Abstract
The management of pathological conditions of the dental pulp is mainly based on the disinfection of root canal system followed by the replacement of the infected or inflamed pulp tissues by inert materials. In immature teeth, the routine root canal treatment often limits the completion of root formation and protection against external root resorption. Hence, alternative biologically based therapies that allow dental pulp regeneration is of high interest for clinicians. Tissue engineering is a multi-disciplinary field focused on the development of materials, techniques and strategies to improve or replace damaged or lost biological functions and tissues. New and exciting perspectives for the development of approaches for the clinical management of dental pulp ere opened by proving that some types of dental stem cells, such as SHED and DPSC, can differentiate into odontoblasts in vivo. This chapter presents recent advances and promising approaches in regeneration of pulp. It also address the future challenges to overcome to make pulp regeneration available in clinic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Dental pulp is a highly vascularized connective tissue encapsulated in mineralized structure formed by enamel, dentin and cementum . It is responsible to keep the homeostasis of the tooth organ and possesses highly responsive sensory nervous system that detects unhealthy stimuli [1]. The apical foramen is the opening at the apex of the root that provides the dental pulp with blood supply for oxygen, nutrients and cells for defence. This limited accessibility and inflexible surroundings makes it difficult for the immune system to eradicate infections in the tissue owing to the lack of a collateral blood supply [2].
Clinically, odontoblasts may survive and continue to produce dentin to protect the pulp in cases of injuries by mild caries, moderate attrition or erosion [3]. On the other hand, dental pulp exposure due to fracture, caries in deep dentin or where the progression of the lesion is aggressive, the survival of primary odontoblasts is frequently compromised demanding root canal treatment . This therapy often involves total pulp extirpation and disinfection followed by compaction of a material within the root canal [4]. Though this is a well-established approach, it is based on the substitution of host tissues for synthetic, and in most of the cases, inert materials [5]. In immature teeth, the routine root canal treatment can allow for infection control, but often does not facilitate the completion of root formation or protects against external root resorption [6]. In these teeth, the root canal treatment is even more challenging due to large apical opening which can have a divergent configuration and does not provide the mechanical stop necessary to confine the filling material within the root canal [6].
Although the success rate of endodontic treatments is relatively high [7], this modality of treatment present some shortcomings such as the high sensitivity to obstruction of the root canal space, breakage of instruments in the canals and perforations [8, 9]. The root canal instrumentation removes significant amount of tooth structure that results in thin dentin walls and large pulp chamber making the remaining structure weaker. Moreover, the devitalization may increase root brittleness increasing the chances of postoperative fracture [10, 11]. In addition, pulpless teeth do not have the ability to sense the aggressions arising from caries. In fact, endodontically treated teeth loss is higher than non-treated ones due to secondary caries [12].
The shift towards the regenerative endodontics may have started with the advent of vital pulp therapy. Clinically, it is based on the indirect pulp treatment in cases of deep dentinal cavities and direct pulp capping where the pulp tissue is exposed [13]. Indirect pulp capping is the procedure wherein the deepest layer of affected dentin is covered with a thin layer of protective agent in order to prevent pulp exposure [13]. In the direct pulp capping, the exposed vital pulp is covered with a protective dressing (such as calcium hydroxide and mineral trioxide aggregate) directly applied over the exposure site to prevent further injury and to stimulate dentin-pulp complex regeneration [14, 15].
One of the methods to treat the pulp of immature teeth is the apexification . This relies on the formation of an apical barrier to close the open apex [4]. For this, calcium hydroxide (Ca(OH)2) must be inserted in the root canal and replaced periodically to stimulate the formation of a calcified structure. Although this strategy has been used clinically for long time, the outcomes are largely unpredictable and fracture rates of nearly 25 % have been reported after long-term Ca(OH)2 treatment due to changes in mechanical properties of dentin [14]. Alternatively, apexification strategies include use of mineral trioxide aggregate (MTA ) that is a material with osteogenic, cementogenic and odontogenic potentials that can be used for perforation repairs, pulp capping and pulpotomy [4]. Unfortunately, apexification by either Ca(OH)2 or MTA may prevent further development of the root [14, 15]. This is specially complex in immature tooth where the apexification does not stimulate the reestablishment of a functional pulp tissue or the continued root development [16].
In existing endodontic treatment, teeth lose substantial amount of sound structure and their ability to detect the secondary infections. There has been no superior synthetic material to replace natural pulp and dentin in terms of chemical composition, physical, mechanical and biological properties. Thus, current research in endodontics has extended its focus on the development of biological therapies that are more biologically effective and mechanically reliable than traditional pulp therapies. Hence, the regeneration of pulp-dentin complex via tissue engineering has the potential to restore tooth functions compromised by the loss of pulp vitality.
Dental Pulp Tissue Engineering
Dental Stem Cells
Stem cells are undifferentiated cells the can differentiate into specialized cells. These cells are responsible for normal tissue renewal, healing and regeneration after injuries [5, 17]. Multipotent stem cells are able to cross lineage boundaries and differentiate into multiple, but limited number of cell types. For instance, a multipotent blood stem cell is a hematopoietic cell that can differentiate into several types of blood cell types (such as neutrophils and lymphocytes) but cannot differentiate into brain cells, bone cells or other non-blood cell types [17].
