Abstract
Background:
Perfusion bioreactors for tissue engineering hold great promises. Indeed, the perfusion of culture medium enhances species transport and mechanically stimulates the cells, thereby increasing cell proliferation and tissue formation. Nonetheless, their development is still hampered by a lack of understanding of the relationship between mechanical cues and tissue growth.
Methods:
Combining tissue engineering, three-dimensional visualization and numerical simulations, we analyze the morphological evolution of neo-tissue in a model bioreactor with respect to the local flow pattern. NIH-3T3 cells were grown under perfusion for one, two and three weeks on a stack of 2 mm polyacetal beads. The model bioreactor was then imaged by X-ray micro-tomography and local tissue morphology was analyzed. To relate experimental observations and mechanical stimulii, a computational fluid dynamics model of flow around spheres in a canal was developed and solved using the finite element method.
Results:
We observe a preferential tissue formation at the bioreactor periphery, and relate it to a channeling effect leading to regions of higher flow intensity. Additionally, we find that circular crater-like tissue patterns form in narrow channel regions at early culture times. Using computational fluid dynamic simulations, we show that the location and morphology of these patterns match those of shear stress maxima. Finally, the morphology of the tissue is qualitatively described as the tissue grows and reorganizes itself.
Conclusion:
Altogether, our study points out the key role of local flow conditions on the tissue morphology developed on a stack of beads in perfusion bioreactors and provides new insights for effective design of hydrodynamic bioreactors for tissue engineering using bead packings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the context of tissue engineering, several types of bioreactors have been designed in an attempt to enhance in vitro cell proliferation and differentiation in a dynamic and controlled environment: spinner flasks [1], rotating wall bioreactors [2], and perfusion systems [3, 4]. Yet an associated challenge is to provide homogeneous nutrients and oxygen supply within the whole volume of such scaffold. Perfusion bioreactors have been shown to give good results in this regard [5, 6], leading to relatively even cell seeding and growth on the porous scaffold [7, 8]. Perfusion bioreactors present a similar basic design consisting of a closed tubing circuit with a culture medium reservoir, a pump, and a growth chamber. They mainly differ in the design of the cell seeded-scaffold residing inside the growth chamber (fiber meshes, beads or cubes stacks, …) and the scaffold materials (coral, titanium, alginate, starch-based material, calcium-phosphate ceramics, …) [3, 9, 10].
Given the complex culture environment in perfusion bioreactors, where the fluid flow stimulates cell proliferation and tissue growth alters in turn the fluid flow, recent investigations have turned to modeling approaches to optimize their tissue production [11].
In hydrodynamic bioreactors, fluid flow alters cell differentiation and proliferation [5, 12] by providing a mechanical stimulation of the cells [3, 13] and enhancing species transport such as nutrients supply and waste removal [14, 15]. Externally applied forces, such as shear stresses, traction stresses or fluid drag [16], are translated into intracellular biochemical signaling, which results in a modification of the cellular response [17]. This process, termed mechanosensitivity, is at the origin of many important biological functions, including bone remodeling [18].
Mechanosensitive mechanisms have been identified such as the activation of stretch-activated ion channels [19, 20], integrin-initiated cytoskeleton deformations [21,22,23], and the stimulation of the primary cilia [24].
More generally, tissue pattern is driven by the complex combination of external signallings [25, 26]: the substrate material and local geometry (either the scaffold or the extracellular matrix), the culture medium (nutrients, growth factors, mitogens, etc.…), and external forces (such as those applied by other cells or fluid shear stress). Besides fluid flow enhanced proliferation, the way connective cells colonize the scaffold is of paramount importance. Despite recent progress in the description of connective tissue growth or repair in static conditions [27,28,29,30,31], the mechanisms underlying its formation in dynamic conditions are still poorly understood. In particular, the morphology of a tissue growing under perfusion of culture medium as well as the distribution of cells within this tissue are key information for the development of bioreactors for tissue engineering.
