Abstract
Leaves from a natural population of Artemisia princeps var. orientalis (Pamp.) H. Hara were collected monthly from April through October and characterized for composition of secondary metabolite compounds and their phytotoxic effects on seed germination and seedling growth of Achyranthes japonica and Lactuca sativa. The compounds were identified using gas chromatography/mass spectrometry (GC/MS) coupled with a solvent-free solid injector (SFSI). GC/MS analyses of all samples revealed qualitative variability in the composition of secondary metabolites. The greatest number of compounds was identified in July (56) followed by September (30) and April (24), and the lowest number was found in June (2) and August (2). Among 92 compounds, the major compounds were various terpenes (23) (mono-, sesqui, di-, and tri-terpenes) followed by heterocyclic compounds (18) and hydrocarbons (14). The higher the concentration of the secondary metabolites, the lower the seed germination and seedling growth of A. japonica and L. sativa. Plant samples collected in July and August were most detrimental. Taken together, variability in the secondary metabolites compounds of A. princeps var. orientalis was verified during different seasons, and the compounds were successfully identified by a combination of SFSI and GC/MS. Notably, the antimicrobial and antioxidative effects were inconsistent throughout the various seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Artemisia princeps (Japanese mugwort), a perennial plant native to China, Japan, and the Republic of Korea, has been used as food and medicine by different populations (Toda 2005). Young leaves of A. princeps have a characteristic greenish aroma, and the leaves are sometimes blanched and added to soups or rice cake in Japanese cuisine (Hosking 1997) or taken as tea (Keys 1976). The green juice of the leaves is also used in traditional Japanese folk medicine to treat skin injuries (Umano et al. 2000). The leaves are used to treat gastrointestinal disorders in the Republic of Korea (Kim et al. 1994). Artemisia has been the object of numerous chemical and biological studies (Toda 2004; Sarath et al. 2007; Choi and Kim 2013). Toda (2005) reported the antioxidative effects of polyphenols generated from the leaves of A. princeps on lipid peroxidation by free radicals in vitro. The anti-inflammatory and analgesic effects of A. princeps along with two other edible plants from the Republic of Korea were reported in mice by Park et al. (1994). The presence of a number of volatile compounds including mono and sesquiterpenes has been well documented in Artemisia species (Ahmed and Misra 1994; Kim et al. 1994). Some of these compounds are referred to as “allelochemicals”, as they are produced by plants, released into the environment, and subsequently affect the growth and development of neighboring plants (Yun and Kil 1992; Einhellig 2002; Chon et al. 2003). Essential oils from the leaves of A. princeps were strongly toxic to wheat seed germination when tested alone at a dose of 500 g/L by Liu et al. (2006). Assessing the allelopathic potential of A. princeps, Yun and Kil (1992) found severely inhibited elongation, dry weight, and caloric content of seedlings of other plants grown in soil underneath A. princeps.
Plants (leaves, flowers, and fruits) are capable of synthesizing thousands of primary and secondary metabolites with diverse biological properties and functions. These compounds play a vital role in the plant life cycle by providing chemical cues to animals, pollinators, and seed disseminators that ensure plant reproductive and evolutionary success (Dudareva and Negre 2005). Artemisia is important in the Republic of Korea because of the presence of attractive monoterpenes and essential oils (Kim 1996). The composition of these volatile compounds varies considerably in different phytogeographical regions (Hall and Langenheim 1987), and these compounds are produced in plant tissues at different developmental stages, including flowering, ripening, and maturation (Goff and Klee 2006). Seasonal variations in volatile compounds from A. princeps var. orientalis (Pamp.) H. Hara have been studied by Kim (1996) and Yun and Choi (2003) using conventional extraction methods such as steam distillation (Umano et al. 2000) or solvent extraction (Yun et al. 2008; Nugroho et al. 2010). However, the major drawbacks of conventional methods are time, consumption of large volumes of solvents, and the cost related to waste disposal. Additionally, target compounds can be oxidized and/or degraded following the lengthy procedure (Abd El-Aty et al. 2008).