The oral environment harbours several types of stem cells [5, 17, 18]. The stem cells from oral sources can be considered a population of mesenchymal stem cells (MSCs) as they present positive expression of STRO-1, Nanog, Oct4, CD73, CD90, CD105, CD106, CD146 and others [19–25]. These cells are capable to differentiate into many types of specialized cells such as adipocytes, neurons, osteoblasts and myocytes. For dental pulp regeneration, the potentially suitable cell are those directly derived from pulp tissue or from the precursor of dental pulp [17, 18, 20, 26, 27].
The apical papilla refers to the soft tissue at the apices of developing permanent teeth that is source of undifferentiated cells in the process of root development [24]. This population of stem cells from the apical papilla (SCAP ) can be only obtained in a limited window of time as after the formation of the crown the dental papilla becomes the dental pulp [24, 28]. The apical portion of the dental papilla is loosely attached to the apex of the developing root and it is separated from the differentiated pulp tissue by a cell rich zone [29]. As the apical papilla is poorly vascularized, the perivascular niche does not appear to be a major source of SCAP [24, 28].
It is possible that SCAP are derived from neural crest cells or at least associated with them [24]. They present positive expression of CD73, CD90, CD105, CD146 and other MSCs markers [24, 28, 30]. SCAP are capable to express positive staining for neural markers such as neurofilament M, neuronal nuclear antigen, nestin and βIII tubulin after being cultured with neurogenic medium for 4 weeks [24]. These cells can also accumulate calcium when cultured under osteogenic medium. Moreover, subpopulations of SCAP with positive expression of STRO-1–positive co-expressed several dentinogenic markers such as bone sialoprotein (BSP) , osteocalcin (OCN) and matrix extracellular phosphoglycoprotein (MEPE) after stimulation with odontogenic conditioning medium [24].
Dental pulp stem cells (DPSC) were firstly derived by enzymatic digestion of dental pulp from adult human impacted third molars in 2000 [31]. These highly proliferative cells have their niche localized in the perivascular and perineural sheath areas and are negative for the odontoblast-specific marker dentin sialophosphoprotein, suggesting an undifferentiated phenotype [23, 31, 32]. DPSC have gene expression profiles similar to bone marrow stem cells (BMSC) for more than 4000 genes [33]. In fact, both BMSC and DPSC present similar expression for several markers such as fibroblast growth factor 2, alkaline phosphatase, type I collagen, osteocalcin, α-smooth muscle actin, vascular cell adhesion molecule 1 and others. Nonetheless, some markers such as bone matrix protein, bone sialoprotein is positive at low levels in BMSC and negative in DPSC [31]. DPSC also express neuronal and glial cell markers, which may be related to their neural crest-cell origin [34].
DPSC can differentiate into multiple cell lineages (e.g. adipocytes, chrondocytes, β cells of islet of pancreas) making these cells a versatile and interesting model for tissue engineering and regeneration [31, 35–38]. DPSC also have the potential to induce angiogenesis/vasculogenesis as a subfraction of a side population was identified to be positive for CD31, CD146, CD34 and vascular endothelial growth factor-2 (VEGF-2 ) . The transplantation of these cells into mouse ischemia resulted in high density of capillary formation and increase in the blood flow into the graft [39]. DPSC express various neural markers making them an interesting model for neural differentiation [38]. The differentiation of DPSC into glial cells and neurons was also confirmed by the upregulation of neural gene markers (GFAP and βIII-tubulin) in the presence of FGF and epidermal growth factor [40]. DPSC presented neuron-like morphological changes and increased gene (Nestin and Tub3) and protein (MAP2) expression after being cultured for 5 days in serum- and growth factor-free medium [41]. After in vivo transplantation into the mesencephalon of chicken embryo, DPSC not only acquired a neuronal morphology but also express neuronal markers [42].
Although both DPSC and SCAP are located in anatomically adjacent sites, they are originated in distinct developmental stages and present differences regarding their growth potential and biochemical characteristics [24, 29, 31]. Morphologically, SCAP are smaller in size, fibroblast-like or stellate in shape, with numerous cytoplasmic processes [43]. They also present higher number of STRO-1–positive cells when compared with DPSC. Compared to DPSC, SCAP present higher expression of the anti-apoptotic protein surviving and human telomerase reverse transcriptase (hTERT) activity , a catalytic subunit of the enzyme telomerase that maintains the telomere length [30]. Considering the proliferation rate, SCAP cultures can present more than double the number of cells compared to DPSCs after an observation period of 4 days [43]. In spite of dentinogenic markers expressed by SCAP after ex vivo expansion, DPSC present higher expression levels of dentin sialoprotein (DSP), matrix extracellular phosphoglycoprotein (MEPE), fibroblast growth factor receptor 3 (FGFR3), transforming growth factor beta receptor II (TGF beta RII) and others [24]. Although both DPSC and SCAP present remarkable characteristics making them attractive models for dental pulp regeneration, the first present a wider window of opportunity to be isolated from fully developed teeth while SCAP can only be retrieved from teeth under development [5, 24, 31, 43].