Several studies adopted a modeling approach to understand the interplay between tissue growth and fluid flow. Numerical simulations of fluid flow through empty porous scaffolds have been performed in order to determine the shear stress field and predict the preferential locations of cell proliferation at the initiation of cell culture [4, 32, 33]. More complex models have been proposed to simulate the evolution of the fluid flow as tissue grows by addition of cell layers of varying thicknesses [34] or by using a moving boundary approach where the interfacial velocity is a function of the local fluid shear stress [35]. Recently, the experimental evidence that the geometry strongly influences the tissue morphology and growth kinetics in static conditions [29, 36] motivated the development of new models. Indeed, tissue growth kinetics under perfusion is determined as a function of both the curvature of the tissue growth front and the fluid shear stress [11, 37], although these predictions are still to be validated against experimental data at the pore scale. Overall, given the complexity of the biophysical phenomena at stakes in perfusion bioreactors, modeling and numerical simulations in simplified controlled settings, appear as valuable investigative tools.
In this study, we propose to take advantage of a model-system inspired from a perfusion bioreactor for bone tissue engineering [3] in order to describe the morphological evolution of neo-tissue with respect to local flow patterns. Our model system makes use of two main simplifications: (i) murine fibroblasts are chosen for their rapid proliferation rate, mechanosensitivity, and ability to produce connective tissue, and (ii) the scaffold is composed of immobile, impermeable and non-absorbable polyacetal beads in order to use a simple geometry and image the bioreactor by X-Ray micro-tomography. We combine three-dimensional visualization by X-Ray microtomography and computational fluid dynamic to answer the following questions: How does the fluid flow pattern determine the spatial distribution of tissue in the bioreactor? What is the morphology of the tissue as it grows?
2 Material and methods
2.1 Cells
Fibroblasts NIH-3T3 (ATCC) were cultured in DMEM Glutamax (Life Technologies, Courtaboeuf Cedex, Villebon-sur-Yvette, France) supplemented with 10% Fetal Bovine Serum (Pan-Biotech, Aidenbach, Germany) and 1% Penicillin Streptomycin (Pan-Biotech), at pH 7.4 under standard cell culture conditions (37 °C, 5% CO2, 100% humidity). Following previous tissue engineering studies using murine fibroblasts [38,39,40], these cells have been chosen for our model system because they belong to the family of connective tissue cells [41]. Additionally, they respond to mechanical stimuli [42], which is relevant to our perfusion bioreactor model and have a rapid proliferation rate.
2.2 Perfusion bioreactor
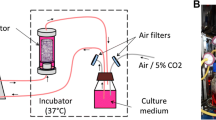
The perfusion bioreactor (Fig. 1) consists of a closed tubing circuit with a media reservoir, a peristaltic pump, and a perfusion chamber [3, 43]. The cylindrical growth chamber (13.8 mm diameter, 5 cm high, made of polycarbonate) is randomly filled by mono disperse polyacetal beads (2 mm diameter). Let us note that bead-based scaffolds have been identified as good candidates for tissue engineering and therefore have been investigated multiple times [4, 32, 39, 40, 44,45,46,47]. The beads are immobilized by two polycarbonate grids (0.5 cm high), inserted in the growth chamber at the bioreactor inlet and outlet. Polyacetal is semi-transparent to X-rays allowing the reconstruction of three-dimensional images of the tissue on the beads. In addition, the scaffold is immobile and non-absorbable: this allows to study tissue growth for a given bead stack arrangement. Finally, note that since the beads volume fraction remains constant, the local organization and the global height of bead stacks are slightly sample-dependent. The chamber is closed by two caps made of polytetrafluoroethylene and a tubing made of silicon (Pharm-Med) connects the bioreactor to the culture medium reservoir. The culture medium pH is kept constant by the constant supply of air enriched with carbon dioxide at 5%.
2.3 Dynamic cell culture in a perfusion bioreactor
The following procedure has been followed in order to seed the cells on the beads before their introduction into the bioreactor [40, 48]. First, a suspension of pre-confluent NIH-3T3 cells (106 cells/ml) in culture medium was gently poured onto the polyacetal beads previously coated with fibronectin (10 μg/ml, 1 h at 37 °C), then left overnight in a CO2 incubator to let the cells adhere to the beads. The seeded scaffold was then gently poured into the perfusion bioreactor and immobilized by two grids to avoid any bead movements during culture medium changes.
