Abstract
For the cartilage repair, the cell sources currently adopted are primarily chondrocytes or mesenchymal stem cells (MSCs). Due to the fact that chondrocytes dedifferentiate during 2-dimensional (2D) expansion, MSCs are generally more studied and considered to have higher potential for cartilage repair purposes. Here we question if the dedifferentiated chondrocytes can regain the chondrogenic potential, to find potential applications in cartilage repair. For this we chose chondrocytes at passage 12 (considered to have sufficiently dedifferentiated) and the expression of chondrogenic phenotypes and matrix syntheses were examined over 14 days. In particular, the chondrogenic potential of MSCs was also compared. Results showed that the dedifferentiated chondrocytes proliferated actively over 14 days with almost 2.5-fold increase relative to MSCs. Moreover, the chondrogenic ability of chondrocytes was significantly higher than that of MSCs, as confirmed by the expression of a series of mRNA levels and the production of cartilage extracellular matrix molecules in 2D-monolayer and 3-dimensional (3D)-spheroid cultures. Of note, the significance was higher in 3D-culture than in 2D-culture. Although more studies are needed such as the use of different cell passages and human cell source, and the chondrogenic confirmation under in vivo conditions, this study showing that the dedifferentiated chondrocytes can also be a suitable cell source for the cell-based cartilage repair, as a counterpart of MSCs, will encourage further studies regarding this issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Articular cartilage damages including joint trauma or osteoarthritis are one of the challenging issues in clinics. Cartilage is almost intractable to regenerate due to the absence of vascularity [1]. Current techniques to treat traumatic cartilage injuries include mosaicplasty and microfracture which can help the repair of the articular cartilage lesions and reduction of pain in joints to some degree. However, these techniques often generate fibrous cartilage and hold the issues of donor-site morbidity, an alternative method needs to be developed [2]. More recently, cell-based therapy is considered one of the most promising approaches to repair the damaged cartilage tissue [3, 4].
Cells used for cartilage therapy include chondrocytes, mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs). Although some studies have demonstrated that the pluripotent stem cells (ESCs and iPSCs) have the potential to undergo efficient chondrogenic differentiation [5, 6], their clinical applications still remain due to biosafety and side effects [7]. Therefore, the chondrocytes and the MSCs are currently considered two primary cell sources for cartilage repair.
Chondrocytes are the cells responsible for the production of cartilage extracellular matrix (ECM) including type II collagen and proteoglycans. Therefore, chondrocytes represent the logical choice for cell-based therapies in cartilage tissue regeneration [8,9,10,11,12]. The most conventional cell-based treatment is autologous chondrocyte implantation (ACI) [8, 13]. This treatment is based on the implantation of expanded autologous chondrocytes that isolated from the non-load-bearing site of the same donor. Due to the relatively low cellularity of the cartilage biopsies, in vitro expansion procedure is necessary to obtain a sufficient number of chondrocytes. Unfortunately, the expansion is associated with a progressive loss of the chondrogenic phenotype resulting in dedifferentiated chondrocytes [14, 15]. Interestingly, some studies have demonstrated that the dedifferentiated chondrocytes can be redifferentiated during culture in gels of alginate and agarose in the absence of the TGF-β superfamily [16, 17].
On the other hand, MSCs are used as another alternative source for cartilage regeneration owing to their abundance in various tissues including bone marrow, adipose tissue, umbilical cord, and synovial membrane, and a vast differentiation potential towards chondrogenesis [18,19,, 19]. Cartilage defects were treated with MSCs alone [20, 21], or their combination with biomaterials to enable tissue-engineered cartilage regeneration [22,23,24,25]. Importantly, it is necessary to supplement the TGF-β superfamily for successful chondrogenic differentiation of MSCs in hydrogel-based or scaffold-free cell cultures [26]. Moreover, cartilage formed by differentiated MSCs displays more typical signs of hypertrophic differentiation than articular chondrocytes [27, 28]. The articular chondrocytes inhibit hypertrophic chondrocyte differentiation of MSCs [29], although the exact mechanism underlying this phenomenon is yet to be clarified.