Stem cells from human exfoliated deciduous teeth (SHED) were firstly isolated in 2003 from normal exfoliated human deciduous incisors and present positive expression of MSCs markers (STRO-1 and CD146), embryonic stem cells markers (Oct4, Nanog), stage-specific embryonic antigens (SSEA -3, SSEA-4) and tumour recognition antigens (TRA -1-60 and TRA-1-81) [22, 44]. The ability that these cells have to cross lineage boundaries expands the potential use of SHED for therapies involving a large number of tissues. SHED can differentiate into adipogenic, chondrogenic, neurons and endothelial cells [6, 22, 27]. SHED can also differentiate into osteoblasts and are positive for TGF-β, FGF and VEGF receptors [27, 45].
SHED present a remarkable advantage over DPSC as they can be retrieved from naturally exfoliated teeth, which are one of the only disposable post-natal human tissues [6, 22, 26, 46]. Although both DPSC and SHED cells are originated from the dental pulp, deciduous teeth are different from permanent teeth with regards to their developmental processes, tissue structure and function. Therefore, it is not a surprise to find out that SHED are distinct from DPSC with respect to their higher proliferation rate, increased cell-population doublings, sphere-like cell-cluster formation and osteoinductive capacity in vivo. SHED apparently represent a population of multipotent stem cells that are perhaps more immature than DPSC [18, 19, 22, 26, 27, 31, 33, 36]. For example, during osteogenic differentiation, SHED present higher levels of alkaline phosphatase activity and osteocalcin production than DPSC [36]. However, the ability to regenerate a dentin/pulp-like complex found in DPSC is also observed in SHED [19, 22, 27, 31].
Dental Stem Cells-Based Pulp Tissue Engineering
The use of synthetic restorative materials as substitutes for dental structures is a practice nearly as old as dentistry itself. To date, most of the procedures performed in dentistry are limited to the replacement of damaged tissues for biocompatible materials that may not present chemical, biological or physico-mechanical characteristics similar to the host tissues. These discrepancies, together with the hostile environment of the oral cavity, can result in frequent need for re-treatment [5]. Particularly in endodontics, despite of the development and introduction of new techniques, instruments and medicaments for the clinical management of the dental pulp, the fundamental principles of clinical practices are not drastically different than those of the time when first root canal instruments and gutta-percha were introduced in the 1800s [26]. Most of the endodontic treatments rely on the disinfection of the root canal followed by a hermetic three-dimensional obturation. Though this is a well-established approach it is based on the substitution of a living tissue for synthetic and, in most of the cases, inert materials.
In regards to biological-based therapies for dental pulp there cell-free and cell-based approaches. The first relies on the chemo attraction of host cells into the root canal [47, 48]. The second is based on the transplantation of responsive stem cells using a suitable scaffold into the root canal to promote tissue formation and regeneration [5, 49–51].
Cell-free approaches can make use of functionalized scaffolds or release of dentin-derived chemotactic factors to induce the migration of host cells from surrounding areas to regenerate the tissue [52]. One example of clinically viable cell-free therapy is the use of the blood clot as a scaffold to attract and maintain periapical (or circulating progenitor) cells inside the root canal [52–54]. The root canal revascularization via blood clotting depends on the establishment of bleeding into the canal system via over instrumentation of the apex [54]. This is an attractive alternative to be performed directly in clinical appointments and eliminate the needs of retrieving, culturing and transplanting cells as required in cell-based approaches [5, 26, 52, 54]. Interestingly, by promoting the bleeding inside the root canal the level of stem cells markers in the blood clot can be up to 600 times higher as compared with levels found in the systemic blood [55]. In fact, revascularization was reported to effectively induce complete root development of immature permanent teeth with apical periodontitis [53]. Other approaches involve the use of functionalized scaffolds and bioactive materials for chemo attraction of periapical cells [56, 57].
Though cell-free approaches are a clinical reality [53], host cells need to migrate long distances inside the root canal space through the apical foramen that narrows with time [52]. Moreover, the types and concentration of cells and composition of the fibrin clot are unpredictable [54]. Nonetheless, the revascularization approach can lead to dentinal wall thickening, apical closure and increased root length [14, 15].
Cell-based strategies aim to develop clinically approachable techniques that induce the regeneration of a functional dental pulp that is capable to deposit mineralized and organized matrix contributing for the development of the root structure. Dental pulp tissue engineering requires the fine orchestration of three elements: cells, scaffolds and morphogenesis/signalling pathways [5, 26, 52]. In this new paradigm, it is rather exciting that stem cells from oral sources play the focal role moving forward the field of regenerative endodontics.
Apart of all stem cells that can be obtained in the oral environment, three of them, namely SCAP, DPSC and SHED have great potential to be used for dental pulp tissue engineering. The first can undergo dentinogenic differentiation in vitro under proper stimulation. Moreover, when SCAP were mixed with hydroxyapatite/tricalcium phosphate (HA/TCP) scaffolds simulating a root canal and implanted in vivo, they presented the ability to regenerate vascularized dental pulp–like tissues in the hollow space and to produce new dentin-like tissue on the walls [24, 29, 30].
