Abstract
BACKGROUND:
Mesenchymal stromal cells (MSCs) are multipotent stem cells that can differentiate into several cell types. In addition, many studies have shown that MSCs modulate the immune response. However, little information is currently available regarding the maintenance of immunomodulatory characteristics of MSCs through passages. Therefore, we investigated and compared cytokine and gene expression levels from adipose (AD) and bone marrow (BM)-derived MSCs relevant to immune modulation from early to late passages.
METHODS:
MSC immunophenotype, growth characteristics, cytokine expressions, and gene expressions were analyzed.
RESULTS:
AD-MSCs and BM-MSCs had similar cell morphologies and surface marker expressions from passage 4 to passage 10. Cytokines secreted by AD-MSCs and BM-MSCs were similar from early to late passages. AD-MSCs and BM-MSCs showed similar immunomodulatory properties in terms of cytokine secretion levels. However, the gene expressions of tumor necrosis factor-stimulated gene (TSG)-6 and human leukocyte antigen (HLA)-G were decreased and gene expressions of galectin-1 and -3 were increased in both AD- and BM-MSCs with repeated passages.
CONCLUSION:
Our study showed that the immunophenotype and expression of immunomodulation-related cytokines of AD-MSCs and BM-MSCs immunomodulation through the passages were not significantly different, even though the gene expressions of both MSCs were different.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mesenchymal stromal cells (MSCs) are multipotent progenitor cells that can differentiate into mesenchymal lineage tissues, including bone, cartilage, and adipose tissue. Many studies have focused on the regenerative properties of MSCs; however, MSCs also have unique immunomodulatory properties. Studies demonstrated that MSCs can suppress the activation and function of various immune cells in the innate and adaptive immune systems. MSCs are able to suppress the proliferation of T cells [1], B cells [2], and natural killer (NK) cells [3], inhibit the differentiation and maturation of dendritic cells (DCs) [4], and promote the generation of regulatory T cells (Treg) [5].
Cell therapy using the immunomodulatory capacity of MSCs has offered therapeutic approaches for diseases such as Crohn’s disease, graft versus host disease, multiple sclerosis, and rheumatoid arthritis [6]. Recently, applied common doses of MSCs ranged from 1 to 5 million MSCs/kg of bodyweight [7]. So, ex vivo expansion of MSCs for clinical applications is required to meet the demand for sufficient cell dosages. With repeated passages, MSCs undergoing in vitro culture expansion and display changes in biological properties associated with cell aging such as enlarged morphology, decreased expression of MSC-specific surface antigens, and a decrease in differentiating potential [8]. The growth kinetics and differentiation potential of MSCs from different cell sources [9, 10], and cytokine expressions of MSCs at early passage 3 (P3) from different sources have been compared [11, 12]. However, there is little information about whether MSC immunomodulatory properties are maintained through the passages. Recently, Zhuang et al. compared the biological properties of umbilical cord-derived MSCs from early (P3) and late (P15) passages [12]. They suggested that senescent MSCs might have stronger immunomodulatory activity than young MSCs [12]. Thus, the immunomodulatory properties of MSCs according to cell passages need further investigation.
In this study, we investigated and compared the phenotypes and cytokine expressions from early to late passages from AD-MSCs and BM-MSCs. We also examined gene expressions relevant to MSC immunomodulation through the passages.
2 Materials and methods
2.1 Culture of human MSCs
BM-MSCs were provided at passage 2 by the Cell Therapy Center, Severance Hospital (Yonsei University College of Medicine, Seoul, Korea) from three donors after taking their consent. AD-MSCs were obtained from two donors (32- and 41-year old females) undergoing plastic surgery, after written informed consent, in accordance with the Institutional Review Boards (4-2010-0236) of Severance Hospital and one donor from ThermoFisher Scientific (R7788115). Cells were plated at a density of 1 × 106 cells per 10-cm cell culture dish (BD Biosciences, San Diego, CA, USA) with DMEM-low glucose supplemented with 10% fetal bovine serum (Corning, NY, USA), 1% glutamine, 1% non-essential amino acids, 0.01% β-mercaptoethanol, and 1% penicillin–streptomycin (all from HyClone, Pittsburgh, PA, USA). MSCs were cultured at 37 °C in an atmosphere containing 5% CO2, and the medium was changed every 3–4 days. Over the course of expansion, we examined the differences in cell morphology under an inverted phase microscope (Nikon ECLIPSE Ti, Tokyo, Japan). All MSCs were cultured, harvested at passage 4, 6, 8, 10 and stored in liquid nitrogen until use.