In the present study, we question if the dedifferentiated chondrocytes can restore the chondrogenic potential to find potential applications in cartilage repair. For this, we chose chondrocytes of passage 12 and examined their chondrogenic potential under 2D-monolayer and 3D-spheroid culture conditions. In general, the chondrocytes are considered to dedifferentiate after an average of about five monolayer passages [30, 31]; thus the passage 12 used in this study is considered to reflect a cell source sufficiently dedifferentiated. For comparison purpose, the MSCs of passage 4 from rats were also used as the passage 3–5 cells have been shown to hold high purity and stable biological characteristics [32, 33]. As a pilot experiment, we briefly analyzed the proliferative potential and chondrogenic marker expressions of the cells.
2 Materials and methods
2.1 Isolation and culture of rat chondrocytes
Rat articular chondrocytes were collected according to the procedures in a previous study [34]. Briefly, the chondrocytes were harvested from articular cartilage of the knees of a Sprague–Dawley rat. The cells were incubated in chondrocyte growth medium, consisting of Dulbecco’s Modified Eagle’s medium (DMEM, 4.5 g/L glucose; Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL) and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 at 37 °C. The cells were used after twelve passages for the following experiments.
2.2 Isolation and culture of rat MSCs
Rat bone marrow-derived MSCs were isolated according to the procedures in a previous study [35]. Briefly, the MSCs were harvested from the excised proximal and distal epiphyses of the femora and tibiae of the Sprague–Dawley rat. These cells were grown and maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 at 37 °C. The cells were used after four passages for the following experiments.
2.3 F-actin observation
The cell growth image was observed by Alexa Fluor 488-conjugated phalloidin (Invitrogen, USA) staining using an inverted fluorescence microscope. Cells grown on each group were fixed with 4% (v/v) paraformaldehyde (PFA) for 30 min, treated with 0.2% Triton X-100 for 5 min, blocked with PBS containing 1% (w/v) bovine serum albumin for 30 min, and then incubated with 20 nM Alexa Fluor 488-conjugated phalloidin diluted in PBS for 30 min. The nuclei of the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. Fluorescence images were obtained using an inverted fluorescence microscope equipped with a DP-72 digital camera (Olympus Co., Tokyo, Japan).
2.4 Cellular proliferation assay
To quantify the proliferation of the cells, the MSCs and the chondrocytes were plated at density of 1 × 104 cells/well in 24-well plates in 2 mL growth medium and cell counting was performed by the trypan blue exclusion test as previously described [36]. Briefly, a uniform suspension containing the cells was added to the trypan blue dye by a 1:1 dilution. The number of viable cells was obtained by counting the cells of four 1 × 1 mm2 squares of a hemocytometer and averaging. The total cell number of viable (unstained) cells was calculated directly as mean values of viable cells. Cell counting results were obtained from three independent samples and presented as average.
2.5 Chondrogenic differentiation in 2D-monolayer and 3D-spheroid cultures
To obtain a chondrogenic differentiation in 2D culture, the MSCs and the chondrocytes were plated at density of 5 × 104 cells/well in 24-well plates in 2 mL chondrogenic differentiation medium, consisting of chondrocyte growth medium supplemented with 1% insulin-transferrin-selenium (PAA Laboratories, Inc.), 37.5 mg/mL ascorbic acid, 100 nM dexamethasone, and 10 ng/mL transforming growth factor-β1 (PeproTech), and incubated for 14 days, respectively. The medium was refreshed every 2 days.
To observe the chondrogenic potential in 3D culture, spheroids were formed by polydimethylsiloxane-based concave micromolds (Prosys® StemFit 3D; Prodizen Inc., Seoul, Korea) with 600 μm diameters as described previously [37]. Briefly, the MSCs and the chondrocytes were seeded at a density of 2 × 106 cells/mL in the concave micromolds and subsequently cultured in 5 mL chondrogenic differentiation medium for 7 days, respectively. The medium was refreshed every 2 days.