DPSC were shown to have great potential to induce pulp regeneration since they were firstly isolated and extensively characterized in the landmark paper from Gronthos et al. [31]. The DPSC isolated were expanded in vitro, mixed with HA/TCP ceramic powder and transplanted into the dorsum of immunocompromised mice. Remarkably, after 6 weeks from implantation, the surface of the ceramic particles was coated by an odontoblast-like layer and dentin-like structure (Fig. 1). The mineralized tissue formed was composed of a highly ordered collagenous matrix containing mainly collagen type I which is the major fibrous component of the dental pulp besides collagen type III [58]. Notably, there was also the formation of a pulp-like tissue permeated with blood vessels and the transplants were positive for human-specific markers for bone sialoprotein, osteocalcin and dentin sialophosphoprotein (DSPP) [31].
Developmental potential of DPSC transplanted with HA/TCP carrier in vivo . (a) After 6 weeks, DPSC generated a dental pulp-like tissue within the HA/TCP carrier (c) with the presence of dentin-like matrix (d), odontoblast-like cells (od) and blood vessels (bv). (b) A magnified view of the dentin matrix (d) highlights the odontoblast-like layer (od) and odontoblast processes (arrow) (c). Polarized light demonstrates perpendicular alignment (dashed lines) of the collagen fibers to the forming surface. Adapted from Gronthos et al. [31] (Copyright (2000) National Academy of Sciences, U.S.A.)
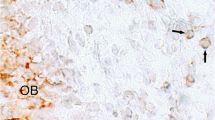
The residual pulp of exfoliated deciduous teeth contains stem cells (SHED) that can regenerate a dental pulp-like tissue that can deposit mineralized tissue [6, 22, 27, 45]. These cells showed their potential to be used for dental pulp tissue regeneration in 2003 in a seminal paper published by Miura et al. There, SHED mixed with HA/TCP were capable to differentiate into odontoblasts after 8 weeks from implantation in immunocompromised mice. Nonetheless, the cells failed to regenerate a complete dentin–pulp-like complex in vivo as previously observed for DPSCs (Fig. 2) [22, 31]. Nonetheless, this ability was reported later [6, 27, 59].
(a) After 8 weeks of transplantation, SHED were able to differentiate into odontoblasts (open arrows) that were responsible for the dentin-like structure (D) formation on the surfaces of the HA/TCP scaffold (HA). (b) In situ hybridization showed that the tissue formed had human origin (open arrows). (c) The black dashed line represents interface between newly formed dentin. (d) bone (B) generated by host cells in the same SHED transplant shows no reactivity to the DSPP antibody. Adapted from Miura et al. [22] (Copyright (2003) National Academy of Sciences, U.S.A.)
Although SCAP is one of the candidates to be used in dental pulp tissue engineering, the majority of the research for such purpose focus on the use of stem cells obtained from the dental pulp itself (DPSC and SHED). Although SCAP are capable of forming odontoblast-like cells to produce dentin in vivo, they are likely to be the source of primary odontoblasts for the root dentin formation. This limits their isolation to the period when the root is not yet fully formed [24]. Thus, third molars are the most attractive source of SCAP as their root completion is only achieved by ages from 18 to 25. DPSC can be obtained from the dental pulp of permanent tooth. Here, pre-molars that are extracted due to orthodontic reasons are also an interesting source of DPSC. SHED can be retrieved from the only naturally disposable post-natal human tissue. This creates a window of approximately 11 years to obtain these stem cells mainly from deciduous central incisors and canines.
The success of cell-based regenerative endodontic strategies depends on techniques that will allow clinicians to create a functional pulp tissue within cleaned and shaped root canal systems [5, 17, 20, 52]. One of the first signs that this could be achieved was reported in 1996. The team led by David Mooney engineered pulp-like tissues in vitro utilizing fibroblast obtained from human adult dental pulp seeded in a synthetic extracellular matrix made of polyglycolic acid fiber-based meshes. Histological analysis performed after 60 days from implantation showed that the cells were capable to proliferate and form a new tissue with general appearance and cell density similar to native dental pulp [60]. In 2004, autogenous transplantation of pulp cells stimulated with bone morphogenetic protein 2 (BMP-2) into amputated pulp of dogs induced the formation of reparative dentin formation [61]. These and other studies shed a light that cell-based regenerative endodontics could be on the horizon of clinicians in the future. Since then, a series of proof-of principles research have been published showing the feasibility of regenerating functional human dental pulp using tissue engineering principles and dental stem cells.
To prove the potential of dental stem cells to induce pulp regeneration in vivo, several reports use the Tooth Slice/Scaffold of Dental Pulp Tissue Engineering model . This strategy allows for the generation of a dental-pulp-like tissue via the transplantation of human dental pulp stem cells seeded in a biodegradable scaffold cast within the pulp chamber of human tooth slices [62]. This model was used to show the ability of SHED to generate dental pulp-like tissue in vivo by the Nör’s lab in 1996. There, SHED were seeded into poly-l-lactic acid (PLLA) scaffolds cast in 1 mm thick tooth slice and transplanted into the subcutaneous space of immunocompromised mice. After 4 weeks, a well-vascularized pulp-like tissue was formed in the pulp chamber space. Notably, there was a layer of odontoblast-like cells expressing dentin sialoprotein (DSP) lining the dentin surface. Although these cells were morphologically similar to odontoblasts, including the eccentric polarized position of the nucleus at the basal part of the cell body, no new dentin deposition was observed [46].