The constructs were perfused at a constant flow rate of 20 ml/min or 30 ml/min for one, two or three weeks (two constructs per experimental condition). The culture medium was changed for the first time after one week then twice a week. At the indicated time points, the constructs, still immobilized by the two grids, were washed with PBS prior being fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) in PBS (Pan-Biotech), then washed again with PBS and H2O prior being stained with 1% osmium tetroxide OsO4 (Sigma-Aldrich) in H2O. The solution is beforehand gently injected multiple times at the top and bottom of each sample through the peripheral envelop of tissue in order to ensure that the core of the samples is fully immersed in the solution. The samples were then dehydrated through a series of ethanol baths of growing concentrations (50, 70, 90, 95, and 100%) before being dried using hexamethyldisilazane (Sigma-Aldrich). Osmium is a heavy metal that stains cell membranes thereby making cells visible by X-ray microtomography.
2.4 Visualization of the cellular phase
The stained cellular phase was imaged by an X-ray tomography system (North Star Imaging, NSI, X50-CT, Rue de la Perdrix, Tremblay-en-France, France) equipped with a molybdenum target and a CMOS image sensor Dexela 2923. Two series of scans were performed with the following characteristics:
-
Physical voxel length of 17 μm with a height of field view of about 2.8–2.9 cm and centered at mid-height of the bioreactor growth chamber. The voltage was 50 kV and the current 400 μA. 900 projections were obtained from each sample over 360 degrees.
-
Physical voxel length of 10.2 μm with a height of field view of about 1:1 cm from the top or the bottom of the bioreactor growth chamber. The voltage was 50 kV and the current 230 μA. 1000 projections were obtained from each sample over 360 degrees.
2.5 Image analysis
The reconstruction was performed with efX-software (NSI) and image processing with ImageJ (version 1.51w) [49].
The mean intensity, that we define as the mean grey value, is measured on the raw data (16 bits) after subtracting the mean intensity of the background noise, measured outside the bioreactor.
The creation of a region of interest (ROI) delimiting the cellular regions in the parietal region and in the core of the bioreactor followed four steps:
Step 1: The mean intensity of the background noise, measured outside the bioreactor wall, was subtracted.
Step 2: The tube was removed by application of an inverted circular mask on all the slices. The mask was created beforehand by drawing two ovals matching the inner perimeter of the tube on the first and last slide of the stack, and adding them to a ROI. The ROI was completed between the first and last slides by interpolation. The associated mask for the tube was then generated and applied to the stack of the samples obtained in step 1.
Step 3: A binary image of the cellular region was obtained in three steps. The thinnest tissue regions were selected by subtracting the background with rolling ball radius set to 1 then 2 pixels on duplicated stacks. Both stacks were added and thresholded to create a binary mask consisting of the regions where the tissue is the thinnest (Step 3a). The largest tissue regions were isolated by thresholding and converted to a mask (Step 3b). Both masks were added in order to obtain the cellular region as binary image (Step 3c).
Step 4: The annular and core regions were delimited by a radius R. A circle of radius R was drawn on the first and last slides with the same center as the centroid of the growth chamber wall and added to a ROI. The ROI was completed between the first and last slides by interpolation. The circular mask of radius R was then generated. For selecting the cellular region close to the bioreactor wall (or in the core), the mask (respectively the inverted mask) was applied to the binarized image defining the cellular region. The cellular region was finally selected with subpixel accuracy and added to a new ROI.
3 Results and discussion
Fibroblasts NIH-3T3 cells were cultured for one, two or three weeks in the bioreactor for two different perfusion flow rates of culture medium (20 or 30 ml/min). The samples were scanned by X-ray micro-tomography and the tridimensional arrangements of the beads and the tissue were obtained after image reconstruction. Preliminary experiments showed that tissue proliferation on glass and polyacetal bead stacks in static conditions (without flow) was negligible compared to dynamic conditions [39, 40], producing too little tissue volume to be resolved by X-ray microtomography. Additionally, mass transport estimates in similar culture conditions and close flow rate indicate that oxygen and nutrient supply is in excess during most of the three weeks of culture [39].
3.1 Channeling effect locally enhances the perfusion flow rate at the bead stack periphery
Preliminary SEM observations (not reported here) of beads seeded with fibroblasts cultured in the perfusion bioreactor up to 3 weeks makes it possible to prove the presence of cells on beads. The grey-scale SEM images do not allow differentiating cells from ECM.