2.6 Quantitative real-time polymerase chain reaction (qPCR)
Quantitative analysis of the chondrocyte-related genes was conducted by qPCR as described previously [38]. RNAs were extracted respectively from the 2D culture samples from day 14 and the 3D culture spheroids from day 7 using TRIzol reagent (Invitrogen). The first strand cDNA was synthesized from the total RNA (1 μg) using a SuperScript first strand synthesis system for real-time PCR (Invitrogen) according to the manufacturer’s instructions. The reaction mixture was made up to 50 μL. Real-time PCR was conducted using SYBR GreenER qPCR SuperMix reagents (Invitrogen). The relative transcript quantities were calculated using the \( 2^{{ - {\Delta \Delta }C_{\text{t}} }} \) method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous reference gene amplified from the samples. The primer sequences of the genes are summarized in Table 1.
2.7 Immunofluorescence staining
The 2D culture samples from day 14 and the 3D spheroids from day 7 were used to detect the expression of type II collagen by immunofluorescence staining. The samples were fixed with 4% PFA for 10 min, blocked with 1% (w/v) bovine serum albumin for 30 min to suppress nonspecific staining, and then incubated with a primary antibody, anti-type II collagen (1:150 dilution, sc-52658; Santa Cruz Biotechnology), for 24 h at 4 °C. The specimens were subsequently incubated with the FITC-conjugated antibody against mouse IgG (1:100 dilution, 115-095-003; Jackson Immunoresearch) for 50 min at room temperature. The nuclei of the cells were counterstained with DAPI for 5 min. The samples were examined with an inverted fluorescence microscope equipped with a DP-72 digital camera (Olympus Co., Tokyo, Japan).
2.8 GAG content assay
The 2D culture samples from day 14 were digested with 300 μg/mL Papain in 20 mM PBS (pH 6.8) at 60 °C for 1 h. After digestion, the sulfated GAG content was measured by dimethyl methylene blue (DMMB, Cat# 8000, Astartebio Ltd., USA) using chondroitin sulfate from bovine trachea (Astartebio Ltd., USA) as described previously [38]. An absorbance was read at 525 nm using a spectrophotometer. The DNA content was determined using the PicoGreen quantitation assay kit (Molecular Probe) with supplied lambda DNA as previously described [39]. The fluorescence intensity at 530 nm with excitation at 485 nm was measured with a multimode microplate plate reader (Spectra Max M2e, Molecular Devices). GAG values were normalized to the DNA content.
2.9 Histological analysis of 3D spheroids
For the assessment of GAG production, the 3D spheroids from day 7 were harvested and fixed with 4% PFA for 10 min, and then stained with Alcian blue (Sigma-Aldrich, USA) for 30 min. Images were observed under an optical microscope.
2.10 Statistical analysis
All conditions were done in triplicate and at least three independent experiments were performed unless otherwise stated. All data are shown as the mean ± standard deviation (SD), or otherwise stated in the figure captions. Statistical comparisons were made using the Student’s t test. p < 0.05 was considered to be statistically significant, with * = p < 0.05; ** = p < 0.01; **** = p < 0.0001.
3 Results
3.1 Cell morphology and proliferation
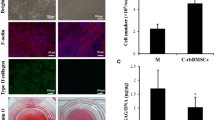
Both chondrocytes at passage 12 and MSCs at passage 4 were cultured in the growth medium, and the cell morphologies were observed. At 1 day after plating, the chondrocytes showed a flattened morphology under a phase-contrast microscope (Fig. 1A). The chondrocytes were then cultured over 14 days. Cells proliferated rapidly and formed almost confluent monolayer by day 7. From day 7 to day 14, the chondrocytes expanded substantially. Under a fluorescence microscope, a number of chondrocytes were well revealed, with an active cytoskeletal development (Fig. 1B, C). The morphology of MSCs at day 1 exhibited mostly a spindle morphology (Fig. 1D). The cells proliferated actively over the culture of 14 days, as visualized on a fluorescence image (Fig. 1E, F). Compared to MSCs, chondrocytes appeared to show more aggregated grow morphology.