The capability of SHED to differentiate into fully functional odontoblasts capable of generating tubular dentin in vivo was observed later (Fig. 3). For this, SHED were seeded a poly-l-lactic acid (PLLA) scaffold cast in a tooth slice and implanted subcutaneously into the dorsum of mice, which received one injection of tetracycline hydrochloride every 5 days. After 32 days from implantation, the dentin disks presented well-defined fluorescent lines originated from the chelation of calcium ions in the newly deposited dentin. The dental pulp generated displayed positive protein expression for dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP-1) . Interestingly, the rate of dentin formation by SHED was far superior (14.1 μm/day) as compared to the one observed for the human dental pulp used as control (3.3 μm/day) [27]. Growth rates of 4–15 μm/day have been reported for primary dentin depending on the stage of development and age of the tooth [63].
SHED differentiate into functional odontoblasts that generate new tubular dentin in vivo. (a) The pictures show samples retrieved from mice after 32 days after implantation. (b) RT-PCR analysis showed the high expression of DSPP and DMP-1 on tissues engineered on tooth slice/scaffolds seeded with SHED. Reprinted from Journal of Dental Research, 89/8, VT Sakai, Z Zhang, Z Dong, KG Neiva, MA Machado, S Shi, CF Santos and JE Nör, SHED differentiate into functional odontoblasts and endothelium, 791–796, Copyright (2010), with permission from SAGE Publications
DPSC were also used to regenerate pulp-like tissue using a similar approach. By seeding DPSC in a collagen-based scaffold along with a growth-factor (DMP-1) it was possible to engineer pulp-like tissue after 6 weeks from subcutaneous transplantation in mice. However, in this study, no odontoblast-like cells were found on the surface of pre-existing dentin surface and the quality of the pulp-like tissue obtained appeared to be less promising as the ones obtained using PLLA scaffold [64]. These drawbacks can be related to the contraction that collagen scaffold experiences leading to a size reduction proportional to the density of cells seeded in it [65].
Although these studies provide an exciting panorama for regenerative endodontics, the use of tooth substrates with few millimetres in thickness does not fully represent the challenges of regenerating a pulp tissue with in complete roots [6]. The larger dimension of the tissue that has to be engineered in addition to the fact that vascularization can be available only through the apex, pose important obstacles to be overcome. Hence, some studies aimed to prove that pulp regeneration can be achieved in three-dimensional environments.
An important step towards stem cells-based regenerative endodontics was obtained by a team of researchers who have seeded swine DPSC into the root segments with 5–6 mm in length and implanted into the jawbone of the adult minipigs. After 6 weeks from implantation, the proliferating cells were filling the space once left by the scaffolds made of collagen or poly(lactic-co-glycolic) acid (PLGA) undergoing degradation. At that time, new extracellular matrix had been occasionally deposited in a polar predentin-like pattern on the canal dentinal walls. After 10 weeks, a continuous layer of cells with columnar or spindle-shaped morphology along with the presence of newly formed organic matrix was observed [66]. An interesting fact about this study was that the swine DPSC used were cryopreserved for 1 year prior to implantation showing that the DPSC do not lose their potential to regenerate dentin/dental pulp-like tissue even after being cryopreserved.
Dental pulp tissue formation using DPSC was also achieved within tooth slices as long as 7 mm in length. Cells mixed with poly-d, l-lactide and glycolide (PLG) scaffolds were inserted into the canal with internal space ranging from 1 to 2.5 mm and one end sealed with MTA. Four months after the implantation into the subcutaneous of mice, the emptied canal was filled with regenerated pulp-like tissue. The entire pulp-like tissue was vascularized with a uniform cell density resembling the natural pulp. Though the odontoblast-like cells were not well organized and did not present characteristics of natural odontoblasts, a layer of mineralized tissue was deposited onto the dentinal walls and the MTA cement used to seal the canal [67]. However, as MTA is known to release calcium ions that induce the formation of calcified barriers [68] it was not possible to determine if DPSC alone could induce such extent of calcified tissue deposition. Nonetheless, that paper offered great perspectives on the capability of using dental stem cells to induce de novo synthesis of vascularized human pulp-like tissue capable to deposit dentin-like tissue onto the surface of root canals [67].
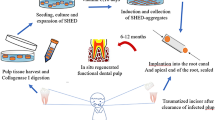
SHED are also capable to regenerate dental pulp within full-length root canals in vivo (Fig. 4). Cells were injected in empty roots using either a self-assembling hydrogel or recombinant human collagen type I. The roots were implanted in the subcutaneous of mice and after 28 days a human pulp-like tissue was observed throughout the extent of the root canals. More excitingly, the engineered pulp was capable of generating new dentin at a rate of approximately 10 μm/day. The high activity of the functional odontoblasts was supported by a high concentration of blood vessels close to the dentinal walls (Fig. 4b) [6]. One fact that deserves to be highlighted in this study is that cells were delivered inside the root canals using injectable scaffolds . As injection is one the most practiced procedures by dentists, the learning curve can be shortened for clinicians to apply this technology in patients.