After two or three weeks of culture, we observe a preferential proliferation at the periphery of the bead stack where the cells form an external tissue envelope (Fig. 2). The difference between the external and internal cell proliferation is shown in Fig. 3 which represents a slice of the bioreactor with a flow rate of 30 ml/min after three weeks. While the newly developed cell-phase certainly lacks the complexity of native tissues, for simplicity, here and in the rest of the article we refer to the cell and ECM continuum as “tissue”. This choice is supported by histological analysis in a quasi-identical system previously published by us, where fibroblast cells were grown on beads in a perfusion bioreactor [39].
Visualization obtained by X-ray micro-tomography of the tissue on the scaffold after three weeks of culture at a flow rate 30 ml/min. A 3D side view. B 2D representative slide (intensity normalized with respect to the maximum intensity). C Sum over the whole stack of pixel intensity in the cellular region (relative to the maximum intensity)
It can be seen that the tissue close to the bioreactor wall (colored in yellow) is denser than the tissue in the core (colored in blue). This phenomenon, observed in all the experiments, is also clearly illustrated in Fig. 3C where the intensity, that we define as the grey value of a pixel, of the cellular phase is summed over the entire stack. In order to support these observations, we quantify the averaged relative difference of intensity between the tissue in the core of the bioreactor and the envelope for all samples cultured three weeks. Two regions are identified: an annular region corresponding mainly to the tissue envelope previously described, and an inner disk region representing the core of the bioreactor. Due to some heterogeneous arrangement of the beads in the vertical direction, the inner region is defined using two different radii R1 and R2. This corresponds to two regions (annular and disk) per radius. The thickness of the annular region amounts to about either one or two bead radii. We first determine the averaged relative difference of intensity between the tissue in the annular region and the tissue in the disk region considering the first radius R1. The results provided in Table 1 are obtained for all samples cultured three weeks. The relative comparison of mean intensity between the core and the periphery are of the order of 50% in most cases. Of particular note, the average of 42.2% is mainly due to the heterogeneous arrangement of the beads in the vertical direction. Indeed, considering the second radius R2, this relative difference amounts to 51.3%.
Additional scans of bioreactor inlets and outlets for experiments carried out with a flow rate of 30 ml/min show that the envelope actually encompasses the whole packing of beads (Fig. 4). We can observe that the pores in the inlet region represented in Fig. 4B are partially blocked. Indeed, the flow of the culture medium is modified: in the center of the inlet section where the permeability is reduced, the flow becomes weaker while it increases at the periphery.
As shown below, this preferential tissue growth is related to the geometrical heterogeneities of the scaffold. These heterogeneities are due to the spatial distribution of the spherical beads at the vicinity of the wall (parietal region). This disordered arrangement in the parietal region is characterized by a local increase of the porosity and permeability values, both being therefore dependent on the radial coordinate. The consequence of these heterogeneities is an increase of the flow rate close to the wall leading to a more intense local flow. This channeling effect [50] gives rise to an increase of both nutrient transport and local shear stress, which results in a stronger mechanical stimulation of the cells in the parietal region and therefore in an increased proliferation. As a matter of fact, Cruel et al. [32] noticed radial heterogeneities of the fluid shear stress at the beads surface for bead granular packings. More specifically, we locate these heterogeneities at the bioreactor periphery. This mechanism can also be described at the bioreactor scale (macroscopic scale) where the averaged filtration velocity V in the direction of the flow (z-component) is governed by Darcy's law [51]:
In Eq. (1), µ is the dynamic viscosity and K is the permeability coefficient in the direction of the flow which depends on the radial coordinate r. For a porous media composed by mono dispersed spherical beads, the permeability is given by the Kozeny-Carman relationship \(\bar{K}\)= \({\bar{\varepsilon }}^{3}{d}^{2}/180 {(1-\bar{\varepsilon })}^{2}\) [47] where \(\bar{\varepsilon }\) represents the average porosity and d the diameter of the beads. Therefore, Eq. (1) clearly shows that this channeling effect is explicitly dependent on the beads diameter. It is important to recall that Darcy's law is relevant if the local Reynolds number (Re = \(\sqrt{\bar{K}} V/\nu\)) is lower than 1 where \(\nu\) represents in the Reynolds number represents the kinematic viscosity. This is the case for our bioreactor where \(\bar{\varepsilon }=0.35\), \(\bar{K}=2\times{10}^{-9}\) m2 and \(Re=0.16\). This estimation has been done for the maximum flow rate (30 ml min−1). Therefore, according to Eq. (1), for a given pressure gradient, the filtration velocity V continuously increases with the permeability K. It is worth mentioning that the thickness of the channeling region is of the order of \(\sqrt{K}\) and that the flow field non-uniformities in this region are negligible if the ratio d/D is very small compared to 1 (with d the beads diameter and D the bioreactor inner diameter) [50]. In our bioreactors, d/D is of the order of 0.1, which makes necessary to take into account the presence of a channeling effect in the analysis.