Cell morphology and proliferation. Chondrocytes and MSCs at day 1, and grown for 7 and 14 days. A, D day 1; B, E day 7; C, F day 14. Phase-contrast microscopy (A and D, scale bar = 100 μm), and fluorescence microscopy with phalloidin (green) and DAPI (blue) double staining (B, C, E and F, scale bar = 140 μm). (Color figure online)
The cell proliferation over the culture period of 14 days was then quantified by a trypan blue exclusion method (Fig. 2). The results showed that both the chondrocytes and the MSCs had an active proliferation rate. During the 14-day culture period, expansion of the chondrocytes was significantly greater than that of MSCs. After 7 days of culture, the MSCs and chondrocytes showed an expansion of 6.5-fold and 10.3-fold, respectively. After 14 days of culture, the expansion was 10.5-fold and 28.7-fold for the MSCs and chondrocytes, respectively.
3.2 Chondrogenic phenotypes expression and matrix production in 2D
The expression of chondrocyte-related genes, SOX9, type II collagen and aggrecan, was firstly assessed in 2D condition at day 14 by qPCR. The expression of genes demonstrated that the chondrocytes showed significantly higher expression of SOX9, type II collagen, and aggrecan than the MSCs, by a significant difference of 6.1-fold, 12.6-fold, and 4.7-fold, respectively (Fig. 3).
Next, the production of cartilage matrix molecules was analyzed. The immunofluorescence staining of type II collagen was conducted, because it is the basis for hyaline cartilage, and a well-documented marker of articular cartilage. The results showed that the chondrocytes have more intense green fluorescence signal with respect to the MSCs (Fig. 4A, B), suggesting that the chondrocytes might secret more cartilage extracellular matrix. The GAG production was also measured (Fig. 4C). The results showed that the production of GAG was significantly higher in the chondrocytes than in the MSCs.
A, B Immunofluorescence images of the MSCs and chondrocytes in 2D culture for 14 days. FITC-conjugated type II collagen (green), DAPI-stained nuclei of cells (blue). Scale bar: 140 μm. C Biochemical assay of GAG production expressed as GAG/DNA. A significant difference between groups was noted at *p < 0.05 (n = 3). (Color figure online)
3.3 Chondrogenic phenotypes expression and matrix production in 3D
Similar to the previous analysis, the expression of chondrocyte-related genes was analyzed in 3D spheroids at day 7 by qPCR. Figure 5 showed that the chondrocytes have significantly greater expression of SOX9, type II collagen, and aggrecan than the MSCs, by a significant difference of 10.3-fold, 25.6-fold, and 48.6-fold, respectively. Meanwhile, the production of cartilage matrix was examined by the immunofluorescence staining of type II collagen and Alcian blue staining of GAG content.
As shown in Fig. 6, the green fluorescence signals were found to be more robust in chondrocytes than in MSCs. The production of GAG was observed to be more positive for Alcian blue staining in chondrocytes than in MSCs.
Histological analysis for the production of cartilage matrix in 3D condition. Immunofluorescence staining of A MSCs and B chondrocytes for type II collagen. FITC-conjugated type II collagen (green), DAPI-stained nuclei of cells (blue). Alcian blue staining of C MSCs and D chondrocytes for GAG production. Scale bar: 140 μm, (A, B); 50 μm (C, D). (Color figure online)
4 Discussion
Cell therapy for cartilage regeneration needs a large number of progenitors with chondrogenic potential. While chondrocytes are one of the major cell sources for this purpose, the dedifferentiation behaviors under 2D expansions are considered to limit the potential uses of chondrocytes. Therefore, substantial effort has been made to overcome the dedifferentiation of chondrocytes, which includes three-dimensional culture systems, like embedding chondrocytes in natural polymer gels [14, 40,41,42] or in synthetic polymer gels [43], and high-density cultures to form spheroid more extensively [44,45,46]. Those culture environments could favor the maintenance of the chondrocyte phenotypes and support the redifferentiation of the dedifferentiated characteristics.
