Abstract
To discriminate economically motivated adulteration in poultry meats and products, species-specific oligonucleotide primer sets for multiplex polymerase chain reaction (PCR) of four poultry meats were newly designed. Sequences for species-specific primers were generated based on the nucleotide variation in mitochondria cytochrome b gene through sequence comparisons. The sizes of species-specific PCR products for turkey, ostrich, chicken, and duck were 217, 330, 516, and 820 bp, respectively. By adjusting PCR conditions and the concentrations of primers, a simultaneous discrimination of four poultry meats was successfully performed. The limits of detection of both single and multiplex PCR for turkey were 0.05 and 0.005 ng for other species, respectively. The developed multiplex PCR method was successfully applied to identify the target species in 34 of poultry meat products and thus was considered to be a useful tool for monitoring poultry meat products.
Similar content being viewed by others
Introduction
The substitution of meat species is quite frequent, due to both unintentional contamination and intentional replacement for economic reasons. An efficient identification of the animal species used in meat products helps in food inspection. Poultry is the most consumed meat around the world, and its consumption has been rapidly increasing in the past decade (www.earth-policy.org/data_center/c24). Efficient detection methods that detect poultry species simultaneously should be available for food control agencies to supervise lawful labeling in poultry meat production.
Recently, molecular genetic approaches for meat species identification have gained full attention because of their high sensitivity and specificity, as well as rapid processing time and low cost (Fajardo et al. 2010). Polymerase chain reaction (PCR) is the most well-developed molecular technique currently available and is applied to detect the presence of species in a meat product. The sizes of amplified DNA fragments are verified by agarose gel electrophoresis (Fajardo et al. 2010). Species-specific oligonucleotide pairs were designed and used to develop single PCR (Haunshi et al. 2009; Amaral et al. 2015), multiplex PCR (Dalmasso et al. 2004; Ghovvati et al. 2009; Kitpipit et al. 2014; Xu et al. 2014), and real-time PCR detection methods to authenticate meat and meat products. PCR using species-specific primers, which are complementary to the DNA of a specific species, enables the verification of animal species by the size of the PCR products. Multiplex PCR is useful for the simultaneous identification of multiple animal species because it is highly repeatable, efficient, and reliable in addition to being time and cost efficient (Dalmasso et al. 2004; Fajardo et al. 2010).
An appropriate selection of genetic markers is critical for the development of a PCR detection method. The use of mitochondrial DNA sequences increases PCR amplification sensitivity because there are several copies of mitochondrial DNA per cell (Fajardo et al. 2010). Since mitochondrial genes have evolved much faster than nuclear genes, they contain more sequence diversity facilitating the identification of phylogenetically related species (Girish et al. 2005). Among mitochondrial genes, the cytochrome b (cytb) gene (Stamoulis et al. 2010; Amaral et al. 2015), the 12S rRNA subunit (Dalmasso et al. 2004; Ghovvati et al. 2009; Stamoulis et al. 2010), the 16S rRNA subunit (Dalmasso et al. 2004; Ghovvati et al. 2009; Xu et al. 2014), and the displacement loop region (Haunshi et al. 2009) are the most commonly used target genes in the development of PCR methods for meat authentication (Fajardo et al. 2010).
The aim of this study was to develop a simultaneous identification method of raw meat from turkey, ostrich, chicken, and duck through the optimization of multiplex PCR assay on mitochondrial cytb genes. Both single and multiplex PCR analyses were performed to evaluate the specificity and sensitivity of the designed primer sets and developed detection method. The assay was applied to 34 samples of commercial poultry meat products, including both food and feed, to examine the possibility of intentional fraud or unintentional admixture among products in the market.
Materials and methods
Samples
Poultry meat samples including turkey (Meleagris gallopavo), ostrich (Struthio camelus), chicken (Gallus gallus), and duck (Anas platyrhynchos) were purchased from either local markets (chicken and duck) or poultry farms (turkey and ostrich) in October 2013. Poultry meat products including pet foods, smoked meat, and canned meat were purchased from a super market in October 2014. Samples were stored at −20 °C until use. All samples were finely ground in liquid nitrogen using a mortar and pestle with 0.03 g of each used for DNA extraction.