The above analysis leads to an important design principle for perfusion bioreactors using beads, or more generally granular scaffolds: the intensity of the channeling effect can be modulated according to specific bioreactor application by adjusting the ratio between the scaffold beads size and chamber diameter. For instance, if one aims to have homogeneous local flow rate across the scaffold, small beads and large chamber diameters should be used. On the contrary, if an application requires a large flow rate at the scaffold surrounding, bigger beads might be more appropriate.
3.2 Shear-stress pattern promotes crater-shaped tissue at early culture time
After one week of culture, the experiments show that whatever the flow rates, the tissue partly covers the beads with a crater-like shape. Indeed, we observe tissue overgrowths on the beads around circular areas devoid of cells and located in the narrowed fluid areas (Fig. 5). In order to investigate this formation of craters, we employ computational fluid dynamics to assess the local wall shear stress induced by the flow on the scaffold beads.
We numerically compute the shear stress field for a stationary laminar viscous flow through an idealized three-dimensional arrangement of nine identical spheres in a cylindrical channel. The radius of the channel is 2.7 mm while the radius of spheres is 1 mm. Flow properties are close to those of water at ambient temperature and the imposed inlet velocity is 0.001 m/s. Under these conditions, the associated Reynolds number is of the order 1 and therefore the fluid flow is laminar. Numerical simulations are performed using Comsol Multiphysics (version 4.3b). Figure 6 represents the isovalues of the shear stress field at the surface of the spheres. As expected, the maxima are located in the narrow fluid regions. Importantly, we find that the locations and form of the shear stress maxima obtained numerically correspond to the locations and the form of the crater-like tissue observed experimentally.
It is well known that the proliferation of mechanosensitive cells, including fibroblasts, is enhanced by the flow-induced shear stress. But this stimulation is clearly limited by the intensity of the stress. For high intensity values, cell detachment (which is also material dependent) [53] can be observed but cellular growth is probably prevented before reaching this limit. In the numerical simulations, the higher flow rate (30 ml/min) the maximum shear stress obtained is about 0.1 Pa which is close to the detrimental value (0.3 Pa) obtained by Truskey and Proulx [53] for fibroblasts NIH-3T3 seeded on glass. Note that this value is of the same order of magnitude as the detrimental shear stress obtained for osteoblasts by Leclerc et al. [54]. Although our numerical simulations ignore the presence of tissue on the beads, they provide useful insights into the determination of the spatial distribution of the tissue by the shear stress field in the perfusion bioreactor. Keeping the global flow rate constant through the growth chamber, the coupling of tissue growth with the laminar flow would lead to an intensification of the flow and an expected increase of the fluid shear stress.
3.3 Biomechanical regulation of tissue morphology evolution
After one week of culture, the tissue has partly covered the beads with a crater-like shape (Fig. 5B–D). After two weeks, it clearly appears in Fig. 7 that the tissue grows by building "bridges" between the beads with concave and smooth interfaces. Such shapes are interpreted as the result of curvature minimization [29, 55, 56]. In other words, this results from the contribution of a tension line between the anchoring points and due to the acto-myosin cable along the edge of the tissue, balanced by a surface tension which comes from the global contractility of the cells [57].
Visualization obtained by X-ray micro-tomography of the tissue on the scaffold after two weeks of culture at a flow rate 20 ml/min. A 3D side view. B 2D representative slide (intensity normalized with respect to the maximum intensity Im). C, D Close-up views, E variation of the relative intensity I/Im associated to each pixel along the yellow line drawn on (D)
Figure 7 also shows that the density of the tissue at the interface with the culture medium is higher than in the center of the "bridges". It seems that this phenomenon is related to the fact that cells in this interfacial region are mechanically stimulated by the shear stress induced by the flow of the medium. Interestingly, Kollmannsberger et al. [31] and Granet et al. [2] showed that cell proliferation preferentially occurs at the tissue-medium interface, where a higher mechanical stimulation results in a higher cell proliferation rate [58]. The graph presented in Fig. 7E shows that this behavior is more pronounced in the parietal region presumably due to the channeling effect (Fig. 7D).
