Abstract
Recent investigations consider adipose-derived stem cells (ASCs) as a promising source of stem cells for clinical therapies. To obtain functional cells with enhanced cytoskeleton and aligned structure, mechanical stimuli are utilized during differentiation of stem cells to the target cells. Since function of muscle cells is associated with cytoskeleton, enhanced structure is especially essential for these cells when employed in tissue engineering. In this study by utilizing a custom-made device, effects of uniaxial tension (1Hz, 10% stretch) on cytoskeleton, cell alignment, cell elastic properties, and expression of smooth muscle cell (SMC) genes in ASCs are investigated. Due to proper availability of ASCs, results can be employed in cardiovascular engineering when production of functional SMCs in arterial reconstruction is required. Results demonstrated that cells were oriented after 24 hours of cyclic stretch with aligned pseudo-podia. Staining of actin filaments confirmed enhanced polymerization and alignment of stress fibers. Such phenomenon resulted in stiffening of cell body which was quantified by atomic force microscopy (AFM). Expression of SM α-actin and SM22 α-actin as SMC associated genes were increased after cyclic stretch while GAPDH was considered as internal control gene. Finally, it was concluded that application of cyclic stretch on ASCs assists differentiation to SMC and enhances functionality of cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mesenchymal stem cells (MSCs) are undifferentiated multi-potent cells which are extractable from different tissues such as bone, umbilical cord and fat. They are capable of differentiation to various phenotypes including osteoblasts, adipocytes, chondrocytes, fibroblasts, and neural cells [1]. However, the limitations of MSCs extraction and the cell available amounts are considered as the common problems in their usage. In this regard, many studies are looking for the new sources of stem cell, focused on the adipose tissue. It has been illustrated that Adipose Derived Stem Cells (ASCs) are more capable of adipogenic, chondrogenic, osteogenic, and myogenic differentiations compared to bone marrow mesenchymal stem cells (BMSCs) [2]. They are also capable of strong extracellular matrix (ECM) synthesis as have been applied in heart valve tissue engineering [3]. ASCs are highly accessible through subcutaneous adipose tissue as an appropriate source of stem cells [4].
Cells function in a dynamic micro-environment with chemical and mechanical stimuli, depending on the tissue in which cells are hosted. Hence, cell behaviour is determined by chemo-mechanical parameters of such environment. For the case of stem cells, the process of differentiation and function of differentiated cells are influenced by the external stimuli. Due to application of adult stem cells in clinical therapies and regenerative medicine, a wide range of experimental studies has been performed to evaluate effects of biochemical and biophysical conditions on cell behavior [5].
Cells adapt to the mechanical stimuli by cytoskeleton remodeling, activation of various signaling pathways, and alteration in the gene expression [5, 6]. Such adaptation influences cellular characteristics including cell, morphology, mechanical properties, and protein synthesis [7, 8]. During exposure to mechanical stimuli, substrate deformation is transmitted from ECM to the cytoskeleton through internal components such as actin filaments, myosin motors, and actin cross-linking proteins. Actin filaments of cytoskeleton are the central contributors to cell remodeling [9].
Biological cells of various tissues are subjected to different types of loading. To investigate cell reactions to these loadings, behavior of cells exposed to mechanical stimuli has been extensively studied in vitro. Examples include effects of cyclic hydrostatic compression on chondrocytes, cyclic planar tension on cardiomyocytes and cyclic uniaxial stretch on smooth muscle cells (SMCs) ([10, 11], Also a complete review can be found in [7]). For the case of stem cells, it has been illustrated that mechanical stimuli regulates differentiation of stem cells. Convenient type of stimulus depends on the type of mechanical loading which the target cells experience in vivo [5, 12].
In the arterial wall media, SMCs are exposed to circumferential cyclic tension due to pulsatile blood pressure[13]. Hence, it is hypothesized that in regenerative medicine of engineered arteries, cyclic tension could mimic environmental conditions for structural remodeling and differentiation of adult stem cells to SMCs. In addition to transforming growth factor-β (tgf-β) and sphingosylphosphorylcholine (SPC) as chemical cytokines [14], cyclic stretch has been found to be effective in expression of SMC associated genes [15]. This loading regime mimics the physiological conditions of SMCs populate within arterial media. It has been demonstrated that vascular SMC genes are up-regulated by exposure of BMSCs to mechanical stretch. While up-regulation of SMC associated genes has been illustrated by application of mechanical loading on BMSCs [16,17,18], such influence has not been characterized for ASCs.