DNA extraction
DNeasy Blood and Tissue Kit (Qiagen, Germany) was used to extract DNA from samples according to the manufacturer’s instructions except that the final elution volume was reduced to 50 μL. The purity of the extracted DNA was confirmed by the ratio of the absorbance at 260 over 280 nm. The DNA was quantified by Maestronano spectrophotometer (Maestrogen, USA), while the quality of the extracted DNA was confirmed on a 1 % agarose gel by electrophoresis at 100 V for 18 min with ethidium bromide (0.25 ng/mL, Sigma, USA). The DNA was visualized under UV light, and a digital image was obtained using a nucleic acid bioimaging instrument (NEO Science, Korea). The range of ratios of absorbance at 260 over 280 nm of extracted DNA from raw meat samples was 1.9–2.1, and the concentrations of DNA were diluted to 50 ng of DNA, a quantity enough for PCR amplification.
Primer design and PCR amplification
Species-specific primers were newly designed from regions of mitochondrial cytb genes using the Primer Designer Program (Version 3.0; Scientific and Educational Software, USA). The sequences of oligonucleotides are listed in Table 1. The mitochondrial cytb gene sequences from the four poultry species were obtained from the National Center for Biotechnology Information (NCBI) Genbank database. The nucleotide sequences were submitted to BLAST, a basic local alignment search tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Primer specificity was assessed using Primer-BLAST tool in the Genbank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Oligonucleotides including the common forward primer (For) and four species-specific reverse primers (Table 1) were synthesized by Bionics Company (Bionics, Korea).
The concentration of each primer was 10 pmol/μL. For a single PCR reaction, 10 pmol of each primer was used. PCR amplifications were performed in a final volume of 25 μL, containing 1 unit of Ampli Gold Taq DNA polymerase (Applied Biosystems, USA), 2.5 μL of 10× buffer (Applied Biosystems), 200 μM of dNTP (Applied Biosystems), 1.5 mM MgCl2, and 50 ng of each template DNA. Amplifications were run using a Thermal Cycler Gene Atlas (Astec, Japan) as follows: pre-denaturation step at 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 61 °C for 30 s, and 72 °C for 45 s, and final extension at 72 °C for 7 min. The PCR products were then stored at 4 °C. PCR products were visualized, and their sizes were confirmed with a 100 bp DNA ladder (Bioneer, Korea) on a 3 % agarose gel (SeaKem, USA) at 100 V for 18 min with ethidium bromide (0.25 ng/mL, Sigma,) staining.
PCR mixtures and PCR reaction conditions for the multiplex PCR were similar as above except that the primers were mixed in a ratio of 2:2:0.3:1.5:1 for For:Tur:Ost:Chi:Duc. Final concentrations of each primer in the final volume (25 μL) of PCR mixture for multiplex PCR were 20, 20, 3, 15, and 10 pmol for For, Tur, Ost, Chi, and Duc, respectively. PCR products were visualized by electrophoresis on a 3 % agarose gel (SeaKem, USA) at 100 V for 50 min with ethidium bromide (0.25 ng/mL, Sigma) staining. The amplified DNA fragments were confirmed on an Agilent 2100 bioanalyzer (Agilent Technologies, USA) with the DNA 1000 Lab Chip kit (Agilent Technologies). The negative control without the template DNA was also run through the whole study.
Sensitivity and specificity of PCR method
The limit of detection (LOD) of both single and multiplex PCR methods was examined to check the sensitivity. Serial dilutions of mixed template DNA ranging from 50 to 0.0005 ng were prepared and tested with each primer set to calculate the sensitivity. Each primer set was tested with all four species to validate the primer specificity. A PCR mixture of the four primer sets was tested with a mixture of the four species in the case of multiplex PCR. A non-template control was included in all reactions.
Monitoring of processed poultry meat products using multiplex PCR
PCR conditions for meat products were the same. Existence of each poultry meat in the processed meat products was confirmed by the existence of correct sizes of PCR products for the specific species. Results from monitoring were compared against the information on the product labels.