2.2 Immunophenotyping
Immunophenotyping of MSCs was conducted at the fourth and tenth passages, and characterized by fluorescence activated cell sorting (FACS) analysis. To accomplish this, the following cell surface antigens were stained with antihuman antibodies (all from BD-Pharmingen, San Diego, CA, USA): surface expression of CD29, CD44, CD73, CD90, CD105 and absence of surface expression of CD14, CD31, CD 34, CD45 and CD106. Additionally, PE-conjugated and FITC-conjugated isotype controls were applied. The cells were stained with the antibodies for 30 min at 4 °C in the dark. Next, cells were collected and analyzed using a Cytomics flow cytometer (Beckman Coulter, Indianapolis, IN, USA). Experiments were performed in triplicate. Obtained data were analyzed using the WinMDI (Scripps Institute, La Jolla, CA, USA).
2.3 Growth characteristics
To compare the growth characteristics of the cells, the population doubling time (period of time required for cells to proliferate or grow) were measured. All cells were plated at a density of 2 × 105 cells in 10-cm cell culture dish (BD Biosciences, San Diego, CA, USA). When the confluent was 80–90%, the cells were harvested and counted by Trypan blue staining (Sigma, St. Louis, MO, USA). The doubling time was calculated and modified based on a previously reported formula [13]. The population doublings were determined at each sub cultivation.
2.4 Cytokine expressions
Supernatant were obtained from every passage of MSCs culture medium after 48 h when the MSCs were plated at 2 × 105/well in 6-well plate (Corning, NY, USA). In our model, MSCs were not treated with any inflammatory stimuli such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon (IFN)-γ compared to other reports [14]. Human IFN-γ, IL-1β, IL-4, IL-6, IL-10, and TNF-α were analyzed using a Human Premixed Multi-Analyte kit (R&D biosystems, Minneapolis, MN, USA). Results were obtained by median fluorescence intensity (MFI) using Luminex machine (Luminex, Billerica, MA, USA). And, human tumor growth factor (TGF)-β1 was measured by sandwich enzyme-linked immunosorbent assay (R&D biosystems, Minneapolis, MN, USA) according to the manufacturer’s protocols. Experiments were performed in triplicate for each sample.
2.5 RNA isolation and real-time PCR
Total RNA was extracted using GeneJET RNA purification kit (Molecular Biology, Waltham, MA, USA), and RNA concentration was determined using a Nano-DropND1000 spectrophotometer (Labtech International, Heathfield, UK). cDNA was synthesized using Maxime RT PreMix (iNtRON, Seongnam, Korea) as follows: an initial denaturation step (at 94 °C for 3 min), 35 cycles of PCR (95 °C for 30 s, 50 °C for 30 s, 72 °C for 15 s) then a final step at 72 °C for 10 min, using the SimpliAmp™ Thermal cycler (Applied Biosystems, Foster, CA, USA). Real-time PCR was performed on ABI Systems 7500 Fast machine (Applied Biosystems, Foster, CA, USA) by using qPCRBIOsyGreen Mix Hi-ROX purchased from PCRBIOSYSTEMS, according to the manufacture’s recommendations. The glyceralde-hyde 3-phosphate dehydrogenase (GAPDH) level was used as an internal control. The resulting cycle threshold (Ct) value was processed based on the comparative Ct method. Primer sequences were previously published and as follows; TNF-stimulated gene (TSG)-6, 5′-GGTGTGTACCACAGAGAAGCA-3′ and 5′-GGGTTGTAGCAATAGGCATCC-3′; human leukocyte antigen (HLA)-G, 5′-GCGGCTACTACAACCAGAGC-3′ and 5′-GCACATGGCACGTGTATCTC-3′; galectin-1, 5′-GGTCTGGTCGCCAGCAACCTGAAT-3′ and 5′-TGAGGCGGTTGGGGAACTTG-3′; galectin-3, 5′-CCAAAGAGGGAATGATGTTGCC-3′ and 5′-TGATTGTACTGCAACAAGTGAGC-3′; GAPDH, 5′-GTGGTCTCCTCTGACTTCAACA-3′ and 5′-CTCTTCCTCTTGTGCTCTTGCT-3′. Each experiment was conducted in triplicate.