Secondary metabolites are easily oxidized by air and/or degraded by heat. Therefore, an extraction technique that isolates the compounds in an intact form without deterioration is needed (Mamede and Pastore 2006). Solvent-free solid injection (SFSI), a vaporization technique, has some advantages over conventional techniques in terms of simplicity and cost effectiveness. SFSI involves directly injecting samples into a gas chromatograph with no prior sample preparation. Therefore, it is an eco-friendly technique because no solvents are consumed (Kim et al. 2006). Additionally, there is a little possibility of losing the volatile compounds/constituents from the tested samples. No detailed characterizations of volatile flavor compounds from A. princeps var. orientalis (Pamp.) H. Hara using an environmentally friendly technique throughout various seasons have been conducted. Thus, the objective of this present study was to: (a) identify the secondary metabolites produced by A. princeps from April through October using gas chromatography/mass spectrometry (GC/MS) coupled with SFSI and (b) evaluate the phytotoxic effects of the compounds on seed germination and seedling growth of both Achyranthes japonica and Lactuca sativa.
Materials and methods
Plant materials
Fresh leaves of A. princeps var. orientalis (Pamp.) H. Hara were collected monthly from April through October from the wild near Suncheon National University, Suncheon, Republic of Korea. The collection site was heavily covered with plant material, and samples were taken from five locations within the sites, sealed in plastic bags, and transported to the laboratory pending analysis.
SFSI
A SFSI device manufactured by Han Jin Precision Co. (Gwangju, Republic of Korea) was used to inject the plant materials into the gas chromatograph equipped with a mass spectrometer. Exactly, 1-mg representative sample of A. princeps leaves was placed into a soft glass capillary tube (1.2 mm i.d. × 30 mm in length). Both ends of the tube were sealed briefly in a gas flame, and the tubes were placed in the SFSI. The tubes were crushed by lowering the injector plunger to carry the sample analytes onto the GC column in a carrier gas. The SFSI was held at the injection port during the preheating phase until the injector plunger reached the top of the injector septum, which allowed a constant pressure of carrier gas to be maintained during analysis. At no point in the SFSI technique was the extraction conditions optimized. Rather, the experimental variables including injector temperatures and preheating times were predicated based on our experience.
GC/MS analysis
An Agilent model 6890 network GC system equipped with a 5973N mass-selective detector (Agilent Technologies, Palo Alto, CA, USA) was used to identify the volatile compounds emitted from A. princeps leaves. Separation was carried out on an HP-5MS fused silica column (30 m × 0.25 mm × 0.25 µm, Agilent Technologies). The injector and detector temperatures were set at 250 and 300 °C, respectively. Oven temperature was held at 50 °C for 5 min, increased to 300 °C at a rate of 5 °C/min, and then held constant for 10 min. High-purity helium (99.9999 %), at a constant flow rate of 1 mL/min, was used as the carrier gas, and the injection was made using a split mode of 10:1. An electron impact mass spectral analysis was carried out at ionization energy of 70 eV at 250 °C. Detection was performed in the scan mode between 10 and 400 amu at 3.71 scans/s.
Qualitative analysis
The tentative identification of the compounds was based on comparing the obtained mass spectra with those of reference compounds in the Wiley 7N mass spectral database (McLafferty and Stauffer 1989). Percentage peak areas were calculated by dividing the ion counts for a particular peak (detected by MS) by the total ion counts for the entire chromatogram, and this value was expressed as percentage.
Phytotoxic effect of emitted compounds from Artemisia leaves
Fifty seeds each of A. japonica and L. sativa (sensitive species largely employed in the allelopathy studies, Araniti et al. 2013; Amini et al. 2014) were placed on filter paper that was layered on moist, absorbent cotton with sufficient moisture in a 1,800 mL glass chamber to assess the time-dependent effect of secondary metabolites on seed germination and radicle elongation. Different quantities of sliced Artemisia leaves (fresh weights of 5, 10, 15, 20, 25, or 30 g) were placed in a glass beaker within the chamber. An identical chamber without sliced leaves was used as a control. The glass chambers were covered with vinyl wrap and placed in a growth chamber maintained at 25 °C during the day (14 h) and 18 °C at night (10 h). Seed germination and radicle elongation were checked according to the method of Kil and Yun (1992).
Results and discussion
SFSI–GC/MS conditions
Based on our experience, a sample preheated for 10 min (preheating time) at 250 °C (injector temperature) was chosen as the favorable condition for SFSI, in which the greatest number of secondary metabolites was emitted (Kim et al. 2006, 2007; Abd El-Aty et al. 2008; Ko et al. 2014). A sampling time of 55 min was selected to provide an acceptable analysis time with the highest yield of compounds for the overall analysis. This is because the sampling profile appeared to depend primarily on the volatility of the compound studied (Hamm et al. 2003).