Finally, it is worth recalling that cell contractility has also an important impact on the tissue morphology. Indeed, the evolution of the tissue depends on the balance between cell–cell and cell-scaffold interactions. After one week of culture, the formation of a few narrower "rings" where the tissue is partly ripped off (Fig. 5B–D) suggests that the tissue is subjected to considerable tensions. Such tears in the tissue have been observed with keratinocytes [59] and fibroblasts [60] forming suspended bridges and have been attributed to cell contractility. In addition, images of bioreactors cultured three weeks clearly show that the tissue has a dense sheet-like shape whatever the location in the bioreactors (Fig. 3), presumably as the result of tissue contraction. This phenomenon is also either initiated or complete after two weeks of culture all along the bioreactors (Fig. 7C). The detachment of the tissue from the scaffold suggests that cell adhesion forces to the substrate are too weak to counterbalance the traction forces due to cell contractility.
4 Conclusion
In this work, we used a combination of tissue engineering, X-ray micro-tomography imaging, and fluid mechanics simulations to relate tissue morphology and local fluid flow conditions in a model perfusion bioreactor. The model bioreactor consisted of fibroblasts cells grown on a scaffold made of a stack of beads in a perfusion chamber.
Our results highlight the heterogeneous distribution of the cells as the consequence of a channeling effect in the bioreactor. Because of the particular arrangement of the bead packing and the subsequent higher porosity and permeability at the periphery of the bioreactor, the flow is more intense in the channel at the periphery. In our model bioreactor, this effect results in a preferred proliferation around the bead stack and the formation of a very dense envelope of tissue. The dimensions of the beads and bioreactor determine the importance of these boundary effects: a small bead to bioreactor diameter ratio means a weak channeling effect.
Furthermore, we identify crater-shaped tissue patterns at early culture times. We show that this morphology resembles the distribution of fluid shear stress on beads, highlighting the importance of local fluid flow on tissue development.
Finally, we find that after one, two and three weeks of culture, the tissue adopts very distinct morphologies, and attribute these observations to the simultaneous growth and contraction of the connective tissue as it grows.
By providing insights into the coupling between tissue development and fluid flow in a perfusion bioreactor, this work has potential implications in the effective design of bioreactors relying on hydrodynamic stimulii for tissue engineering.
References
Vunjak-Novakovic G, Freed LE, Biron RJ, Langer R. Effects of mixing on the composition and morphology of tissue-engineered cartilage. AIChE J. 1996;42:850–60.
Granet C, Laroche N, Vico L, Alexandre C, Lafage-Proust MH. Rotating-wall vessels, promising bioreactors for osteoblastic cell culture: comparison with other 3d conditions. Med Biol Eng Comput. 1998;36:513–9.
David B, Bonnefont-Rousselot D, Oudina K, Degat MC, Deschepper M, Viateau V, et al. A perfusion bioreactor for engineering bone constructs: an in vitro and in vivo study. Tissue Eng Part C Methods. 2011;17:505–16.
Yeatts AB, Fisher JP. Tubular perfusion system for the long-term dynamic culture of human mesenchymal stem cells. Tissue Eng Part C Methods. 2011;17:337–48.
Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, et al. Fluid flow increases mineralized matrix deposition in 3d perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99:12600–5.
Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171–81.
Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107:3299–304.
Grayson WL, Marolt D, Bhumiratana S, Fröhlich M, Guo XE, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108:1159–70.
Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16:5517–27.
Robeson MJ. Model of a tubular perfusion bioreactor using computational fluid dynamics. [Electron Theses Dissertation], Louisville, USA: University of Louisville; 2015.
Sanaei P, Cummings LJ, Waters SL, Griffiths IM. Curvature-and fluid-stress-driven tissue growth in a tissue-engineering scaffold pore. Biomech Model Mechanobiol. 2019;18:589–605.
Glowacki J, Mizuno S, Greenberger JS. Perfusion enhances functions of bone marrow stromal cells in three-dimensional culture. Cell Transplant. 1998;7:319–26.
Scaglione S, Wendt D, Miggino S, Papadimitropoulos A, Fato M, Quarto R, et al. Effects of fluid flow and calcium phosphate coating on human bone marrow stromal cells cultured in a defined 2d model system. J Biomed Mater Res A. 2018;86:411–9.