Dental pulp tissue engineering with SHED injected into human root canals and transplanted into immunodeficient mice. (a) Low-magnification and (b) high-magnification images of tissues formed when SHED mixed with scaffolds (Puramatrix™, rhCollagen type I groups) were injected into full-length root canals of human premolars. A vascularized connective tissue occupied the full extension of the root canal. Cell densification and many blood vessels were observed along dentin walls. Scaffolds (Puramatrix™) injected into the root canals without cells were used as controls for SHED. Freshly extracted human premolars were used as tissue controls. Black arrows point to blood vessels close to the odontoblastic layer. Reprinted from Journal of Dental Research, 92/11, V Rosa, Z Zhang, RHM Grande and JE Nör, Dental Pulp Tissue Engineering in Full-length Human Root Canals, 970–975, Copyright (2013), with permission from SAGE Publications
Future Challenges
Stem cells-based therapies offer new perspectives targeting dental pulp regeneration and further development of root structure [17]. Besides that, the two branches of the tissue engineering triad (the development of suitable scaffolds to deliver cells and fine tuning of signalling pathways) still offer several challanges to be solved in order to improve the outcomes.
Scaffolds are frameworks that provide the support for cells to proliferate, differentiate and generate the desired tissue [5, 6]. Ideally, a scaffold must allow cell attachment and migration, permit the localized and sustained delivery of growth factors and enable the influx of oxygen to maintain the high metabolic demands of cells and tissues in formation [5].
The scaffolds physical and mechanical characteristics must be compatible with the surrounding tissues to support specific demands in vivo [69–71]. It is widely known that stiffness of the substrate play an important role in differentiation of stem cells thus. In fact, the physical and mechanical properties of the scaffolds can have direct impact in cell differentiation through mechano transduction [5, 69, 72]. Interestingly, the increase of scaffold’ stiffness favours the differentiation of MSCs into neurons, myoblasts, and osteoblasts in that order [72]. DPSC seeded on soft matrices present upregulation of collagen I but downregulation of markers such as DSPP, DMP-1 [73]. In addition, physical features of the scaffolds (e.g. quantity and extension of pores) change the specific surface modifying its permeability and mechanical properties, influencing cell differentiation and tissue formation [5, 25, 74]. Noticeably, higher number and extension of pores can contribute significantly for cellularity but compromise scaffold strength [74, 75]. Pore interconnectivity also plays a crucial role to sustain tissue growth [75, 76]. Also, the rate of scaffold degradation is important to achieve success in tissue engineering therapies. The scaffold should ideally reabsorb once it has served its purpose of providing a template for tissue regeneration. The degradation ought happen at a rate compatible with the new tissue formation [5, 27, 71, 73, 76]. The by-products generated cannot be toxic and must be easily cleared or resorbed to minimize the risk of inflammatory response [77].
The third pillar to be tuned for successful tissue engineering is cell signalling [5, 52]. This is a complex system of communication that governs cell activities individually and it can be dramatically changed upon cellular interactions or external stimulation. This could be observed when proteins present in dentin disks [78], dentin extract in EDTA [79] or a tooth-germ conditioned extract [80] were found to supplement the scaffolds as a mechanism of cellular induction. For example, there is evidence that the TGF-1 is released from the dentin after injuries [81] and dentin itself can induce the odontogenic differentiation by releasing embedded growth factors like transforming growth factor-β1 (TGF-β1) [82]. Moreover, BMP’s are involved in the odontoblastic differentiation processes [59, 83] and both BMP-2 and 7 present inductive effects in reparative dentinogenesis [84, 85]. Nonetheless, SHED undergoing odontoblastic differentiation responds strongly to BMP-2 as compared to BMP-7 [59]. This complex system where molecules can trigger different responses creates a very challenging environment for researchers developing cell-based therapies.
Other challenge that needs to be addressed is the cost of therapies when available at large. Comparatively to routine root canal treatments, regenerative endodontics requires additional procedures and qualified manpower in order to isolate, expand, preserve and prepare cells for use. Undoubtedly, these add extra cost to the process that eventually will be directed to patients preventing popularization of the treatments. There are lots of research efforts to optimize process and minimize the number of procedures impacting the economic burden. History has shown that most of the revolutionary technologies became vertiginously popular as they also became more affordable. Hopefully, this also can be true for tissue engineering in endodontics (Fig. 5).
