After preparation, the cultured DPSCs are implanted into the internal root canal cavity, and the crown is closed using materials selected to prevent the infection of the DPSCs. The following sections will focus on the treatment of the pulp cavity, root canal preparation, disinfection, flushing agent selection, selecting materials for crown sealing.
4.1 Treatment of the dental pulp cavity
The primary manipulation for successful pulp vascular regeneration is to thoroughly eliminate microbes and necrotic tissue from the pulp cavity. Under local anesthesia, dentists exploit a rubber barrier to isolate pulpitis teeth and then eradicate the saprophytic pulp, followed by careful meterage of the working length of the root canal. After repeatedly rinsing the pulp cavity, doctors stretch the sterile paper tip to dry the pulp cavity, add the root canal medicine, and then seal the tooth temporarily for 2–6 weeks. Full and thorough root canal disinfection, microenvironmental matrix promotion (inducing blood clot formation), and tight crown closure are essential elements for new tissues to generate in the root canal.
4.2 Root canal washing
Flushing plays an important role in primary root canal disinfection. Flushing agents should have the greatest bactericidal and bacteriostatic effect and have the least cytotoxicity to stem cells and fibroblasts to maintain their vitality, proliferation, and differentiation abilities. At present, there are several kinds of flushing agents in clinical application:(1) hydrogen peroxide: Its foaming effect can remove root canal exudation and necrotic tissue, and has a hemostatic function. (2) Sodium hypochlorite: Sodium hypochlorite is the most extensively applied root canal flushing AGENT, which can dissolve the organic material in the saprophytic pulp tissue and smear layer, combined with high-efficiency bacteriostasis and low cytotoxicity [53]. (3) Chlorhexidine has a broad-spectrum antibacterial effect, similar to sodium hypochlorite. In particular, it can inhibit gram-positive bacterial infection. (4) Ethylenediamine Tetraacetic Acid (EDTA) is a potent chelating agent and is regularly used in combination with other flushing agents, such as sodium hypochlorite solution, in clinical application. Chelation using metal ions is necessary for bacterial growth; therefore, it has certain anti-microbial properties [54,55,56,57,58,59,60,61,62,63]. (5) MTAD is a new type of root canal flushing solution, which comprises doxycycline, citric acid, and detergent (polysorbitol ester-80). MTAD can effectively remove the smear layer, does not destroy the physical properties of dentin, and has a strong anti-microbial effect.
However, several studies found that the flushing agents can affect the regeneration of DPSCs. Ring et al. found that sodium hypochlorite is not biocompatible and will kill DPSCs, preventing their adhesion to the root canal surface [56]. Chen et al. supported the view that if sodium hypochlorite is used in dental pulp vascular regeneration, it should be mixed with normal saline to remove the residual toxicity that can weaken the regeneration reaction [58]. A recent study has showed that chlorhexidine irrigation has cytotoxic influences on human stem cells and interferes with the association of DPSCs to the pulp cavity wall. In addition to root canal flushing fluid, root canal irrigation also affects the success rate of pulp vascular regeneration [56]. Da et al. recommended using the Endovac system for sodium hypochlorite root tip negative pressure washing [59]. Nielsen et al. believed that negative pressure flushing of the Endovac system can break the gas plug in the apical region (the root canal is 3 mm from the root tip and a mass of air column produced by the hydrolysis of organic tissue by sodium hypochlorite solution), thus removing the debris [60].
4.3 Preparation of the root canal system
Many scholars agree that there is no need for mechanical preparation of the root canal system in the treatment of pulp revascularization, to maintain stem cell activity and avoid weakening the root wall [61,62,63,64,65]. Mechanical preparation might also destroy stem cells present in the apical region [66,67,68]. Chen et al. also supported the use of minimal root canal preparation in the root canal crown to facilitate the elimination of saprophytic pulp, thus ensuring no interference with pulp vascular regeneration [69]. However, it remains a challenge to transplant exogenous stem cells into the narrow and curved root canal. Novel equipment for stem cell transplantation is necessary to facilitate such surgery.
4.4 Disinfection of the root canal
Endodontic disinfection drugs affect the success of pulp vascular regeneration. Usually, Hoshino paste, consisting of tri-antibiotics minocycline, ciprofloxacin, and metronidazole, is recommended as a root canal disinfectant [70]. Comparative studies using these antibiotics, alone or in combination, found that for infected dentin and pulp periapical lesions, combined use can have a continuous bactericidal effect on all bacterial samples. Moreover, Sato et al. believed that removing the bacteria in the necrotic pulp of young permanent teeth created an environment conducive to the regeneration of blood vessels and cells [71]. However, the side effects, including discoloration of the crown, bacterial resistance, and allergic reactions, limited their application [72].