Results and discussion
Single PCR specificity and sensitivity
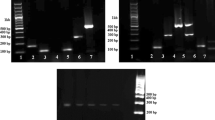
Single PCR reactions were carried out on DNA extracted from raw poultry meat samples to verify the specificity of the primers. The sizes of PCR products were 217, 330, 516, and 820 bp, each from the corresponding species: turkey, ostrich, chicken, and duck, respectively (Fig. 1). The primer sets were able to produce DNA fragments for only the targeted poultry species. Each set of primers was tested in single PCR with non-target species as the negative control, and there was no cross-contamination (Fig. 2). Positive amplifications were obtained for all four primer sets (Fig. 2). It was observed that the designed primers were specific to the poultry species for which they were designed. The detection limits for single PCR were 0.05 ng for turkey and 0.0005 ng for the others (Fig. 2).
Sensitivity of the single PCR analysis of four poultry meat samples, respectively (turkey, 217 bp; ostrich, 330 bp; chicken, 516 bp; duck, 820 bp). The sensitivity of the PCR method was determined by diluting 10-folds of 50 ng of each template DNA [(A) agarose gel electrophoresis; (B) bioanalyzer]. Lanes in (A): M, 100 bp DNA ladder marker; 1, 50 ng; 2, 5 ng; 3, 0.5 ng; 4, 0.05 ng; 5, 0.005 ng; 6, 0.0005 ng; and 7, non-template control. Peaks in (B): M, 100 bp DNA ladder marker; T, turkey; O, ostrich; C, chicken; and D, duck
Multiplex PCR specificity and sensitivity
The primer sets (Table 1) retained the same specificity in multiplex PCR as the specificity in single PCR (Fig. 3). The electrophoresis pattern clearly shows the absence of cross-contamination. Positive results were obtained for all species, so there was no false-negative reaction. The LOD in multiplex PCR was 0.05 ng for turkey and 0.005 ng for the others (Fig. 4). Chicken and ostrich meats were simultaneously detected with pork, horse, and beef meats using direct-multiplex PCR method reported by Kitpipit et al. (2014). The LOD was measured in DNA concentration, which was more accurate, in this study compared to the mitochondrial DNA that was used in a study by Kitpipit et al. (2014). Multiplex PCR did not compromise the sensitivity of species-specific PCR reaction. Thus, monitoring of processed poultry meat products would be possible using this multiplex PCR detection method.
Sensitivity of the multiplex PCR analysis of four poultry meat samples (turkey, 217 bp; ostrich, 330 bp; chicken, 516 bp; duck, 820 bp). The sensitivity of the PCR method was determined by diluting 10-folds of 50 ng of each template DNA [(A) agarose gel electrophoresis; (B) bioanalyzer]. Lanes in (A): M, 100 bp DNA ladder marker; 1, 50 ng; 2, 5 ng; 3, 0.5 ng; 4, 0.05 ng; 5, 0.005 ng; 6, 0.0005 ng; and 7, non-template control. Peaks in (B): M, 100 bp DNA ladder marker; T, turkey; O, ostrich; C, chicken; and D, duck
Monitoring of processed poultry meat products using multiplex PCR
The applicability of the multiplex PCR method was tested with 23 poultry meat products, including a smoked turkey, a smoked chicken, 4 smoked duck products, a canned chicken breast, a turkey jerky, 2 duck jerkies, 4 chicken jerkies, 3 chicken nuggets, a grilled short rib patty, a hamburger patty, a sausage, and 3 pressed ham products, as well as 11 pet foods including 3 chicken meat pet foods, 2 duck meat pet foods, 5 turkey meat pet foods, and a duck and chicken meat pet food (Table 2). PCR results were consistent and reproducible across the different types of samples monitored in this study and agreed with the indication on product labels. The sizes of PCR product from the meat products were identical to those from the raw poultry meat samples (data not shown). Meyer et al. (1993) reported that the average size of DNA fragments following heat and pressure processing was approximately 300 bp. The required amplicon sizes for gel visualization were usually over 130 bp (Bottero et al. 2003). The sizes of PCR products for ostrich, chicken, and duck in this study were larger than the reported average size of 300 bp (Meyer et al. 1993), but the method developed in this study was able to detect poultry species in processed poultry meat product with high specificities. There were samples that contained pork (samples 17, 18, 20, 25, 26), beef (sample 17), or lamb (sample 33), but none of them were cross-reacted with the primers used for the developed multiplex PCR. Girish et al. (2004) reported that the mitochondrial DNA was relatively stable during the heat treatment and thus gave a higher possibility of PCR detection of DNA fragment in processed meat products. Rastogi et al. (2007) stated that mitochondrial DNA markers would be more efficient than nuclear markers in species identification and authentication purpose. All of the 34 meat product samples and pet foods that declared the presence of specific meat showed positive results for those species.