The aim of this study is to quantify the effects of uniaxial cyclic stretch on the morphology, mechanical properties, and SMC gene expression of ASCs. Due to high accessibility of ASCs, results can be applied in vascular engineering.
2 Methods and materials
2.1 Cell isolation and culture
ASCs were extracted from adipose tissue according to recommended protocols [19]. The adipose tissue was derived during orthopaedic surgery of human anterior cruciate ligament (ACL) reconstruction. Tissue was rinsed in the digesting solution containing 2mg/ml of type-I Collagenase in PBS. Then, it was centrifuged at 1400 rpm for 5 minutes. The supernatant solution containing adipose cells was incubated for 30 minutes at 37° Centigrade for further digestion. Subsequently, the solution was centrifuged at 2000 rpm for 5 minutes and the remaining pellet was re-suspended and transferred to DMEM culture medium (Invitrogen, USA) containing 15% FBS (Fetal Bovine Serum) (Gibco, USA) and 1% Penicillin-Streptomycin. After 24 hours, morphologically homogeneous ASCs were attached while adipose cells were suspended and discarded.
Cells were cultured in DMEM-LG (Low Glucose Dulbecco’s Modified Eagle’s Medium) supplemented with 10% FBS, and incubated at 37° with 5% CO2. The culture medium was replaced every 3 days and cells from third passage were used for experiments. Cells were characterized using flow cytometry by their stem cell–surface antigens. Cells were positive for CD105, CD166, CD90, and CD44 as stem cell markers and negative for CD34, and CD45 as hematopoietic markers. ASCs express similar surface antigens similar to BMSCs while maintain for extended period as undifferentiated cells [ 20].
2.2 Mechanical stimulation
A custom-made device was utilized for application of cyclic stretch on cultured cells attached on an elastic membrane (Fig. 1). The device was modified for a long time stretch by design of a new detachable cell culture chamber while protecting cells from environmental contamination. Transparent elastic membranes of medical grade Polydimethylsiloxane (PDMS) were produced by heat compression of Silicone (Wackers Chemie AG, Germany). Since hydrophobic surface of PDMS membranes is not suitable for cell culture [21], several coating materials such as fibronectin, type-I collagen, bFGF, and gelatin are mostly recommended for surface modification [22]. PDMS surface wettability was modified by gelatin coating to avoid cell detachment during cyclic stretch. For this purpose, a solution of 0.2% type-A gelatin from porcine skin (Sigma, USA) in deionized water was autoclaved and filtered by 0.2µm pore-size filter and incubated at 4° for 24 hours on PDMS surface.
Viable cells were counted by Trypan Blue using hemocytometer (HBC, Germany) and seeded with density of 10,000 Cells/cm2 on two samples of coated silicones. Cells were incubated for 24 hours to adhere properly. After attachment with spreading pseudo-podia, elastic membranes of test group were assembled into the grippers of the stretching device and exposed to cyclic stretch with 10% amplitude and frequency of 1 Hz to mimic microenvironment of SMCs within the arterial wall.
2.3 Cell morphology and orientation
Cell images were obtained from test and control groups before and after loading using a digital camera installed on a phase-contrast inverted optical microscope. Images of cells were processed using Image Processing Toolbox of Matlab (MathWorks Software, USA). The colour images were transformed to grey scale images. Using segmentation algorithms segmented cells distinct from background were isolated in a black-white format (binary image). Appropriate digital filters were applied for precise border detection of cell images and removal of image artefacts. The resultant images were exploited for an analysis of 2-Dimentional Fast Fourier Transform (2D-FFT) in ImageJ Software (v. 1.44a) to quantify cell alignment. The 2D-FFT Power Spectrum of an image demonstrates average angle for the majority of lines and can be employed to calculate orientation of cells in the segmented images [23]. Since cyclic stretch alters cell morphology and orientation [24], the morphological evaluation of ASCs after loading is valuable for study of cell responses to environmental stimuli through remodelling and adaptation.
