Abstract
Six perennial groundcovers including Alchemilla mollis, Nepeta × faassenii, Phlox subulata, Sedum acre, Solidago cutleri, and Thymus praecox were investigated for the allelopathic potential of their respective foliar tissues via evaluation of volatile constituents produced by foliage. These groundcovers were selected for further laboratory evaluation because of superior performance as weed-suppressive groundcovers in previous field experiments. Foliar volatile components of N. × faassenii exhibited the strongest inhibitory effects on seedling growth of curly cress (Lepidium sativum), but S. cutleri also showed allelopathic potential by reducing shoot growth of curly cress seedlings with extracted volatiles. Although A. mollis and P. subulata exhibited strong weed-suppressive traits in past field experiments, weed suppression is apparently associated with either competition for resources or other allelopathic mechanisms rather than an allelopathic effect caused by volatiles. Volatiles of N. × faassenii were further evaluated with gas chromatography coupled to mass spectrometry (GC-MS). A total of 21 chemical constituents were identified in the volatile cocktail; 17 components were identified from a direct crude leaf sample extraction, including sabinene, β-pinene, β-myrcene, 2-(2-ethoxyethoxy)-ethanol, 1,8-cineole, ocimene, neryl Acetate, 4aα,7α,7aα-nepetalactone, α-copaene, trans-caryophyllene, alloaromadendrene, 4aβ,7α,7aβ-nepetalactone, germacrene D, β-farnesene, χ-cadinene, germacrene B, and β-sesquiphellandrene. Five additional constituents were identified in a methanolic extract of dried of N. × faassenii foliage, but not the volatile cocktail collected from N. × faassenii foliage. These included methyl benzoate, 2,4-decadienal, neryl acetate, isodihydronepetalactone, and caryophyllene oxide. Three components, 2-(2-ethoxyethoxy)-ethanol, alloaromadendrene, and χ-cadinene, were not only detected in both the volatile mixture and the methanolic extract, but also in an aqueous foliar extract that exhibited potential allelopathic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbaceous perennial groundcovers have been popularly used in home gardens and landscapes because of their ability to rapidly cover the soil surface and prevent erosion, as well as for their aesthetic appeal. Certain herbaceous perennials evaluated in past field experiments have shown potential as weed-suppressive groundcovers for utilization in many urban landscapes including commercial properties, municipal settings, and even along roadsides (Eom et al., 2005).
Weed-suppressive groundcovers can be used in both private landscapes and roadside settings, and generally form dense foliar canopies, preventing light transmittance to the soil surface beneath the groundcover, thereby limiting weed seed germination and seedling establishment (Eom et al., 2005). The height and density of the groundcover canopy contribute to its ability to suppress weeds, and allelochemicals from vegetative plant parts may also contribute to weed suppression (Eom 2004). Establishment of groundcovers is limited by various environmental factors that influence plant growth, including physical soil condition, salinity, drought, previously established plant species, and temperature extremes (Usón and Poch 2000). Furthermore, relatively large-scale landscape settings are often affected by uncontrollable and undesirable environmental factors. Although we selected groundcovers that exhibited a strong ability to suppress weeds over time because of their competitive interference with weeds, the growth and establishment of groundcovers in difficult urban landscape settings is dependent upon many factors. Therefore, groundcovers that exhibit inherent allelopathic as well as competitive traits may be more successful in suppressing surrounding vegetation, especially on a long-term basis.
Allelochemicals are released from plants into the environment in several ways, including volatilization from leaf tissues, leaching of nonvolatiles from foliage by rainfall, exudation from living roots, or decomposition of residues by soil microorganisms (Shiraishi et al., 2002; Rai et al., 2003; Kobayashi, 2004). Persistence and activity of allelochemicals released under natural conditions or field settings are influenced by the environment, specifically meteorological and rhizospheric conditions, as well as physical distance from the source of production (Jose and Gillespie, 1998; Romero-Romero et al., 2002). Unlike other mechanisms of allelopathy, studies on volatile effects in plant–plant interactions have been less often investigated, although some significant effects have been associated with volatiles produced by mugwort (Artemisia vulgaris) and other species as well (Bradow and Connick, 1990; Barney et al., 2005). The evaluation of certain herbaceous ornamentals for their ability to suppress weeds through allelopathic interference has been reported (Shiraishi et al., 2002). Several groundcovers, including Oxalis brasiliensis, Phlox subulata, and Lycoris radiate were observed to be strongly weed-suppressive when foliar tissues were bioassayed in the laboratory under controlled conditions.
As little information is available regarding the allelopathic potential of such groundcovers, we selected six of our top-performing weed-suppressive species for further evaluation in the laboratory. In our field evaluation, certain groundcovers, including Alchemilla mollis, Nepeta × faassenii, P. subulata, and Thymus praecox, were superior performers in terms of establishment and/or weed suppression (Eom et al., 2005). In addition, Solidago cutleri and Sedum acre were included for further investigation of allelopathic traits because these two groundcovers and related species are relatively stress-tolerant and establish well in field settings (Eom et al., 2005). We specifically sought to determine if these groundcovers exhibited allelopathic potential in in vitro bioassays by examining the activity of the foliar volatiles they produced. Certain herbaceous perennials, such as mints and common potherbs, emit large quantities of volatiles, likely associated with plant defense against pests or insect attraction. Because N. × faassenii exhibited strong potential allelopathic activity in these assays, we focused on isolation and evaluation of its volatile constituents.