Identification of secondary metabolites emitted from Artemisia leaves
Monthly identification of compounds emitted from the leaves of A. princeps was carried out with a GC/MS coupled with SFSI from April through October to demonstrate the variability in composition and concentration of the secondary metabolites. A total of 94 compounds including acids, alcohols, aldehyde, ketones, amides, esters, pyrazines, pyrroles, pyridines, hydrocarbons, and others were identified (Table 1). The presence of heterocyclic compounds suggested the self-protecting capability of the plants. Pyrazine and pyrroles, identified as odorous substances in many plants (Rothschild et al. 1984), act as warning signals for herbivores to the presence of dangerous chemicals, and in other situations as an attractant for edible fruit which simultaneously assists in seed dispersal (Rothschild et al. 2005). Hashidoko et al. (1989) suggested a specific and significant role for sesquiterpenes in plant protection against microbial invasion if tissue is injured.
In the present study, dibutyl phthalate and squalene were found in blank samples, indicating that these compounds were generated from the capillary tube and/or injector line and, thus, were excluded from the total number of compounds. The reported number of compounds detected in the current study was a bit lower than the 132 compounds identified by Umano et al. 2000. This difference might be attributed to the extraction technique; steam distillation under reduced pressure followed by dichloromethane extraction and simultaneous purging and extraction.
Correlation between seasonal variations and secondary metabolites
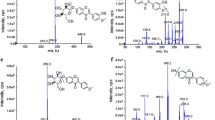
Representative GC/MS chromatograms for May and July are shown in Fig. 1. The compounds emitted from Artemisia were not consistent throughout the seasons of spring, summer, and fall. As shown in Table 1, the greatest number of compounds was emitted in July (56), followed by September (30) and April (24). The number of compounds decreased substantially to 12 in May and declined drastically to six in October and two each in June and August. Clearly, the plant vegetative stages (July and September) were the richest in secondary metabolites compared to the fading stage in October. As secondary metabolite contents varied throughout the year, we expected that the biological effects would also vary (Roussis et al. 2000; Angelopoulou et al. 2002). In this context, Yun et al. (2008) reported that Artemisia collected in spring are useful as food or food additives and those collected in summer are useful for the pharmaceutical industry. We agreed with their findings regarding the summer season; however, we differed with respect to the spring season, which is discussed below.
Variation in antimicrobial and antioxidant activities throughout the seasons
Yun et al. (2008) found that the antimicrobial activity of Artemisia was weaker in April and October and more pronounced in July, August, and September. In contrast, they found that extracts of plants collected in May and June were most effective for DPPH radical scavenging activity and those from plants collected in August, September, and October showed the weakest activity. Those authors evaluated the entire extract without any characterization. The major compounds in April (>1 %) were 1,2-benzenedicarboxylic acid and 3-nitro (5.17 %), a secondary metabolite with good antimicrobial activity against Staphylococcus aureus, S. epidermis, Bacillus subtilis, Candida albicans, and Aspergillus niger (Kavitha et al. 2010) and 3-methylbutanal, which is an aldehyde derived from leucine degradation, responsible for a plant flavor similar to cheese. We suggest that plant samples collected in early spring season (April) may also have a pharmacological effect (antimicrobial effect) in addition to being a food source.
1,2-Benzenediol (2.56 %), a compound that contributes to the antioxidant properties of plant/medicinal herbs (Manorenjitha et al. 2013); 1,2-benzenedicarboxylic acid, 3-nitro (1.75 %), a compound with antimicrobial activity (Kavitha et al. 2010), and nonacosane (1.07 %), a compound with antibacterial effects (Shobha and Agrawal 2007), were the major compounds emitted in May. Additionally, some minor compounds, including vitamin E (0.69 %), were detected which has an antioxidative function (Venkata Raman et al. 2012). Several biological activities have been attributed to beta-caryophyllene (0.68 %), including anti-inflammatory, antimicrobial, antioxidant, anticarcinogenic, and local anesthetic activities (Legault and Pichette 2007). Taken together, the sum of the major and minor compounds contributing to the antioxidant effects of Artemisia in May was higher than those contributing to antimicrobial action.
No major compounds were detected in June, August, or October that could contribute either to antimicrobial or antioxidant effects.
Most of the major compounds detected in July, including eicosane (5.4 %), beta-caryophyllene (1.65 %), and alpha-caryophyllene (1.14 %) have antimicrobial effects (Legault and Pichette 2007; Keawsa-ard and Kongtaweelert 2012; Venkata Raman et al. 2012). Notably, the greatest number of compounds was generated in July and it may be that all minor compounds had cumulative and synergistic effects.