2.6 Statistics
Results were expressed as mean ± SEM. All data were analyzed by one-way ANOVA or multiple comparison adjusted with Bonferroni method using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). p value of less than 0.05 was considered statistically significant.
3 Results
3.1 AD-MSC and BM-MSC phenotypes through passages
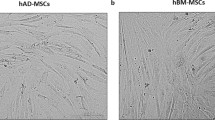
Flow cytometric analyses were performed with AD-MSCs and BM-MSCs. Results showed that the two types of MSCs displayed similar immunophenotypic patterns. CD14, CD31, CD34, CD45, and CD106, which are known as hematopoietic and endothelial cell surface markers, were negative in all passages for AD-MSCs and BM-MSCs. However, negative markers were slightly expressed in the late passage (P10) of MSCs (Fig. 1A, B). But, they were not statistically significant. Negative markers were expressed in less than 1% of MSCs. CD29, CD44, CD73, CD90, and CD105, which are known as typical MSC surface markers were positive in both MSC types. The expression of CD29 was slightly increased in P10 compared to P4 in AD-MSCs, and the expressions of CD90 and CD105 were slightly decreased in P10 compared to P4 in BM-MSCs (Fig. 1A, B), but not statistically significantly. In addition, spindle-shaped fibroblastic morphological features of the MSCs were similar at both the early and late passage (Fig. 1C). The doubling time for human MSCs was prolonged along the cell passage, but it was not statistically significantly (Fig. 1D).
Comparative immunophenotypes and cellular properties of AD-MSCs and BM-MSCs through cell passages. A, B MSCs were stained with surface antibodies and analyzed by flow cytometry. AD-MSCs and BM-MSCs were negative for CD14, CD31, CD34, CD45, CD106, and positive for CD29, CD44, CD73, CD90, and CD105. In AD-MSCs, CD29 expression was slightly increased in P10 compared to AD-MSC P4, but this was not statistically significant. In BM-MSCs, the expressions of CD90 and CD105 were slightly decreased in P10 compared to the P4. But, this was not statistically significant. C Photographs of AD- and BM-MSCs showed spindle-shaped fibroblastic morphological features in early (P4) and late passages (P10). Scale bar = 40 μm. D Doubling times for AD- and BM-MSCs were slightly delayed after repeated passages. Doubling time was determined during each sub cultivation. All values are mean ± SE of the mean (SEM)
3.2 Cytokine levels of AD-MSCs and BM-MSCs through passages
To investigate the cytokine secretion of AD-MSCs and BM-MSCs, we determined the levels of IFN-γ, IL-1β, IL-4, IL-6, IL-10, TNF-α, and TGF-β1 in the supernatant of 2 × 105 cells per well of 6-well plate using a multiple cytokine array kit. Results showed TGF-β1 levels were highly secreted by both AD-MSCs and BM-MSCs through all passages (Fig. 2A). IL-10 levels were similar in all AD- and BM-MSCs passages (Fig. 2B), and the levels of TNF-α and IFN-γ were also similar in all passages of both MSC types (Fig. 2C, D). IL-4 levels were slightly increased with repeated passage of AD- and BM-MSCs, but not significantly (Fig. 2E). IL-6 levels were significantly increased in BM-MSC P6 compared to P4, but it was not significantly increased in AD-MSCs (Fig. 2F). IL-1β levels were expressed at a very low level from P4 to P10, and not detected in BM-MSCs from P6 to P10 in vitro (Fig. 2G).