On the other hand, as an additional cell source for cartilage repair, the MSCs, either derived from bone marrow or adipose tissues, have recently been considered to be a more potential cell source than the chondrocytes. However, the hypertrophy of chondrogenic MSCs could often lead to an ossification of the repaired tissue [47,48,49,50], often raising a concern on the extensive clinical applications of MSCs for the cartilage repair.
Here we examined the proliferative and chondrogenic potential of dedifferentiated chondrocytes, to provide useful information for future cartilage repair. The cell proliferation data demonstrated that the chondrocytes have excellent expansion capacity (even higher than the MSCs), suggesting the excellent potential for cell therapy. From literatures, human chondrocytes cultured on 2D dishes were shown to have a high proliferative capacity through optimizing the culture and cryopreservation conditions of cells [14, 51,52,53]. To enable successful cell therapy, a prerequisite is the large population of cells; thus, the cellular capacity to expand rapidly is considered an important asset of cell sources to be clinically available.
However, the dedifferentiated chondrocytes following about five monolayer passages were shown to fail to produce cartilage matrix and lose their phenotypes [30, 31]. Even so, when the dedifferentiated chondrocytes were re-cultured in 3D environments such as a gel [8, 16], a pellet [54], or a 3D scaffold [55], they were shown to regain sufficient chondrogenic potential, suggesting the dedifferentiated characteristics of chondrocytes could be overcome and thus the cell source might be used with a clinical relevance. All these culture systems are based on high-density cell cultures preventing the cells from adhesion and therefore creating a suitable environment for mimicking embryonic cartilage development [56].
Although this study was conducted limitedly in terms of using one-passage cells and in vitro assays, the results showed the chondrocytes at a passage of 12 expressed substantial levels of chondrogenic genes (SOX9, type II collagen, and aggrecan) and matrix molecules (type II collagen protein and GAGs), even higher than the MSCs. In fact, from clinical reports, patients younger than 45 years of age have shown significantly better improvements than those older than 45 years in the repair of articular cartilage when autologous chondrocytes were used rather than the MSCs [57].
In conclusion, this study demonstrates that the proliferation and chondrogenic potential of the dedifferentiated rat chondrocytes was higher than that of bone marrow MSCs over the culture period of 14 days, from a set of comparison experiments, and the potential was more obvious in 3D-culture conditions. It is thus considered that the chondrocytes might be a more suitable source for the cell-based cartilage repair, as a counterpart of the MSCs. However, more in-depth in vitro and in vivo studies are still needed to confirm the potential of dedifferentiated chondrocytes in comparison to the MSCs. For example, detailed characterization of the passage-dependent dedifferentiation of chondrocytes, the mechanistic study of their redifferentiation under specified conditions (like 3D gels and high density cultures), and the use of human cells with in vivo findings, will help extending the clinical usefulness of chondrocytes as an alternative cell source of MSCs.
References
Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–63.
Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop. 2017;14:A1–3.
Park DY, Min BH, Lee HJ, Kim YJ, Choi BH. Repair of partial thickness cartilage defects using cartilage extracellular matrix membrane-based chondrocyte delivery system in human Ex Vivo model. Tissue Eng Regen Med. 2016;13:182–90.
Jang JH, Lee JS, Lee EY, Lee EA, Son YS. Disc-type hyaline cartilage reconstruction using 3D-cell sheet culture of human bone marrow stromal cells and human costal chondrocytes and maintenance of its shape and phenotype after transplantation. Tissue Eng Regen Med. 2016;13:352–63.
Yamashita A, Nishikawa S, Rancourt DE. Identification of five developmental processes during chondrogenic differentiation of embryonic stem cells. PLoS One. 2010;5:e10998.
Wei Y, Zeng W, Wan R, Wang J, Zhou Q, Qiu S, et al. Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur Cell Mater. 2012;23:1–12.
Mousavinejad M, Andrews PW, Shoraki EK. Current biosafety considerations in stem cell therapy. Cell J. 2016;18:281–7.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95.