Dental pulp tissue engineered inside root canal using SHED cells (a) and natural dental pulp from a young premolar (b). The engineered tissue occupies the apical portion (c). Immunohistochemistry with PCNA (proliferating cell nuclear activity) and Factor VIII show a proliferative tissue with established blood network (d, e). Reprinted from Dental Materials, 28/04, B Cavalcanti, A Della Bona and JE Nör, Tissue engineering: From research to dental clinics, 341–348, Copyright (2012), with permission from Elsevier
References
Nanci A (2007) Ten cate’s oral histology-pageburst on vitalsource: development, structure, and function. Elsevier Health Sciences, St. Louis
Huang GTJ (2011) Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Frontiers in Bioscience (Elite Ed) 3:788–800
Zhang W, Yelick PC (2010) Vital pulp therapy—current progress of dental pulp regeneration and revascularization. Int J Dent 2010, 856087
Ingle JI (2008) Ingle’s endodontics 6. PMPH-USA, Shelton, CT
Rosa V, Della Bona A, Cavalcanti BN, Nor JE (2012) Tissue engineering: from research to dental clinics. Dent Mater 28(4):341–348
Rosa V, Zhang Z, Grande RH, Nor JE (2013) Dental pulp tissue engineering in full-length human root canals. J Dent Res 92(11):970–975
Kojima K, Inamoto K, Nagamatsu K, Hara A, Nakata K, Morita I et al (2004) Success rate of endodontic treatment of teeth with vital and nonvital pulps. A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97(1):95–99
McGuigan MB, Louca C, Duncan HF (2013) The impact of fractured endodontic instruments on treatment outcome. Br Dent J 214(6):285–289
Alhadainy HA (1994) Root perforations. A review of literature. Oral Surg Oral Med Oral Pathol 78(3):368–374
Dammaschke T, Steven D, Kaup M, Ott KHR (2003) Long-term survival of root-canal-treated teeth: a retrospective study over 10 years. J Endod 29(10):638–643
Reeh ES, Messer HH, Douglas WH (1989) Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod 15(11):512–516
Caplan DJ, Cai J, Yin G, White BA (2005) Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent 65(2):90–96
Fuks AB (2008) Vital pulp therapy with new materials for primary teeth: new directions and treatment perspectives. J Endod 34(7 Suppl):S18–S24
Jeeruphan T, Jantarat J, Yanpiset K, Suwannapan L, Khewsawai P, Hargreaves KM (2012) Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J Endod 38(10):1330–1336
Bose R, Nummikoski P, Hargreaves K (2009) A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 35(10):1343–1349
Cvek M (1992) Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Dent Traumatol 8(2):45–55
Rosa V (2013) What and where are the stem cells for dentistry? Singapore Dent J 34(1):13–18
Bojic S, Volarevic V, Ljujic B, Stojkovic M (2014) Dental stem cells: characteristics and potential. Histol Histopathol 29(6):699–706
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A et al (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81(8):531–535
Huang GT (2011) Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Front Biosci (Elite Ed) 3:788–800
Kemoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F et al (2007) Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res 329(2):283–294
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG et al (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100(10):5807–5812
Shi S, Gronthos S (2003) Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18(4):696–704
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S et al (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34(2):166–171
Xie H, Cao T, Viana Gomes JC, Castro Neto AH, Rosa V (2015) Two and three-dimensional graphene substrates to magnify osteogenic differentiation of periodontal ligament stem cells. Carbon 93:266–275
Rosa V, Botero TM, Nor JE (2011) Regenerative endodontics in light of the stem cell paradigm. Int Dent J 61(Suppl 1):23–28
Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S et al (2010) SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89(8):791–796
Ruparel NB, de Almeida JF, Henry MA, Diogenes A (2013) Characterization of a stem cell of apical papilla cell line: effect of passage on cellular phenotype. J Endod 39(3):357–363
Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34(6):645–651
Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C et al (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1, e79
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97(25):13625–13630
Tecles O, Laurent P, Zygouritsas S, Burger AS, Camps J, Dejou J et al (2005) Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol 50(2):103–108
Shi S, Robey PG, Gronthos S (2001) Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 29(6):532–539
Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH et al (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127(8):1671–1679
Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B et al (2009) Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int 55(5):323–332
Koyama N, Okubo Y, Nakao K, Bessho K (2009) Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg 67(3):501–506
Govindasamy V, Ronald VS, Abdullah AN, Nathan KR, Ab Aziz ZA, Abdullah M et al (2011) Differentiation of dental pulp stem cells into islet-like aggregates. J Dent Res 90(5):646–652
Kim BC, Bae H, Kwon IK, Lee EJ, Park JH, Khademhosseini A et al (2012) Osteoblastic/cementoblastic and neural differentiation of dental stem cells and their applications to tissue engineering and regenerative medicine. Tissue Eng Part B Rev 18(3):235–244
Iohara K, Zheng L, Wake H, Ito M, Nabekura J, Wakita H et al (2008) A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells 26(9):2408–2418
Takeyasu M, Nozaki T, Daito M (2006) Differentiation of dental pulp stem cells into a neural lineage. Pediatr Dent J 16:154
Zainal Ariffin SH, Kermani S, Zainol Abidin IZ, Megat Abdul Wahab R, Yamamoto Z, Senafi S et al (2013) Differentiation of dental pulp stem cells into neuron-like cells in serum-free medium. Stem Cells Int 2013, 250740
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 26(7):1787–1795
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P et al (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol 56(7):709–721
Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV et al (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 184(3–4):105–116