2.4 RNA preparation, cDNA synthesis and quantitative real time RT-PCR
Expression of SMC associated genes was investigated in the cells of test samples after 24 hours cyclic stretch and compared to the gene expression in control samples after same duration. Smooth muscle (SM) α-actin and SM-22 α-actin genes which are expressed at early-stage of stem cell differentiation to SMC were analysed [25]. Real Time RT-PCR method was used to study intensity of expression of SMC genes due to application of mechanical stimuli. Since ASCs in regular culture conditions express those genes in small degree, it is necessary to quantify such expression after application of cyclic load through real time PCR [26].
Total RNA was extracted using chloroform and phenol extraction protocols. Cells in samples were lysed with 1ml RNXTM (-Plus) (CinnaGen Inc., Iran) and added by 20% chloroform. Isopropanol was added to precipitate the RNA. The lysed solutions were centrifuged between each step for 15 minutes at 4°C and 12,000 rpm. To wash the RNA pellet, 75% ethanol was added and centrifuged at 7500 rpm for 8 minutes. The final pellet was suspended in diethyl pyrocarbonate (DEPC)-treated water. Lastly, RNA purity was quantified by optical density in 26nm wavelength (OD260) measurement using BioPhotometer (Eppendorf, Germany). Purity of RNA was approved for cDNA synthesis when OD260/OD280≥1.8. First strand cDNA was synthesized from 2µg of total RNA using PrimeScript RT Reagent (Perfect Real Time; Takara, Japan), and the quantitative analysis was performed by SYBR Premix Ex Taq II (Perfect Real Time; Takara, Japan) using ABI Prism 7500 Fast Real Time RT-PCR System (Applied Biosystems, USA).
The primers (TAG Copenhagen A/S, Denmark) were selected according to previous published data on BMSCs for evaluating expression of SMC genes [27], and checked in database of National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/BLAST/) to assure the uniqueness of primer sequences (Table 1). Both β-actin and GAPDH were candidates of internal controls for gene expression.
2.5 Actin filament staining
To examine influence of mechanical loading on cell cytoskeleton, arrangement of actin filaments was analyzed in both control and test groups. After 24 hours incubation following cyclic stretch, actin staining was used to investigate cytoskeletal structure. For this purpose, ASCs were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 (Merck, Germany), and blocked from non-specific binding with 1% BSA (bovine serum albumin) (Merck, Germany). Then actin filaments were stained by incubating cells for 30 minutes in Phalloidin-FITC (Sigma, Germany) [28]. An inverted fluorescence microscope was utilized to observe actin filaments with excitation at 495nm and emission at 513nm in green.
2.6 Evaluation of whole-cell mechanical property
Cell cytoskeleton determines mechanical properties of the cell body through arrangement of actin stress fibers [29]. Uniaxial cyclic stretch influences actin alignment within cells [24], hence quantification of alterations in mechanical properties of the cell body due to the loading is of importance. Indentation of the cells with atomic force microscopy (AFM) assists better understanding of physical mechanisms involved in cell biology [29]. At 24 hours after termination of cyclic stretch, cells in both control and test group were subjected to indentation for evaluation of cell apparent elasticity. Cells were fixed with 0.5% glutaraldehyde for 1 minute and washed twice for 5 minutes, and then air dried for cell probing [30]. A DME DualScopeTM scanning probe microscope (DS 95-200/50; DME, Denmark) was utilized. Both apparent elasticity and surface topography data were captured by AFM contact mode using a blunted pyramidal tip DC cantilever (DME, DS 95-x). Cantilever force constant was from 0.07 to 0.4N/m with silicon tips (NanoWorld AG, Switzerland). Equation 1 defines the force-indentation relation for cantilevers with pyramid tips according to Hertz model defined by assumptions of elastic, isotropic and homogenous material properties for the probed material.
Where δ defines indentation, α is half angle of probe tip, and ν represents the Poisson’s ratio for cell which has been assumed to be 0.45 due to assumption of incompressibility. The indentation is prescribed to be less than 10% of the cell height to diminish substrate contribution in determining apparent cell elasticity (E) [31].