Kim JM, Han JR, Shetty AA, Kim SJ, Choi NY, Park JS. Comparison between total knee arthroplasty and MCIC (autologous bone marrow mesenchymal-cell-induced-chondrogenesis) for the treatment of osteoarthritis of the knee. Tissue Eng Regen Med. 2014;11:405–13.
do Amaral RJ, Matsiko A, Tomazette MR, Rocha WK, Cordeiro-Spinetti E, Levingstone TJ, et al. Platelet-rich plasma releasate differently stimulates cellular commitment toward the chondrogenic lineage according to concentration. J Tissue Eng. 2015;6:2041731415594127.
Im GI. Tissue engineering for osteochondral defects. Tissue Eng Regen Med. 2008;5:552–8.
Sancho-Tello M, Martorell S, Mata Roig M, Milián L, Gámiz-González MA, Gómez Ribelles JL, et al. Human platelet-rich plasma improves the nesting and differentiation of human chondrocytes cultured in stabilized porous chitosan scaffolds. J Tissue Eng. 2017;8:2041731417697545.
Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB Jr, Micheli LJ, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;436:237–45.
Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313–21.
Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res. 2008;26:1230–7.
Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, et al. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994;212:97–104.
Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–58.
Guha Thakurta S, Budhiraja G, Subramanian A. Growth factor and ultrasound-assisted bioreactor synergism for human mesenchymal stem cell chondrogenesis. J Tissue Eng. 2015;6:2041731414566529.
Blashki D, Murphy MB, Ferrari M, Simmons PJ, Tasciotti E. Mesenchymal stem cells from cortical bone demonstrate increased clonal incidence, potency, and developmental capacity compared to their bone marrow-derived counterparts. J Tissue Eng. 2016;7:2041731416661196.
Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cells. 2007;25:2964–71.
Emadedin M, Aghdami N, Taghiyar L, Fazeli R, Moghadasali R, Jahangir S, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15:422–8.
Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y, et al. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–9.
Zheng L, Fan HS, Sun J, Chen XN, Wang G, Zhang L, et al. Chondrogenic differentiation of mesenchymal stem cells induced by collagen-based hydrogel: an in vivo study. J Biomed Mater Res A. 2010;93:783–92.
Zhou XZ, Leung VY, Dong QR, Cheung KM, Chan D, Lu WW. Mesenchymal stem cell-based repair of articular cartilage with polyglycolic acid-hydroxyapatite biphasic scaffold. Int J Artif Organs. 2008;31:480–9.
Seo SJ, Mahapatra C, Singh RK, Knowles JC, Kim HW. Strategies for osteochondral repair: focus on scaffolds. J Tissue Eng. 2014;5:2041731414541850.
Freyria AM, Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury. 2012;43:259–65.
Leijten JC, Emons J, Sticht C, van Gool S, Decker E, Uitterlinden A, et al. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64:3302–12.
Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–88.
Jikko A, Kato Y, Hiranuma H, Fuchihata H. Inhibition of chondrocyte terminal differentiation and matrix calcification by soluble factors released by articular chondrocytes. Calcif Tissue Int. 1999;65:276–9.
Kang SW, Yoo SP, Kim BS. Effect of chondrocyte passage number on histological aspects of tissue-engineered cartilage. Biomed Mater Eng. 2007;17:269–76.
Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–8.
Li X, Zhang Y, Qi G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells. Cytotechnology. 2013;65:323–34.
Osipova EY, Shamanskaya TV, Kurakina OA, Nikitina VA, Purbueva BB, Ustugov AY, et al. Biological characteristics of mesenchymal stem cells during ex vivo expansion. Br J Med Med Res. 2011;1:85–95.
Jin GZ, Kim JJ, Park JH, Seo SJ, Kim JH, Lee EJ, et al. Biphasic nanofibrous constructs with seeded cell layers for osteochondral repair. Tissue Eng Part C Methods. 2014;20:895–904.