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K et al (2008) SHED repair critical-size calvarial defects in mice. Oral Dis 14(5):428–434
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S et al (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34(8):962–969
Smith JG, Smith AJ, Shelton RM, Cooper PR (2012) Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp Cell Res 318(18):2397–2406
Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR (2012) Dentine as a bioactive extracellular matrix. Arch Oral Biol 57(2):109–121
Nakashima M, Iohara K (2011) Regeneration of dental pulp by stem cells. Adv Dent Res 23(3):313–319
Yen AH, Sharpe PT (2008) Stem cells and tooth tissue engineering. Cell Tissue Res 331(1):359–372
Albuquerque MT, Valera MC, Nakashima M, Nor JE, Bottino MC (2014) Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 93(12):1222–1231
Botero TM, Nör JE (2015) Tissue engineering strategies for endodontic regeneration. In: Vishwakarma A, Sharpe P, Songtao S, Ramalingam M (eds) Stem cell biology and tissue engineering in dental sciences, 1st edn. Academic, London, pp 419–430
Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF (2009) Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod 35(5):745–749
Murray PE, Garcia-Godoy F, Hargreaves KM (2007) Regenerative endodontics: a review of current status and a call for action. J Endod 33(4):377–390
Lovelace TW, Henry MA, Hargreaves KM, Diogenes A (2011) Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod 37(2):133–138
Galler KM, Aulisa L, Regan KR, D’Souza RN, Hartgerink JD (2010) Self-assembling multidomain peptide hydrogels: designed susceptibility to enzymatic cleavage allows enhanced cell migration and spreading. J Am Chem Soc 132(9):3217–3223
Liu X, Wang X, Wang X, Ren H, He J, Qiao L et al (2013) Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater 9(6):6798–6805
Linde A (1985) The extracellular matrix of the dental pulp and dentin. J Dent Res 64:523–529
Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nor JE (2010) Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res 89(6):603–608
Mooney DJ, Powell C, Piana J, Rutherford B (1996) Engineering dental pulp-like tissue in vitro. Biotechnol Prog 12(6):865–868
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A (2004) Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 83(8):590–595
Sakai VT, Cordeiro MM, Dong Z, Zhang Z, Zeitlin BD, Nor JE (2011) Tooth slice/scaffold model of dental pulp tissue engineering. Adv Dent Res 23(3):325–332
Kawasaki K, Tanaka S, Ishikawa T (1977) On the incremental lines in human dentine as revealed by tetracycline labeling. J Anat 123(Pt 2):427–436
Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J et al (2008) In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod 34(4):421–426
Huang GT, Sonoyama W, Chen J, Park SH (2006) In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res 324(2):225–236
Kodonas K, Gogos C, Papadimitriou S, Kouzi-Koliakou K, Tziafas D (2012) Experimental formation of dentin-like structure in the root canal implant model using cryopreserved swine dental pulp progenitor cells. J Endod 38(7):913–919
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS et al (2010) Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16(2):605–615
Natu VP, Dubey N, Loke GC, Tan TS, Ng WH, Yong CW et al (2015) Bioactivity, physical and chemical properties of MTA mixed with propylene glycol. J Appl Oral Sci 23(4):405–411
Kemppainen JM, Hollister SJ (2010) Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res A 94(1):9–18
Saito E, Kang H, Taboas JM, Diggs A, Flanagan CL, Hollister SJ (2010) Experimental and computational characterization of designed and fabricated 50:50 PLGA porous scaffolds for human trabecular bone applications. J Mater Sci Mater Med 21(8):2371–2383
Qazi TH, Mooney DJ, Pumberger M, Geissler S, Duda GN (2015) Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials 53:502–521
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Lu Q, Pandya M, Rufaihah AJ, Rosa V, Tong HJ, Seliktar D et al (2015) Modulation of dental pulp stem cell odontogenesis in a tunable peg-fibrinogen hydrogel system. Stem Cells Int 2015:525367
Salerno A, Zeppetelli S, Di Maio E, Iannace S, Netti PA (2011) Processing/structure/property relationship of multi-scaled PCL and PCL-HA composite scaffolds prepared via gas foaming and NaCl reverse templating. Biotechnol Bioeng 108(4):963–976
Loh QL, Choong C (2013) Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng B Rev 19(6):485–502
Salerno A, Iannace S, Netti PA (2008) Open-pore biodegradable foams prepared via gas foaming and microparticulate templating. Macromol Biosci 8(7):655–664
Nof M, Shea LD (2002) Drug-releasing scaffolds fabricated from drug-loaded microspheres. J Biomed Mater Res 59(2):349–356
Goncalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nor JE (2007) Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod 33(7):811–814
Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH (2005) In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim 41(7):232–238
Yu J, Deng Z, Shi J, Zhai H, Nie X, Zhuang H et al (2006) Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng 12(11):3097–3105
Magloire H, Romeas A, Melin M, Couble ML, Bleicher F, Farges JC (2001) Molecular regulation of odontoblast activity under dentin injury. Adv Dent Res 15:46–50
Tziafas D, Kodonas K (2010) Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod 36(5):781–789
Nakashima M, Reddi AH (2003) The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol 21(9):1025–1032
Nakashima M (1994) Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Arch Oral Biol 39(12):1085–1089
Goldberg M, Six N, Decup F, Buch D, Soheili Majd E, Lasfargues JJ et al (2001) Application of bioactive molecules in pulp-capping situations. Adv Dent Res 15:91–95
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Dubey, N., Min, Ks., Rosa, V. (2016). Dental Stem Cells for Pulp Regeneration. In: Zavan, B., Bressan, E. (eds) Dental Stem Cells: Regenerative Potential. Stem Cell Biology and Regenerative Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-33299-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-33299-4_8
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-33297-0
Online ISBN: 978-3-319-33299-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)