2.7 Experimental procedure
Extracted human ASCs from third passage were seeded on gelatin-coated PDMS. After 24 hours incubation for proper attachment, membranes of test groups were exposed to 10% uniaxial cyclic stretch at frequency of 1 Hz for 24 hours. After test, morphological parameters, apparent elastic modulus and SMC gene expression were evaluated and compared to control (no load) group to study effects of cyclic stretch on cell functions. Cells in control groups were cultured at similar biological conditions without exposure to loading. For each variable, at least four tests were performed for statistical verification. AFM indentation was carried out on cells within five fields of view, and indentation was performed on three locations of the each cell body. Phase contrast images were obtained from 4 fields of view for morphology and orientation assessment. Morphological parameters of each image were measured by a computer assisted code. Statistical analysis was carried out to investigate differences of the parameters in test and control groups. Data were presented in mean±SD, and statistical significant difference was defined by p<0.05.
3 Results
3.1 Cell morphology and orientation
Results indicated alignment of ASCs after 24 hours of cyclic stretch, whereas cells of the control group were distributed randomly (Fig. 2D vs. A). Results of actin staining in test group indicated fiber orientation in addition to enhanced polymerization of actin filaments as results of cyclic stretch (Fig. 2E vs. B). Quantified 2D-FFT analysis of cell images showed that cells of test groups have been aligned with the angle of 70±10 degrees compared to load axis, in contrast to the random orientation of cells in control groups (Fig. 2F vs. C). This has been observed in the MSCs under mechanical loading in previous studies [32].
Effects of cyclic stretch on cell orientation, Top control group; A random orientation of ASCs, B actin filament staining, C 2D-FFT power spectrum describing random distribution of cells by a tinny circular halation, Bottom Test group after 24h cyclic stretch; D oriented ASCs, E actin filament orientation, F 2D-FFT power spectrum demonstrating cell orientation (app. α=70°) by an elliptical halation
Actin filaments of test group are oriented as illustrated in phase contrast images. Cells exposed to mechanical stretch remodel their cytoskeleton to minimize energy distortion by alignment of actin filaments and the consequent actin polymerization, leading to thicker bundles of stress fibers [33, 34], as it was exemplified by brighter stained actin filaments in test group (Fig. 2E).
3.2 Cell indentation by AFM
As illustrated by AFM topography (Fig. 3), un-stretched ASCs have three or more leading edges spreading in different directions. However in stretched cells, the leading edges are aligned in specific direction imitating spindle-shaped striated SMCs [35]. This is in good agreement with fluorescence images obtained from stained actin filaments demonstrating orientation of spreading edges and actin filaments of cytoskeleton (Fig. 2E).
Cells in control group have thin, sheet-like extensions known as lamellipodia regardless of cell direction, however mechanical strain gives polarity to leading edges and inhibit lamellipodia extensions. Based on the evidences, inhibition of lamellipodia restricts cell migration unless in cell direction [36, 37].
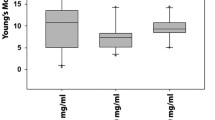
AFM indentation depth was significantly reduced as illustrated in Fig. 4A (99.28±7.08nm vs. 104.81±4.62nm). Consequently, whole-cell mechanical property, measured by AFM indentation demonstrated 8.5% rise of apparent elasticity (cell stiffening) in test group compared to no-load control group (553563±59623Pa vs. 510852±35421Pa), [Fig. 4B]. Cell stiffening due to the cyclic stretch was statistically significant (P<0.05).
3.3 SMC gene expression
Results of Quantified gene expression demonstrated biophysical cues for up-regulation of SMC genes describing probable phenotype changes. Although both GAPDH and SM β-actin were evaluated as endogenous controls, SM β-actin was excluded illustrating variation of 1.733 to 3.474 fold after cyclic stretch with respect to GAPDH. Hence, real time PCR demonstrated that after application of cyclic loading, SMC early-stage genes (SM α-actin and SM22 α-actin) were elevated considering GAPDH as house-keeping genes. As illustrated in Fig. 5, cyclic stretch for a period of 24 hours caused 13.039 and 4.933 folds elevations in SM α-actin and SM22 α-actin, respectively. This indicates that ASCs are markedly prone to differentiation to SMCs due to cyclic tension.