Human AD- and BM-MSCs cytokine expression through passages. A The levels of TGF-β1 were higher in BM-MSC P4 than late passage. But, this was not significantly different in AD- and BM-MSCs through passages. B–E, G Human IL-10, TNF-α, IFN-γ, IL-4, and IL-1β were scantly produced from P4, 6, 8, and 10 by AD- and BM-MSCs. F IL-6 was highly expressed in both MSC types. This was significantly higher in BM-MSC P6 compared to P4, however there was no significant difference between the other passages. Experiments were performed in triplicate for each sample. All values are the mean ± SEM. *p < 0.05 was considered statistically significant versus P4 of each origin, ns means not significant
3.3 The expression of immunomodulatory genes in AD-MSCs and BM-MSCs through all passages
To assess the immunomodulatory genes of MSCs through all passages, we analyzed gene expression profiles related to immunomodulation with real time PCR that included TSG-6, galectin-1, galectin-3, and HLA-G. The relative quantification of gene expression from MSCs was normalized to the internal control, GAPDH. The expression of TSG-6 was decreased in both AD- and BM-MSCs after repeated passages compared to each MSCs P4 (Fig. 3A–C), and HLA-G expression was also decreased (Fig. 3J–L). Meanwhile, the normalized expressions of TSG-6 and HLA-G were very low through passages. However, the expressions of galectin-1 and galectin-3 were increased in both AD- and BM-MSCs after repeated passages compared to P4 for each MSC type (Fig. 3D–F, G–I). And, the normalized expressions of galectin-1 and galectin-3 were strongly higher than those of TSG-6 and HLA-G showing 100-fold higher expression.
Gene expressions relevant to immune modulation of AD- and BM-MSCs through passages. A–L TSG-6 and HLA-G expressions were decreased, whereas galectin-1 and galectin-3 expressions were increased or maintained throughout passages in AD-MSCs and BM-MSCs. Data is shown as normalized gene expression (A–B, D–E, G–H, J–K) compared to the housekeeping gene, GAPDH, and as relative gene expression (C, F, I, L) compared to AD- and BM-MSC P4. Each experiment was conducted in triplicate. All values are the mean ± SEM. *p < 0.05 was considered statistically significant versus P4 of each origin, ns means not significant
4 Discussion
MSCs are a useful source of adult progenitor cells for cell therapy due to their immunomodulatory capacities. To date, many studies showed that MSCs can affect the activation and function of T cells, B cells, NK cells, regulatory T cells and DC in the innate and adaptive immune systems [1,2,3,4,5]. MSCs have been characterized in relation to their capacity to produce a range of cytokines that affect immune modulation [6]. Many clinical trials have been performed using MSC immunomodulatory properties. In clinical trials, early passage MSCs were preferred for therapeutic efficacy [15]. In future, large numbers of MSCs will be needed to be produced through ex vivo expansion to meet high clinical cell dose demands. If MSCs retain their immunomodulatory properties during late passage expansion, more cultured MSCs from one patient could be obtained. There is little data showing if there are differences in immunomodulatory function after repeated passages. Therefore, evidence for MSC immunomodulatory property maintenance from early to late passage by cell origin (AD-MSC or BM-MSC) is clinically useful.
BM-MSCs have been most widely used in clinical applications, however AD-MSCs have become an attractive alternative to BM-MSCs due to ease of tissue collection, high yield of initial cells and robust in vitro proliferative capacity [10]. Adipose can be obtained via either liposuction aspirates or excised fat under local anesthesia. AD-MSCs from adipose tissue are easier to obtain in larger volumes, adipose harvest is less painful, presents lower risk and adipose yields more stem cells compared to bone marrow [15]. There are 50 times more MSCs in 1 g of fat compared to 1 g of aspirated bone marrow [16].
To assess MSCs consistent immunomodulation capacity, we investigated and compared surface marker, cytokine and gene expressions related to MSC immunomodulatory function from early and late passages. There has been no study investigating immunomodulatory property differences between AD-MSCs and BM-MSCs from early to late passages.
According to our data, surface molecule expression of AD-MSCs and BM-MSCs was not significantly changed, and original cell morphology and population doubling time were not significantly different regardless of repeated passages. MSCs have a limited lifetime and undergo replicative senescence during in vitro culture, as indicated by enlarged and irregular cell shapes and interruption of proliferation [17]. AD- and BM-MSCs exhibited replicative senescence when they reached passage 10 on average. MSCs are theoretically capable of long-term culture in vitro without losing their fundamental stem cell properties.
According to our data, the levels of TNF-α, IFN-γ, IL-1β and IL-4 were very low in all AD- and BM-MSC passages, and IL-6 level was similar in AD- and BM-MSCs except P6 for BM-MSCs. TNF-α and IFN-γ are pro-inflammatory cytokines that induce inflammatory responses in cells and tissues, which are important to increase cell surface HLA I and II class antigen expression, and to reinforce T-lymphocyte activities [18]. IL-4 is a lymphocyte growth and survival factor, which plays a critical role in regulating T cell differentiation during the immune response. IL-6 is involved in the immunoregulatory mechanism mediated by MSCs through a partial inhibition of DC differentiation [19]. The low expression of TNF-α, IFN-γ and IL-4, and maintenance of IL-6 expression may play an important role in maintaining MSC immunomodulation.