Scheper T, Faurie R, Thommel J. Advances in biochemical engineering/biotechnology, vol. 109. Berlin: Springer; 2007.
Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–6.
Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20.
Ladoux B, Nicolas A. Physically based principles of cell adhesion mechanosensitivity in tissues. Rep Prog Phys. 2012;75:116601.
Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J Cell Physiol. 1990;143:100–4.
Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–4.
Li Z, Lee H, Zhu C. Molecular mechanisms of mechanotransduction in integrin-mediated cell-matrix adhesion. Exp Cell Res. 2016;349:85–94.
Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–69.
McCoy RJ, O’Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng Part B Rev. 2010;16:587–601.
Brosig M. Mechanotransduction in fibroblasts. [Doctoral dissertation]. Basel, Switzerland: University of Basel; 2011.
Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–35.
Korossis S, Bolland F, Kearney J, Fisher J, Ingham E. Bioreactors in tissue engineering. Topics Tissue Eng. 2005;2:1–23.
Ladoux B, Mège RM. Mechanobiology of collective cell behaviours. Nat Rev Mol Cell Biol. 2017;18:743–57.
Bidan CM, Kollmannsberger P, Gering V, Ehrig S, Joly P, Petersen A, et al. Gradual conversion of cellular stress patterns into pre-stressed matrix architecture during in vitro tissue growth. J R Soc Interface. 2016;13:20160136.
Sakar MS, Eyckmans J, Pieters R, Eberli D, Nelson BJ, Chen CS. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat Commun. 2016;7:11036.
Rumpler M, Woesz A, Dunlop JW, van Dongen JT, Fratzl P. The effect of geometry on three-dimensional tissue growth. J R Soc Interface. 2008;5:1173–80.
Sharma P, Ng C, Jana A, Padhi A, Szymanski P, Lee JSH, et al. Aligned fibers direct collective cell migration to engineer closing and nonclosing wound gaps. Mol Biol Cell. 2017;28:2579–88.
Kollmannsberger P, Bidan CM, Dunlop JW, Fratzl P, Vogel V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci Adv. 2018;4:eaao4881.
Cruel M, Bensidhoum M, Nouguier-Lehon C, Dessombz O, Becquart P, Petite H, et al. Numerical study of granular scaffold efficiency to convert fluid flow into mechanical stimulation in bone tissue engineering. Tissue Eng Part C Methods. 2015;21:863–71.
Hossain MS, Bergstrom DJ, Chen XB. Prediction of cell growth rate over scaffold strands inside a perfusion bioreactor. Biomech Model Mechanobiol. 2015;14:333–44.
Lesman A, Blinder Y, Levenberg S. Modeling of flow-induced shear stress applied on 3D cellular scaffolds: Implications for vascular tissue engineering. Biotechnol Bioeng. 2010;105:645–54.
Nava MM, Raimondi MT, Pietrabissa R. Multiphysics 3D model of tissue growth under interstitial perfusion in a tissue-engineering bioreactor. Biomech Model Mechanobiol. 2013;12:1169–79.
Bidan CM, Kommareddy KP, Rumpler M, Kollmannsberger P, Bréchet YJ, Fratzl P, et al. How linear tension converts to curvature: geometric control of bone tissue growth. PLoS One. 2012;7:e36336.
Guyot Y, Papantoniou I, Luyten FP, Geris L. Coupling curvature-dependent and shear stress-stimulated neotissue growth in dynamic bioreactor cultures: a 3D computational model of a complete scaffold. Biomech Model Mechanobiol. 2016;15:169–80.
Chabanon M, Duval H, Francais O, Lepioufle B, Perrin E, Goyeau B, et al. Discrete model combined with mimetic microfluidic chips to study cell growth in porous scaffold under flow conditions. Comput Methods Biomech Biomed Engin. 2012;15:S25–6.
Chabanon M, Duval H, Grenier J, Beauchesne C, Goyeau B, David B. Histological method to study the effect of shear stress on cell proliferation and tissue morphology in a bioreactor. Tissue Eng Regen Med. 2019;16:225–35.
Thibeaux R, Duval H, Smaniotto B, Vennat E, Néron D, David B. Assessment of the interplay between scaffold geometry, induced shear stresses, and cell proliferation within a packed bed perfusion bioreactor. Biotechnol Prog. 2019;35:e2880.