Calcium hydroxide has been used successfully used in general root canal disinfection. Compared with other antibiotic pastes, calcium hydroxide has antibacterial properties, does not discolor teeth, and promotes growth factors and biomolecular secretion from dentin. Several studies demonstrated that calcium hydroxide can promote root elongation, root canal wall thickening, and pulp vascular regeneration [72]. However, some studies suggested that the high PH value of calcium hydroxide could kill the stem cells in the remaining pulp or apical papilla, or change the nature of dentin [73, 74].
4.5 Root canal coronal sealing
To avoid the reinfection of the root canal caused by bacteria and to achieve pulp vascular regeneration, strict coronal sealing filling and sealing should be carried out [73]. According to the advice of most scholars, double-layer materials can be closed above blood clots, such as coronal filling using mineral trioxide aggregate (MTA) and resin bonding [62, 65, 69, 75,76,77,78]. Some scholars also recommend the sealing of three layers of materials, i.e., adding a layer of glass ions between the MTA and the composite resin as the second layer of closed materials, to seal the coronal more closely [79]. MTA is often in direct contact with the dental pulp tissue and blood clots within the root canal, reproduce organization in dental pulp vascular regeneration surgery, has beneficial biocompatibility, sealing stability, and strong bacteriostasis, as well as good hydrophilicity and unique hard-solid properties. However, the curing time of MTA is long, the cost is high, and the tooth tissue is easily discolored. Khetarpal et al. suggested the use of tricalcium silicate (Biodentine) instead of MTA placed above the blood clot [80]. Biodentine is a kind of dentin substitute with good biological activity and sealing characteristics. The time required to achieve hard fixation is shorter than that of MTA, the clinical operation process is simpler and the possibility of the color change of the coronal is avoided.
5 Summary
Using regenerative dental pulp therapy, it is possible to restore the intact structure of the affected teeth. The three-dimensional dental pulp-like structure formed by DPSCs could be transplanted in vivo and provides an induced micro-environment for blood flow reconstruction and nerve regeneration, thus ensuring the survival and function of the transplanted tissue. To achieve successful dental pulp regeneration, suitable seed cells, a scaffold, and a regenerative microenvironment are necessary to ensure the survival, migration, proliferation, and differentiation of stem cells. Moreover, the preparation method of a root canal, the selection of disinfection preparation, physical and chemical washing methods, and the selection of washing preparation will lay a good foundation for the successful transplantation of stem cells in the clinic. The most challenging issue is how to optimize and integrate each composition process into a whole, and finally realize the regeneration of the pulp dentin complex. Thus, a multi-disciplinary and comprehensive research process is necessary for future dental pulp regeneration.
References
Ng YL, Mann V, Gulabivala K. Tooth survival following non-surgical root canal treatment: a systematic review of the literature. Int Endod J. 2010;43:171–89.
Torabinejad M, Corr R, Handysides R, Shabahang S. Outcomes of nonsurgical retreatment and endodontic surgery: a systematic review. J Endod. 2009;35:930–7.
Fonzar F, Fonzar A, Buttolo P, Worthington HV, Esposito M. The prognosis of root canal therapy: a 10-year retrospective cohort study on 411 patients with 1175 endodontically treated teeth. Eur J Oral Implantol. 2009;2:201–8.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56:709–21.
El Backly RM, Marei MK. Dental pulp stem cells in tissue engineering and regenerative medicine: Opportunities for translational research. In: El-Badri N, editor. Advances in stem cell therapy. Stem cell biology and regenerative medicine. Humana Press, Cham; 2017. p. 171-96.
Luo L, He Y, Wang X, Key B, Lee BH, Li H, et al. Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells Int. 2018;2018:1731289.
D'Alimonte I, Nargi E, Lannutti A, Marchisio M, Pierdomenico L, Costanzo G, et al. Adenosine A1 receptor stimulation enhances osteogenic differentiation of human dental pulp-derived mesenchymal stem cells via WNT signaling. Stem Cell Res. 2013;11:611–24.
Gronthos S, Arthur A, Bartold PM, Shi S. A method to isolate and culture expand human dental pulp stem cells. Methods Mol Biol. 2011;698:107–21.
Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs those from other sources their biology and role in regenerative medicine. J Dent Res. 2009;88:792-806.
Li JH, Liu DY, Zhang FM, Wang F, Zhang WK, Zhang ZT. Human dental pulp stem cell is a promising autologous seed cell for bone tissue engineering. Chin Med J (Engl). 2011;124:4022–8.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d'Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004;19:2388–98.