We identified some compounds in September that could contribute either to antimicrobial effects such as eicosane (8.3 %), gamma-sitosterol (1.87 %), and methyl commate (1.48 %; Venkata Raman et al. 2012) or antioxidant effects such as beta-amyrin acetate (2.21 %; Fabiyi et al. 2012) and stigmasterol (1 %; Venkata Raman et al. 2012). 10-Demethylsqualene was found at a high level (6.32 %); however, we could not identify its biological activity in the databases. Anti-inflammatory, antidiabetic, and anticancer effects have been reported for alpha-amyrin (1.08 %; Venkata Raman et al. 2012). Taken together, the sum of the compounds responsible for antimicrobial activities exceeded those with antioxidant effects, indicating strong antimicrobial and weak antioxidant effects.
Phytotoxic effects
Many chemical components are allelochemicals, which influence either plant–plant or plant–microbial interactions (Eom et al. 2006). Barney et al. (2005) suggested a potential role for the presence of bioactive terpenoids in mugwort (Artemisia vulgaris) establishment and proliferation in introduced habitats. On the other hand, Kegode et al. (2012) attributed the reduction in Solanum melanocerasum plant height and fresh weight to the allelopathic potential effect of Artemisia biennis. Furthermore, Araniti et al. (2013) found that aqueous and methanolic extract of Artemisia arborescens strongly inhibited both germination and root growth of lettuce (L. sativa L.). In this study, we tested the effects of emitted compounds from A. princeps var. orientalis on A. japonica and L. sativa. The effects of the secondary metabolites differed depending on the growing season. Neither the amount nor season had a substantial effect on relative germination ratio of L. sativa. In contrast, a concentration-dependent inhibitory effect was observed in A. japonica when the amount of Artemisia was increased, and a severe effect was detected in the summer (particularly August). This result indicates that L. sativa, as a crop, is more resistant than A. japonica, a representative weed, in response to Artemisia secondary metabolites derived from different times of the year (Fig. 2). It is not clear why the effect was most detrimental in August, as only two compounds were generated from Artemisia at that time. Could it possible that the emitted compounds are responsible for the effect? This is the subject of a future study. Another explanation is that the plant collected in summer might be rich in essential oil, which reported to have potential phytotoxicity on seed germination and root growth of other plants (Singh et al. 2008; Araniti et al. 2013).
The Artemisia concentration had no effect on the relative elongation ratio of L. sativa, whereas the inhibitory effect on A. japonica was concentration dependent. Notably, the summer season samples affected both plants (Fig. 3). The number of secondary metabolites that were emitted during July and September may be the main reason. It is possible that identified or unidentified components may influence the overall phytotoxic potential of mugwort secondary metabolites emitted over time in an additive/synergistic manner as suggested by Barney et al. (2005).
Seasonal variations in the composition of compounds emitted from A. princeps var. orientalis (Pamp.) H. Hara and their phytotoxic effects were examined without extraction. The plant materials were injected directly into the GC/MS through a SFSI. As a result, significant seasonal variations in the composition of secondary metabolites were observed. The Artemisia plant material showed significant phytotoxic effects on seed germination and seedling growth of A. japonica and L. sativa.