In conclusion, four poultry species were identifiable on a practical basis using multiplex PCR technique. The developed method was relatively quick, accurate, and sensitive for the poultry species identification. It provided a very low detection limit (LOD of 0.05–0.005 ng) for raw poultry meat samples. This test method could be useful for a rapid analysis of raw poultry meats and poultry meat products available for use to researches and quality control in food inspection agencies.
References
Amaral JS, Santos CG, Melo VS, Costa J, Oliveira MBPP, Mafra I (2015) Identification of duck, partridge, pheasant, quail, chicken and turkey meats by species-specific PCR assays to assess the authenticity of traditional game meat Alheira sausages. Food Control 47:190–195
Bottero MT, Dalmasso A, Nucera D, Turi RM, Rosati S, Squadrone S, Goria M, Civera T (2003) Development of a PCR assay for the detection of animal tissues in ruminant feeds. J Food Protect 66:2307–2312
Dalmasso A, Fontanella E, Piatti P, Civera T, Rosati S, Bottero MT (2004) A multiplex PCR assay for the identification of animal species in feedstuffs. Mol Cell Probes 18:81–87
Fajardo V, Gonzalez I, Rojas M, Garcia T, Martin R (2010) A review of current PCR-based methodologies for the authentication of meats from game animal species. Trends Food Sci Technol 21:408–421
Ghovvati S, Nassiri MR, Mirhoseini SZ, Heravi Moussavi A, Javadmanesh A (2009) Fraud identification in industrial meat products by multiplex PCR assay. Food Control 20:696–699
Girish PS, Anjaneyulu ASR, Viswas KN, Anand M, Rajkumar N, Shivakumar BM, Bhaskar S (2004) Sequence analysis of mitochondrial 12S rRNA gene can identify meat species. Meat Sci 66:551–556
Girish PS, Anjaneyulu ASR, Viswas KN, Shivakumar BM, Anand M, Patel M, Sharma B (2005) Meat species identification by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) of mitochondrial 12S rRNA gene. Meat Sci 70:107–112
Haunshi S, Basumatary R, Girish PS, Doley S, Bardoloi RK, Kumar A (2009) Identification of chicken, duck, pigeon and pig meat by species-specific markers of mitochondrial origin. Meat Sci 83:454–459
Kitpipit T, Sittichan K, Thanakiatkrai P (2014) Direct-multiplex PCR assay for meat species identification in food products. Food Chem 163:77–82
Meyer R, Candria U, Luthy J (1993) Detection of pork in heated meat products by the PCR. J AOAC Int 77:617–622
Rastogi GR, Dharne MS, Walujkar S, Kumar A, Patole MS, Shouche YS (2007) Species identification and authentication of tissue of animal origin using mitochondrial and nuclear markers. Meat Sci 76:666–674
Stamoulis P, Stmatis C, Sarafidou T, Mamuris Z (2010) Development and application of molecular markers for poultry meat identification in food chain. Food Control 21:1061–1065
Xu J, Zhao W, Zhu M, Wen Y, Xie T, He X, Zhang Y, Cao S, Niu L, Zhang H, Zhong T (2014) Molecular identification of adulteration in mutton based on mitochondrial 16S rRNA gene. Mitochondr DNA. doi:10.3109/19401736.2014.908377
Acknowledgments
This research was supported by a Grant (14162MFDS971) from the Ministry of Food and Drug Safety in Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Hong, Y., Kim, JH. et al. Multiplex PCR for simultaneous identification of turkey, ostrich, chicken, and duck. J Korean Soc Appl Biol Chem 58, 887–893 (2015). https://doi.org/10.1007/s13765-015-0118-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0118-7