Sui BD, Hu CH, Zheng CX, Jin Y. Microenvironmental views on mesenchymal stem cell differentiation in aging. J Dent Res. 2016;95:1333–40.
Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421–6.
Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. J Endod. 2011;37:265–8.
Shivashankar VY, Johns DA, Maroli RK, Sekar M, Chandrasekaran R, Karthikeyan S, et al. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex a triple blind randomized clinical trial. J Clin Diagn Res. 2017;11:ZC34-9.
Gaviño Orduña JF, Caviedes-Bucheli J, Manzanares Céspedes MC, Berástegui Jimeno E, Martín Biedma B, Segura-Egea JJ, et al. Use of platelet-rich plasma in endodontic procedures in adults: regeneration or repair? a report of 3 cases with 5 years of follow-up. J Endod. 2017;43:1294–301.
Kim HJ, Kim UJ, Leisk GG, Bayan C, Georgakoudi I, Kaplan DL. Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromol Biosci. 2007;7:643–55.
Yuan Z, Nie H, Wang S, Lee CH, Li A, Fu SY, et al. Biomaterial selection for tooth regeneration. Tissue Eng Part B Rev. 2011;17:373–88.
Yamada Y, Nakamura-Yamada S, Umemura-Kubota E, Baba S. Diagnostic cytokines and comparative analysis secreted from exfoliated deciduous teeth, dental pulp, and bone marrow derived mesenchymal stem cells for functional cell-based therapy. Int J Mol Sci. 2019;20:5900.
Aranha AM, Zhang Z, Neiva KG, Costa CA, Hebling J, Nör JE. Hypoxia enhances the angiogenic potential of human dental pulp cells. J Endod. 2010;36:1633–7.
Zimna A, Kurpisz M. Hypoxia-inducible Factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015:549412.
Goldberg M, Njeh A, Uzunoglu E. Is pulp inflammation a prerequisite for pulp healing and regeneration? Mediators Inflamm. 2015;2015:347649.
Yamamura T. Differentiation of pulpal cells and inductive influences of various matrices with reference to pulpal wound healing. J Dent Res. 1985;64:530-40.
Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013–6.
Mathieu S, El-Battari A, Dejou J, About I. Role of injured endothelial cells in the recruitment of human pulp cells. Arch Oral Biol. 2005;50:109–13.
Tran-Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. J Dent Res. 2006;85:819–23.
Alkharobi H, Beattie J, Meade J, Devine D, El-Gendy R. Dental pulp cells isolated from teeth with superficial caries retain an inflammatory phenotype and display an enhanced matrix mineralization potential. Front Physiol. 2017;8:244.
Zhou Y, Fan W, Xiao Y. The effect of hypoxia on the stemness and differentiation capacity of PDLC and DPC. Biomed Res Int. 2014;2014:890675.
Zhou J, Sun C. SENP1/HIF-1α axis works in angiogenesis of human dental pulp stem cells. J Biochem Mol Toxicol. 2020;34:e22436.
Yuan C, Wang P, Zhu L, Dissanayaka WL, Green DW, Tong EH, et al. Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Eng Part A. 2015;21:1163–72.
Vijayaraghavan R, Mathian VM, Sundaram AM, Karunakaran R, Vinodh S. Triple antibiotic paste in root canal therapy. J Pharm Bioallied Sci. 2012;4:S230–3.
Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009;59:150–7.
Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33:2109–18.
Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17:1911–20.
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–15.
Iohara K, Zheng L, Ito M, Ishizaka R, Nakamura H, Into T, et al. Regeneration of dental pulp after pulpotomy by transplantation of CD31(-)/CD146(-) side population cells from a canine tooth. Regen Med. 2009;4:377–85.
Kuo TF, Huang AT, Chang HH, Lin FH, Chen ST, Chen RS, et al. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. J Biomed Mater Res A. 2008;86:1062–8.
Sui B, Chen C, Kou X, Li B, Xuan K, Shi S, et al. Pulp stem cell-mediated functional pulp regeneration. J Dent Res. 2019;98:27–35.
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–5.
Kodonas K, Gogos C, Papadimitriou S, Kouzi-Koliakou K, Tziafas D. Experimental formation of dentin-like structure in the root canal implant model using cryopreserved swine dental pulp progenitor cells. J Endod. 2012;38:913–9.
Iohara K, Murakami M, Nakata K, Nakashima M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol. 2014;52:39–45.
Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440–8.
Kim SG, Zheng Y, Zhou J, Chen M, Embree MC, Song K, et al. Dentin and dental pulp regeneration by the patient’s endogenous cells. Endod Topics. 2013;28:106–17.
Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133–8.