References
Abd El-Aty AM, Kim IK, Kim MR, Lee CH, Shim JH (2008) Determination of volatile organic compounds generated from fresh, white and red Panax ginseng (C. A. Meyer) using a direct sample injection technique. Biomed Chromatogr 22(5):556–562
Ahmed A, Misra LN (1994) Terpenoids from Artemisia annua and constituents of its essential oil. Phytochemistry 37:183–186
Amini S, Azizi M, Joharchi MR, Shafei MN, Moradinezhad F, Fujii Y (2014) Determination of allelopathic potential in some medicinal and wild plant species of Iran by dish pack method. Theor Exp Plant Physiol 26:189–199
Angelopoulou D, Demetzos C, Perdetzoglou D (2002) Diurnal and seasonal variation of the essential oil labdanes and clerodanes from Cistus monspeliensis L. leaves. Biochem Syst Ecol 30:189–203
Araniti F, Lupini A, Sorgonà A, Conforti F, Marrelli M, Statti GA, Menichini F, Abenavoli MR (2013) Allelopathic potential of Artemisia arborescens: isolation, identification and quantification of phytotoxic compounds through fractionation-guided bioassays. Nat Prod Res 27:880–887
Barney JN, Hay AG, Weston LA (2005) Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J Chem Ecol 31(2):247–265
Choi EJ, Kim GH (2013) Antioxidant and anticancer activity of Artemisia princeps var. orientalis extract in HepG2 and Hep3B hepatocellular carcinoma cells. Chin J Cancer Res 25(5):536–543
Chon SU, Kim YM, Lee JC (2003) Herbicidal potential and qualification of causative allelochemicals from several compositae weeds. Weed Res 43:444–450
Dudareva N, Negre F (2005) Practical application of research into the regulation of plant volatile emission. Plant Biol 8:113–118
Einhellig FA (2002) The physiology of allelochemical action: clues and views. In: Reigosa MJ, Pedrol N (eds) Allelopathy, from molecules to ecosystems. Science Publishers, Enfield
Eom SH, Yang HS, Weston LA (2006) An evaluation of the allelopathic potential of selected perennial groundcovers: foliar volatiles of catmint (Nepeta × faassenii) inhibit seedling growth. J Chem Ecol 32:1835–1848
Fabiyi OA, Atolani O, Adeyemi OS, Olatunji GA (2012) Antioxidant and Cytotoxicity of β-Amyrin acetate fraction from Bridelia ferruginea Leaves. Asian Pac J Trop Biomed 2:S981–S984
Goff SA, Klee HJ (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science 311:815–819
Hall GD, Langenheim JH (1987) Geographic variation in leaf monoterpenes of Sequoia sempervirens. Biochem Syst Ecol 15:31–43
Hamm S, Lesellier E, Bleton J, Tchapla A (2003) Optimization of headspace solid phase microextraction for gas chromatography/mass spectrometry analysis of widely different volatility and polarity terpenoids in olibanum. J Chromatogr A 1018:73–83
Hashidoko Y, Tahara S, Mizutani J (1989) Antimicrobial sesquiterpene from damaged Rosa rugosa leaves. Phytochemistry 28(2):425–430
Hosking R (1997) A dictionary of Japanese food. Tuttle Publishing, Tokyo, Japan, p 175. ISBN 978-0804820424
Kavitha A, Prabhakar P, Narasimhulu M, Vijayalakshmi R, Venkateswarlu Y, Rao KV, Raju VB (2010) Isolation, characterization and biological evaluation of bioactive metabolites from Nocardia levis MK-VL_113. Microbiol Res 165(3):199–210
Keawsa-ard S, Kongtaweelert S (2012) Antioxidant, antibacterial, anticancer activities and chemical constituents of the essential oil from Mesua ferrea leaves. Chiang Mai J Sci 39(3):455–463
Kegode G, Ciernia M, Vlieger D (2012) Allelopathic potential of Artemisia biennis (biennial wormwood). Agric Sci 3:582–587. doi:10.4236/as.2012.34070
Keys JD (1976) Chinese herbs, 1st edn. Charles E. Tuttle, Tokyo, p 219
Kil BS, Yun KW (1992) Allelopathic effects of water extracts of Artemisia princeps var. orientalis on selected plant species. J Chem Ecol 18:39–51
Kim JH (1996) Seasonal variation in concentration and composition of monoterpenes from Artemisia princeps var. orientalis. Korean J Ecol 19(4):321–328
Kim YS, Kim MN, Kim JO, Lee JH (1994) The effect of hot water-extract and flavor compounds of Mugwort on microbial growth. Korean Soc Food Nutr 23:994–1000
Kim MR, Abd El-Aty AM, Kim IS, Shim JH (2006) Determination of volatile flavor components in danggui cultivars by solvent free injection and hydrodistillation followed by gas chromatographic–mass spectrometric analysis. J Chromatogr A 1116(1–2):259–264