Jin GZ, Kim JH, Park JH, Choi SJ, Kim HW, Wall I. Performance of evacuated calcium phosphate microcarriers loaded with mesenchymal stem cells within a rat calvarium defect. J Mater Sci Mater Med. 2012;23:1739–48.
Jin GZ, Park JH, Lee EJ, Wall IB, Kim HW. Utilizing PCL microcarriers for high-purity isolation of primary endothelial cells for tissue engineering. Tissue Eng Part C Methods. 2014;20:761–8.
Lee SI, Yeo SI, Kim BB, Ko Y, Park JB. Formation of size-controllable spheroids using gingiva-derived stem cells and concave microwells: Morphology and viability tests. Biomed Rep. 2016;4:97–101.
Jin GZ, Kim HW. Porous microcarrier-enabled three-dimensional culture of chondrocytes for cartilage engineering: a feasibility study. Tissue Eng Regen Med. 2016;13:235–41.
Olmos Buitrago J, Perez RA, El-Fiqi A, Singh RK, Kim JH, Kim HW. Core–shell fibrous stem cell carriers incorporating osteogenic nanoparticulate cues for bone tissue engineering. Acta Biomater. 2015;28:183–92.
Meinhart J, Fussenegger M, Höbling W. Stabilization of fibrin-chondrocyte constructs for cartilage reconstruction. Ann Plast Surg. 1999;42:673–8.
Häuselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, et al. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107:17–27.
Park KD, Hwang JY, Kim C, Kang JY, Chun HJ, Han DK. Dedifferentiated chondrocyte culture using alginate microbead prepared from microfluidic technique. Tissue Eng Regen Med. 2009;6:353–9.
Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng. 2004;86:747–55.
Greco KV, Iqbal AJ, Rattazzi L, Nalesso G, Moradi-Bidhendi N, Moore AR, et al. High density micromass cultures of a human chondrocyte cell line: a reliable assay system to reveal the modulatory functions of pharmacological agents. Biochem Pharmacol. 2011;82:1919–29.
Zhang Z, McCaffery JM, Spencer RG, Francomano CA. Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J Anat. 2004;205:229–37.
Lewis NS, Lewis EE, Mullin M, Wheadon H, Dalby MJ, Berry CC. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. J Tissue Eng. 2017;8:2041731417704428.
Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–402.
Mwale F, Girard-Lauriault PL, Wang HT, Lerouge S, Antoniou J, Wertheimer MR. Suppression of genes related to hypertrophy and osteogenesis in committed human mesenchymal stem cells cultured on novel nitrogen-rich plasma polymer coatings. Tissue Eng. 2006;12:2639–47.
Mwale F, Stachura D, Roughley P, Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791–8.
Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–66.
Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue Eng. 2004;10:109–18.
Neri S, Mariani E, Cattini L, Facchini A. Long-term in vitro expansion of osteoarthritic human articular chondrocytes do not alter genetic stability: a microsatellite instability analysis. J Cell Physiol. 2011;226:2579–85.
Xia Z, Duan X, Murray D, Triffitt JT, Price AJ. A method of isolating viable chondrocytes with proliferative capacity from cryopreserved human articular cartilage. Cell Tissue Bank. 2013;14:267–76.
Jakob M, Démarteau O, Schäfer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–77.
Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, et al. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23:1187–95.
Quintana L, zur Nieden NI, Semino CE. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15:29–41.
Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–6.
Acknowledgement
The present research was conducted by the research fund of Dankook University in 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors (GZJ and HWK) declare no conflict of interests exists.
Ethical statement
The cell isolation from Sprague–Dawley rats was according to the consent from Dankook University Institutional Animal Care and Use Committee (DKU-IRB-2014-039).
Rights and permissions
About this article
Cite this article
Jin, GZ., Kim, HW. Chondrogenic Potential of Dedifferentiated Rat Chondrocytes Reevaluated in Two- and Three-Dimensional Culture Conditions. Tissue Eng Regen Med 15, 163–172 (2018). https://doi.org/10.1007/s13770-017-0094-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0094-6