Our results showed IL-10 levels were similar in all AD- and BM-MSC passages, and TGF-β1 levels were highly produced in both AD- and BM-MSCs passages. IL-10 plays an important role in MSC-mediated immunosuppression. IL-10 is an anti-inflammatory cytokine with multiple effects in immunomodulation and inflammation. It has been shown to suppress cytokine secretion, antigen presentation and CD4+ T cell activation. IL-10 predominantly inhibited the induction of the pro-inflammatory cytokines TNF-α, IL-1β, IL-12 and IFN-γ [20]. In addition, TGF-β1 is a powerful pleiotropic immunosuppressive and anti-inflammatory cytokine critical for immune modulation. It can decrease inflammatory responses by promoting inhibition of IL-1β and TNF-α and modulating proliferation of T cell subtypes such as Th1, Th17, and Treg [21]. Therefore, IL-10 and TGF-β1 maintenance may play a key role in immunosuppression of MSCs regardless of passage.
In terms of cytokine secretion levels, AD-MSCs and BM-MSCs showed similar patterns. This study showed that the expression of immunomodulation-related cytokines of AD-MSC and BM-MSC through the passages were not significantly different. MSCs late passage (P10) still produced immunomodulatory cytokines similar to MSCs P4. MSCs produce and secrete various soluble/trophic factors, e.g. secretome, exosome, and microvesicles and MSC-derived soluble factors can maintain the function of MSCs [22, 23].
Previously, there has been no report on gene expression relevant to immunomodulation in MSCs from all passages. Thus, we investigated the expression of genes including TSG-6, galectin-1 and -3, and HLA-G in MSCs through early to late passages. A significant decrease in TSG-6 expression was observed in both AD- and BM-MSCs. TSG-6 is a glycoprotein expressed by cells in response to pro-inflammatory cytokines, and it exerts anti-inflammatory actions through multiple mechanisms, one of which is the modulation of myeloid cells into a tolerogenic phenotype [24]. It inhibits the presentation of inflammatory chemokines to the cell surface, thus providing a mechanism through which chemokine expression is regulated [24]. In addition, the expression of HLA-G was significantly decreased in both types of MSC through early to late passages. HLA-G exerts an anti-inflammatory effect by enhancing CD4+ CD25+ FoxP3+ Treg populations [25], increasing the level of IL-10 on DCs, and modulating the activity of NK cells [26]. Therefore, this suggests that reduction of TSG-6 and HLA-G decreases anti-inflammatory potency in MSCs over repeated passages.
However, the expressions of galectin-1 and -3 were significantly increased in both MSCs through all passages. Galectin-1 can modulate a variety of immune response by enhancing FoxP3+ Treg populations, increasing anti-inflammatory cytokines including IL-4 and IL-10 in T cell and inducing Th1 and Th17 cell apoptosis [27]. Moreover, a stable knockdown of galectin-1 in MSCs restored the proliferation of CD4+ and CD8+ T cells [28], also modulating macrophage polarization through enhancing arginase-1 and decreasing iNOS activity [29]. In addition, galectin-3 is a candidate gene that influences the suppressive function of MSCs on T cell proliferation to alloantigens, secreted high levels in MSCs culture supernatant [30]. Galectin-3 deficient mice showed more severe disease activity in DSS-induced colitis and galectin-3 inhibited colonic mucosa inflammation by inducing Treg [31]. According to our data, the normalized expressions of galectin-1 and -3 were strongly higher than those of TSG-6 and HLA-G showing 100-fold higher expression. Therefore, the influence of galectin-1 and galectin-3 should be further investigated in immunomodulatory properties of MSCs with repeated passages.
The present study is limited in that the number of cases is not big enough compared to each sub-cultured AD-MSCs and BM-MSCs from three donors. However, in this study, we observed immunomodulatory cytokine and gene expressions of both MSCs from passage 4 to 10 at every 2 passage intervals for each donor and repeated all the experiments in triplicate to minimize the error. The study compared the differences of cytokine and gene expressions relevant to immune modulation between AD-MSCs and BM-MSCs from the early to late passages first. In the future, additional work will be needed for more patients with different gender and age. Also, the functional and mechanistic studies should be investigated further in the expressions of TSG-6, HLA-G, galectin-1 and -3 relevant to immunomodulation of MSCs.