Kim IK, Abd El-Aty AM, Shin HC, Lee HB, Kim IS, Shim JH (2007) Analysis of volatile compounds in fresh healthy and diseased peppers (Capsicum annuum L.) using solvent free solid injection coupled with gas chromatography-flame ionization detector and confirmation with mass spectrometry. J Pharm Biomed Anal 45:487–494
Ko AY, Musfiqur Rahman M, Abd El-Aty AM, Jang J, Choi JH, Mamun MI, Shim JH (2014) Identification of volatile organic compounds generated from healthy and infected powdered chili using solvent-free solid injection coupled with GC/MS: application to adulteration. Food Chem 156:326–332
Legault J, Pichette A (2007) Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol 59(12):1643–1647
Liu CH, Mishra RX, Tan RX, Tang C, Yang H, Shen YF (2006) Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour Technol 97:1969–1973
Mamede MEO, Pastore GM (2006) Study of methods for the extraction of volatile compounds from fermented grape must. Food Chem 96(4):586–590
Manorenjitha MS, Norita AK, Norhisham S, Asmawi MZ (2013) GC–MS analysis of bioactive components of Ficus religiosa (linn.) stem. Int J Pharma Biosci 4(2):99–103
McLafferty FW, Stauffer DB (1989) The Wiley/NBS registry of mass spectral data. Wiley, New York
Nugroho A, Lee KR, Alam MB, Choi JS, Park HJ (2010) Isolation and quantitative analysis of peroxynitrite scavengers from Artemisia princeps var. orientalis. Arch Pharmacal Res 33(5):703–708
Park JC, Yu YB, Lee JH, Kim NJ (1994) Studies on the chemical components and biological activities of edible plants in Korea (VI)-Anti-inflammatory and analgesic effects of Cedrela sinensis, Oenanthe javanica and Artemisia princeps var. orientalis. Korean Soc Food Nutr 23(1):116–119
Rothschild M, Moore BP, Brown WV (1984) Pyrazines as warning odour components in the Monarch butterfly, Danaus plexippus, and in moths of the genera Zygaena and Amata (Lepidoptera). Biol J Linn Soc 23(4):375–380
Rothschild M, Bergström G, Wängberg S (2005) Cannabis sativa: volatile compounds from pollen and entire male and female plants of two variants, Northern Lights and Hawaiian Indica. Bot J Linn Soc 147:387–397
Roussis V, Tsoukatou M, Petrakis PV, Chinou I, Skoula M, Harborne JB (2000) Volatile constituents of four Helichrysum species growing in Greece. Biochem Syst Ecol 28:163–175
Sarath VJ, So CS, Won YD, Gollapudi S (2007) Artemisia princeps var. orientalis induces apoptosis in human breast cancer MCF-7 cells. Anticancer Res 27(6B):3891–3898
Shobha RP, Agrawal R (2007) Volatile compounds of therapeutic importance produced by Leuconostoc paramesenteroides, a native laboratory isolate. Turk J Biol 31:35–40
Singh HP, Kaur S, Mittal S, Batish DR, Kohli RK (2008) Phytotoxicity of major constituents of the volatile oil from leaves of Artemisia scoparia Waldst. & Kit. Z Naturforsch C 63:663–666
Toda S (2004) Inhibitory effects of polyphenols in leaves of Artemisia princeps PAMP on protein fragmentation by Cu(II)–H2O2 in vitro. J Med Food 7(1):52–54
Toda S (2005) Antioxidative effects of polyphenols from leaves of Artemisia princeps Pamp. on lipid peroxidation in vitro. J Food Biochem 29:305–312
Umano K, Hagi Y, Nakahara K, Shoji A, Shibamoto T (2000) Volatile chemicals identified in extracts from leaves of Japanese mugwort (Artemisia princeps Pamp.). J Agric Food Chem 48:3463–3469
Venkata Raman B, Samuel LA, Pardha Saradhi M, Narashimha Rao B, Naga Vamsi krishna A, Sudhakar M, Radhakrishnan TM (2012) Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J Pharm Clin Res 5:99–106
Yun KW, Choi SK (2003) Seasonal variation in allelopathic potential of Artemisia princeps var. orientalis on plants and microbes. J Plant Biol 46(2):105–110
Yun KW, Kil BS (1992) Assessment of allelopathic potential in Artemisia princeps var. orientalis residues. J Chem Ecol 18:1933–1940
Yun KW, Jung HJ, Kim JH (2008) The influence of the growth season on the antimicrobial and antioxidative activity in Artemisia princeps var. orientalis. Ind Crops Prod 27:69–74
Acknowledgments
This study was supported by the MSIP (Ministry of Science, ICT and Future Planning).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mamun, M.I.R., Abd El-Aty, A.M., Musfiqur Rahman, M. et al. Characterization of secondary metabolite compounds correlated with the seasons in Artemisia princeps var. orientalis (Pamp.) H. Hara leaves using direct sample injection and gas chromatography–mass spectrometry: contribution to phytotoxicity. J Korean Soc Appl Biol Chem 58, 173–183 (2015). https://doi.org/10.1007/s13765-015-0020-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0020-3