4 Discussion
In this study we intended to investigate the effects of the cyclic stretch on the ASCs morphology and the SMC gene expression. It has been shown that cyclic stretch (1Hz, 10% amplitude) modulates alignment of ASCs and their pseudopodia; and reduces unintended migration by reducing cell lamellipodia. Aligned cells with premeditated migration are vital in tissue engineering, especially for arterial wall with organized lamellar structure [38]. Function of engineered cells is enhanced when they are exposed to the micro-environmental stimuli that biological cells experience in vivo [5, 12]. Mechanical incentives result in cell alignment, cytoskeletal arrangement and stress fiber generation leading to enhanced functionality of cells through improvement of ECM synthesis, and deliberate cell migration and motility. Cytoskeletal enhancement is particularly indispensable in muscle-like cells such as SMCs in which force generation and mechanical properties are essential for their biological function. Generation of stress fibers together with their alignment are associated with stiffening of the cell body [39, 40]. Cell elongation and stiffening in response to cyclic stretch is caused by actin alignment and elevation of SMC protein content [37, 41]. Load dependent cell orientation and morphological variation are correlated with cell type, loading conditions (amplitude, duration, frequency), and mechanical properties of cell body [42].
Application of 24 hours cyclic stretch on ASCs intensifies expression of two early-stage SMC associated genes. This phenomenon is in agreement with previous studies indicating that cyclic stretch up-regulates expression of SMC genes in BMSCs [16]. Although TGF-β1 is the most recommended cytokine promoting differentiation of ASCs and BMSCs to SMCs [26, 27], application of cyclic uniaxial stretch is also an efficient method for up-regulating SMC genes. This cell differentiation could affect cell elasticity [43]. Besides, cell differentiation due to external load is accompanied by structural remodeling, through enhancement of cell cytoskeleton fibers which are key factors in functionality of muscle cells. For this reason, mechanical stimuli might enhance function of these cells once they go through the same mechanical conditions in hosting tissues. This is specifically practical in tissue reconstruction when functional cells are required to contribute in accelerated ECM synthesis through enhanced cell cytoskeleton and improved cell function.
In conclusion, when arterial reconstruction is intended, uniaxial stretch might be employed during incubation of stem cells with growth factors to obtain functional SMCs. It was shown that ASCs are capable of appropriate response to uniaxial cyclic stretch for expression of SMC associated genes and enhancement of cell behavior. Due to high accessibility of ASCs, this approach facilitates production of functional SMCs through differentiation process. As a result, combined effects of biochemical stimuli together with proper biophysical stimuli are recommended for efficient differentiation of stem cells to target cells in cell therapy and regenerative medicine.
References
Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1:51–8.
Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem cells. 2012;30:804–10.
Colazzo F, Sarathchandra P, Smolenski RT, Chester AH, Tseng YT, Czernuszka JT, et al. Extracellular matrix production by adipose-derived stem cells: implications for heart valve tissue engineering. Biomaterials. 2011;32:119–27.
Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep. 2011;7:269–91.
Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, et al. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol (Camb). 2012;4:1008–18.
Panadero J, Lanceros-Mendez S, Ribelles JG. Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016;33:1–12.
Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18:436–44.
Cardwell RD, Kluge JA, Thayer PS, Guelcher SA, Dahlgren LA, Kaplan DL, et al. Static and cyclic mechanical loading of mesenchymal stem cells on elastomeric, electrospun polyurethane meshes. J Biomech Eng. 2015;137:071010.
Carlier MF, Pernier J, Montaville P, Shekhar S, Kühn S. Cytoskeleton dynamics and motility group. Control of polarized assembly of actin filaments in cell motility. Cell Mol Life Sci. 2015;72:3051–67.
Heng BC, Haider HKh, Sim EK, Cao T, Ng SC. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc Res. 2004;62:34–42.
Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–60.
Maul TM, Chew DW, Nieponice A, Vorp DA. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2011;10:939–53.
Tarbell JM, Shi ZD, Dunn J, Jo H. Fluid mechanics, arterial disease, and gene expression. Ann Rev Fluid Mech. 2014;46:591.
Firth AL, Yuan JX-J. Human models for smooth muscle cell differentiation. Focus on “A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells”. Am J Physiol Cell Physiol. 2013;304:C287–8.