Wang X, Thibodeau B, Trope M, Lin LM, Huang GT. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36:56–63.
Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32:3189–209.
Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, et al. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 2017;8:61.
Xuan K, Li B, Guo H, Sun W, Kou X, He X, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10:eaaf3227.
Yang J, Yuan G, Chen Z. Pulp regeneration: current approaches and future challenges. Front Physiol. 2016;7:58.
Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98.
Srivastava N, Chandra S. Effect of endodontic smear layer and various solvents on the calcium ion diffusion through radicular dentin–an in vitro study. J Indian Soc Pedod Prev Dent. 1999;17:101–6.
Aktener BO, Bilkay U. Smear layer removal with different concentrations of EDTA-ethylenediamine mixtures. J Endod. 1993;19:228–31.
Ring KC, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. The comparison of the effect of endodontic irrigation on cell adherence to root canal dentin. J Endod. 2008;34:1474–9.
Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37:1109–15.
Chen SC, Chueh LH, Hsiao CK, Wu HP, Chiang CP. First untoward events and reasons for tooth extraction after nonsurgical endodontic treatment in Taiwan. J Endod. 2008;34:671–4.
da Silva LA, Nelson-Filho P, da Silva RA, Flores DS, Heilborn C, Johnson JD, et al. Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs’ teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:779–87.
Nielsen BA, Craig Baumgartner J. Comparison of the EndoVac system to needle irrigation of root canals. J Endod. 2007;33:611-5.
Gelman R, Park H. Pulp revascularization in an immature necrotic tooth: a case report. Pediatr Dent. 2012;34:496–9.
Kim DS, Park HJ, Yeom JH, Seo JS, Ryu GJ, Park KH, et al. Long-term follow-ups of revascularized immature necrotic teeth: three case reports. Int J Oral Sci. 2012;4:109–13.
Cehreli ZC, Sara S, Aksoy B. Revascularization of immature permanent incisors after severe extrusive luxation injury. Tex Dent J. 2012;129:675–81.
Dabbagh B, Alvaro E, Vu DD, Rizkallah J, Schwartz S. Clinical complications in the revascularization of immature necrotic permanent teeth. Pediatr Dent. 2012;34:414–7.
Forghani M, Parisay I, Maghsoudlou A. Apexogenesis and revascularization treatment procedures for two traumatized immature permanent maxillary incisors: a case report. Restor Dent Endod. 2013;38:178–81.
Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod. 2011;37:562–7.
Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod. 2009;35:745–9.
Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008;34:919-25.
Chen MY, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, Lin LM. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 2012;45:294–305.
Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–30.
Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118–24.
Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343–9.
Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200.
Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–7.
Cehreli ZC, Sara S, Aksoy B. Revascularization of immature permanent incisors after severe extrusive luxation injury. J Mich Dent Assoc. 2013;95:58–62.
Soares Ade J, Lins FF, Nagata JY, Gomes BP, Zaia AA, Ferraz CC, et al. Pulp revascularization after root canal decontamination with calcium hydroxide and 2% chlorhexidine gel. J Endod. 2013;39:417–20.
Aggarwal V, Miglani S, Singla M. Conventional apexification and revascularization induced maturogenesis of two non-vital, immature teeth in same patient: 24 months follow up of a case. J Conserv Dent. 2012;15:68–72.
Garcia-Godoy F, Murray PE. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol. 2012;28:33–41.
Kottoor J, Velmurugan N. Revascularization for a necrotic immature permanent lateral incisor: a case report and literature review. Int J Paediatr Dent. 2013;23:310–6.
Khetarpal A, Chaudhary S, Talwar S, Ravi R, Verma M. Revascularization of immature permanent tooth with periapical lesion using a new biomaterial-A case report. 2013;1:20-2.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFA0104800), the Fundamental Research Funds for the Central Universities (YJ201878), Technology Innovation Research and Development Project of Chengdu (2019-YF05-00705-SN), Key Project of Sichuan province (2019YFS0311, 2019YFS0515), and the Nature Science Foundation of China (81600912, 31601113). The authors gratefully acknowledge all the faculties of the State Key Laboratory of Oral Diseases and Ministry of Education & National Engineering Laboratory for Oral Regenerative Medicine, West China Hospital of Stomatology, Sichuan University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declaed that there is no conflicts of interest.
Ethical Statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hua-Nien Lee and Cheng Liang contribute to this article equally.
Rights and permissions
About this article
Cite this article
Lee, HN., Liang, C., Liao, L. et al. Advances in Research on Stem Cell-Based Pulp Regeneration. Tissue Eng Regen Med 18, 931–940 (2021). https://doi.org/10.1007/s13770-021-00389-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00389-2