In conclusion, the phenotype and cytokine expression for both AD-MSCs and BM-MSCs were similarly maintained from early to late passages, i.e. passage 10, even though gene expressions relevant to MSC immune modulation were different through the passages.
Change history
12 January 2019
Unfortunately, the online published article has error in Figure?3. The name of protein, ?Gelectin-1? should be corrected to ?Galectin-3?. The corrected Fig.?3 is placed in the following page.
12 January 2019
Unfortunately, the online published article has error in Figure?3. The name of protein, ?Gelectin-1? should be corrected to ?Galectin-3?. The corrected Fig.?3 is placed in the following page.
References
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72.
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85.
Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7.
Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–18.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96.
Subbanna PK. Mesenchymal stem cells for treating GVHD: in vivo fate and optimal dose. Med Hypotheses. 2007;69:469–70.
Wagner W, Bork S, Lepperdinger G, Joussen S, Ma N, Strunk D, et al. How to track cellular aging of mesenchymal stromal cells? Aging (Albany NY). 2010;2:224–30.
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–97.
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301.
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–6.
Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11:166–74.
Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115–25.
Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 2016;85:51–60.
Pawitan JA. Prospect of adipose tissue derived mesenchymal stem cells in regenerative medicine. Cell Tissue Transpl Ther. 2009;2:7–9.
Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–41.
Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213.
Thejaswi K, Amarnath M, Srinivas G, Jerald MK, Avinash Raj T, Singh S. Immune modulatory responses of mesenchymal stem cells from different sources in cultures and in vivo. Cell Tissue Transpl Ther. 2012;4:1–13.
Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–32.
Varma TK, Toliver-Kinsky TE, Lin CY, Koutrouvelis AP, Nichols JE, Sherwood ER. Cellular mechanisms that cause suppressed gamma interferon secretion in endotoxin-tolerant mice. Infect Immun. 2001;69:5249–63.
Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–53.
Tsai TL, Li WJ. Identification of bone marrow-derived soluble factors regulating human mesenchymal stem cells for bone regeneration. Stem Cell Reports. 2017;8:387–400.
Teshima T, Matsumoto H, Koyama H. Soluble factors from adipose tissue-derived mesenchymal stem cells promote canine hepatocellular carcinoma cell proliferation and invasion. PLoS One. 2018;13:e0191539.
Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci U S A. 2016;113:158–63.
Kapasi K, Albert SE, Yie S, Zavazava N, Librach CL. HLA-G has a concentration-dependent effect on the generation of an allo-CTL response. Immunology. 2000;101:191–200.
Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–44.
Woodward EA, Prêle CM, Nicholson SE, Kolesnik TB, Hart PH. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology. 2010;131:118–27.
Gieseke F, Böhringer J, Bussolari R, Dominici M, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–9.
Wang J, Xia J, Zhang F, Shi Y, Wu Y, Pu H, et al. Galectin-1-secreting neural stem cells elicit long-term neuroprotection against ischemic brain injury. Sci Rep. 2015;5:9621.
Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010;71:267–74.
Tsai HF, Wu CS, Chen YL, Liao HJ, Chyuan IT, Hsu PN. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J Mol Med (Berl). 2016;94:545–56.
Acknowledgements
This study was supported by a Grant of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2058120). The authors are grateful to Dong-Su Jang, (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
BM-MSCs were provided at passage 2 by the Cell Therapy Center, Severance Hospital (Yonsei University College of Medicine, Seoul, Korea) from three donors after taking their consent. AD-MSCs were obtained from two donors (32- and 41-year old females) undergoing plastic surgery, after written informed consent, in accordance with the Institutional Review Boards (4-2010-0236) of Severance Hospital and one donor from ThermoFisher Scientific (R7788115).
Rights and permissions
About this article
Cite this article
Mun, C.H., Kang, MI., Shin, Y.D. et al. The Expression of Immunomodulation-Related Cytokines and Genes of Adipose- and Bone Marrow-Derived Human Mesenchymal Stromal Cells from Early to Late Passages. Tissue Eng Regen Med 15, 771–779 (2018). https://doi.org/10.1007/s13770-018-0147-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-018-0147-5