Dan P, Velot É, Decot V, Menu P. The role of mechanical stimuli in the vascular differentiation of mesenchymal stem cells. J Cell Sci. 2015;128:2415–22.
Kurpinski K, Park J, Thakar RG, Li S. Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain. Mol Cell Biomech. 2006;3:21–34.
Koobatian MT, Liang MS, Swartz DD, Andreadis ST. Differential effects of culture senescence and mechanical stimulation on the proliferation and leiomyogenic differentiation of MSC from different sources: implications for engineering vascular grafts. Tissue Eng Part A. 2015;21:1364–75.
Yao R, Wong JY. The effects of mechanical stimulation on controlling and maintaining marrow stromal cell differentiation into vascular smooth muscle cells. J Biomech Eng. 2015;137:020907.
Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9.
Mizuno H, Hyakusoku H. Mesengenic potential and future clinical perspective of human processed lipoaspirate cells. J Nippon Med Sch. 2003;70:300–6.
Khorasani MT, Mirzadeh H, Kermani Z. Wettability of porous polydimethylsiloxane surface: morphology study. Appl Surf Sci. 2005;242:339–45.
Song G, Ju Y, Soyama H. Growth and proliferation of bone marrow mesenchymal stem cells affected by type I collagen, fibronectin and bFGF. Mater Sci Eng C. 2008;28:1467–71.
Palmer BM, Bizios R. Quantitative characterization of vascular endothelial cell morphology and orientation using Fourier transform analysis. J Biomech Eng. 1997;119:159–65.
Lee WC, Maul TM, Vorp DA, Rubin JP, Marra KG. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6:265–73.
Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–77.
Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, DiMuzio PJ. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 2011;168:306–14.
Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–68.
Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA. 2006;103:16095–100.
Li QS, Lee GY, Ong CN, Lim CT. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. 2008;374:609–13.
Hutter JL, Chen J, Wan WK, Uniyal S, Leabu M, Chan BM. Atomic force microscopy investigation of the dependence of cellular elastic moduli on glutaraldehyde fixation. J Microsc. 2005;219:61–8.
Rico F, Roca-Cusachs P, Gavara N, Farré R, Rotger M, Navajas D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;72:021914.
Goli-Malekabadi Z, Tafazzoli-Shadpour M, Rabbani M, Janmaleki M. Effect of uniaxial stretch on morphology and cytoskeleton of human mesenchymal stem cells: static vs. dynamic loading. Biomed Tech (Berl). 2011;56:259–65.
Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–92.
Albinsson S, Bhattachariya A, Hellstrand P. Stretch-dependent smooth muscle differentiation in the portal vein—role of actin polymerization, calcium signaling, and microRNAs. Microcirculation. 2014;21:230–8.
Alford PW, Nesmith AP, Seywerd JN, Grosberg A, Parker KK. Vascular smooth muscle contractility depends on cell shape. Integr Biol (Camb). 2011;3:1063–70.
Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L958–65.
Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577–90.
Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–9.
Azeloglu EU, Costa KD. Atomic force microscopy in mechanobiology: measuring microelastic heterogeneity of living cells. Atomic Force Microsc Biomed Res Methods Protoc. 2011;736:303–29.
Rodriguez ML, McGarry PJ, Sniadecki NJ. Review on cell mechanics: experimental and modeling approaches. Appl Mech Rev. 2013;65:060801.
Steward RL Jr, Rosner SR, Fredberg JJ. Emergent behaviors. Cell mechanics structure-based mechanics of tissues and organs. New York: Springer; 2016. p. 41–55.
Zhang L, Kahn CJ, Chen HQ, Tran N, Wang X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol Int. 2008;32:344–52.
Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–702.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
Adipose tissue is excised from patients during orthopaedic knee surgery with informed consent considering ethical issues.
Rights and permissions
About this article
Cite this article
Rabbani, M., Tafazzoli-Shadpour, M., Shokrgozar, M.A. et al. Cyclic Stretch Effects on Adipose-Derived Stem Cell Stiffness, Morphology and Smooth Muscle Cell Gene Expression. Tissue Eng Regen Med 14, 279–286 (2017). https://doi.org/10.1007/s13770-017-0033-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0033-6