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Fibroblasts and their transformations: the connective-tissue cell family. In: Alberts B, Johnson A, Lewis J, Raff J, Robert K, Walter P, editors. Molecular biology of the cell. 4th ed. New York: Garland Science; 2002.
Wang JHC, Thampatty BP, Lin JS, Im HJ. Mechanoregulation of gene expression in fibroblasts. Gene. 2007;391:1–15.
David B, Petite H, Myrtil V. Reacteur pour la mise en oeuvre d’un procédé de culture de tissus osseux. WO Patent App. PCT/FR2008/000,846; 2009.
Yeatts AB, Gordon CN, Fisher JP. Formation of an aggregated alginate construct in a tubular perfusion system. Tissue Eng Part C Methods. 2011;17:1171–8.
Orr DE, Burg KJL. Design of a modular bioreactor to incorporate both perfusion flow and hydrostatic compression for tissue engineering applications. Ann Biomed Eng. 2008;36:1228–41.
Lakhkar NJ, Park JH, Mordan NJ, Salih V, Wall IB, Kim HW, et al. Titanium phosphate glass microspheres for bone tissue engineering. Acta Biomater. 2012;8:4181–90.
Vrana NE, Dupret-Bories A, Schultz P, Debry C, Vautier D, Lavalle P. Titanium microbead-based porous implants: bead size controls cell response and host integration. Adv Healthc Mater. 2014;3:79–87.
Thibeaux R, Perrin E, Smaniotto B, Duval H, Vennat E, Neron D, et al. Using x-ray computed tomography for quantification of cell proliferation within a perfusion bioreactor. Comput Methods Biomech Biomed Engin. 2015;18:S2072–3.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Kaviany M. Principles of heat transfer in porous media. Berlin: Springer Science & Business Media; 2012.
Darcy H. Les fontaines publiques de la ville de Dijon: exposition et application. Victor Dalmont; 1856.
Carman PC. Fluid flow through granular beds. Trans Inst Chem Eng. 1937;15:150–66.
Truskey GA, Proulx TL. Relationship between 3T3 cell spreading and the strength of adhesion on glass and silane surfaces. Biomaterials. 1993;14:243–54.
Leclerc E, David B, Griscom L, Lepioufle B, Fujii T, Layrolle P, et al. Study of osteoblastic cells in a microfluidic environment. Biomaterials. 2006;27:586–95.
Bidan CM, Kommareddy KP, Rumpler M, Kollmannsberger P, Fratzl P, Dunlop JW. Geometry as a factor for tissue growth: towards shape optimization of tissue engineering scaffolds. Adv Healthc Mater. 2013;2:186–94.
Kollmannsberger P, Bidan CM, Dunlop JW, Fratzl P. The physics of tissue patterning and extracellular matrix organisation: how cells join forces. Soft Matter. 2011;7:9549–60.
Bischofs IB, Klein F, Lehnert D, Bastmeyer M, Schwarz US. Filamentous network mechanics and active contractility determine cell and tissue shape. Biophys J. 2008;95:3488–96.
Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–9.
Vedula SRK, Hirata H, Nai MH, Brugués A, Toyama Y, Trepat X, et al. Epithelial bridges maintain tissue integrity during collective cell migration. Nat Mater. 2014;13:87–96.
Rossier OM, Gauthier N, Biais N, Vonnegut W, Fardin MA, Avigan P, et al. Force generated by actomyosin contraction builds bridges between adhesive contacts. EMBO J. 2010;29:1055–68.
Acknowledgements
This work has been financially supported by the French Agence Nationale de la Recherche through the Investissements d'avenir program (ANR-10-EQPX-37 MATMECA Grant) and the Fondation pour la Recherche Médicale (FRM FDT201805005480). We thank Pascal Silberzan (Institut Curie) for kindly providing the NIH-3T3 cell line and Stéphane Roux (LMT, ENS Paris-Saclay) for his kind help regarding image post-processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare and all co-authors confirm agreement with the final statement. All authors have been appropriately disclosed according to the policy of the Journal.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beauchesne, C.C., Chabanon, M., Smaniotto, B. et al. Channeling Effect and Tissue Morphology in a Perfusion Bioreactor Imaged by X-Ray Microtomography. Tissue Eng Regen Med 17, 301–311 (2020). https://doi.org/10.1007/s13770-020-00246-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00246-8