Methods and Materials
Groundcover Growth
Six groundcovers and a commercial tomato plant (Solanum lycopersicum L.) were propagated for use in our volatile assays. The tomato plant was used as a positive control for production of foliar volatiles. S. acre L. (Sedum “Acre”) was propagated from 5-cm stem cuttings. Seeds of other perennial groundcovers—A. mollis L. (lady's mantle), N. × faassenii L. (catmint “Walker's Low”), P. subulata L. (creeping phlox “Emerald blue”), S. cutleri L. (ornamental goldenrod), and T. praecox L. (creeping thyme) were purchased from Jelitto Staudensamen GmbH (Schwarmstedt, Germany). Selected groundcovers had previously exhibited strong weed suppression (A. mollis, N. × faassenii, and P. subulata) in field and roadside experiments (Eom et al., 2005) or excellent establishment (S. cutleri, T. praecox, and S. acre) in landscape settings. Stratification of seed was required for uniform germination; therefore, seed was initially placed in moist, filter-paper-lined Petri dishes (4 ml of distilled H2O/Petri dish) for 6 d at a 4°C dark condition, and then moved to a lighted growth chamber [150 μmol m−2 s−1 photosynthetic photon flux (PPF) overhead light intensity] at 25°C for 7 d according to instructions available in the Jelitto catalogue, 2000. Groundcovers were sown into Metro-mix® 510 growth media (Scotts Co., Marysville, OH, USA) and later transplanted into a soil mixture comprised of 50% local soil (Hudson silt clay loam), 25% sand, and 25% Metro-mix® 510 growth media based on volume. All groundcovers in 25 pots of each species for this experiment were grown in a propagation house for 12 mo. Plants were maintained under a 14-hr photoperiod with a light intensity of 200 μmol m−2 s−1 PPF from supplemental overhead lighting. Daytime temperatures in the greenhouse were maintained within 24–28°C, whereas nighttime temperatures were maintained at 18–22°C. Plants were fertilized biweekly with full-strength Hoagland's solution (200–300 ml/pot).
Volatile Bioassay
In an assay similar to that developed in our laboratory to assess volatile activity in mugwort tissue (Barney et al., 2005), mature leaves of each groundcover species were excised and directly placed in a plastic ziplock bag submerged in ice water. In the laboratory shortly after collection, leaf samples were folded and wrapped in cheesecloth at amounts of 0, 5, 10, and 20 g of tissue (fresh weight). Each wrapped sample was suspended in a capped 500-ml glass flask in which curly cress (Lepidium sativum L.) seeds (20/bottle) were placed on moistened filter paper (Whatman #4) wetted with 5 ml distilled water (Figure 1). Selection of seedling indicators for use in the bioassay requires that the selected species exhibit a high level of germination and uniform growth over time (Bertin et al., 2003). Curly cress meets these requirements (Bertin et al., 2003) and does not require seed pretreatment or stratification; seeds rapidly germinated after storage in a dark, cold room (5°C) upon transfer to a moistened filter paper (4 ml of distilled H2O/Petri dish) in parafilm-sealed Petri dishes and a light regime of 150 μmol m−2 s−1 PPF overhead light intensity. As a control treatment, foliar tissues of tomato (S. lycopersicum L.), commonly known to produce volatile constituents from its foliage (Smith et al., 1996; Farag and Paré, 2002; Feng et al., 2004), were utilized for comparative purposes. Fully expanded leaves of tomato grown for 30 d were selected, and treatments were arranged in a completely randomized design maintained in a growth chamber at 28°C daily temperature and 200 μmol m−2 s−1 PPF overhead light intensity. After 72 hr of growth in enclosed environments, length of curly cress seedling roots and shoots were measured. All treatments were replicated four times.
Sample Preparations for Analyzing Nepeta Foliar Constituents
A direct crude leaf volatile extract was made to analyze the volatile constituents from fresh leaves of N. × faassenii grown in a greenhouse. A potted N. × faassenii plant was brought into the laboratory where volatile analysis was performed with a gas chromatograph coupled to a mass spectrometer at room temperature and ambient conditions. Detached fully expanded young leaves were immediately submitted for volatile analyses as described below. In addition, both aqueous and methanolic extracts were formulated by using dried leaves. Ground foliage (100 g) was extracted with 1500 ml distilled water or HPLC (high-performance liquid chromatography)-grade methanol with a rotating shaker maintained in the dark at 5°C for 48 hr. Both extracts were filtered through eight layers of cheesecloth to remove coarse particulates, and centrifuged at 25,000 rpm for 20 min (RC5C®, Sorvall Instruments, Du Pont, Wilmington, MA, USA) to remove the fine particulates. Final filtration was performed with #4 and #42 filter papers (Whatman) placed in a Büchner funnel. The methanol solvent was vacuum-evaporated at 38°C, and the remaining constituents were analyzed by gas chromatography-mass spectrometry (GC-MS). The aqueous water extract was reduced to dryness with a vacuum rotary evaporator at 38°C, and the residue was resolved in 250 ml distilled deionized water. The aqueous extract was partitioned sequentially × 6 with 250 ml HPLC-grade solvents that included n-hexane, diethyl ether, and ethyl acetate by using a separatory funnel. After evaporation of solvents, activity of those residues was assessed by measuring the growth of 72 hr cress seedlings in comparison to an aqueous control with a standard Petri dish germination assay. Each concentrated fraction dissolved in its initial solvent was applied to filter paper in Petri dish. The Petri dish was placed into a 40°C oven for 48 hr to allow solvent evaporation. Ten cress seeds were arranged in glass Petri dishes containing 1 ml distilled water and #1 Whatman filter paper. After parafilm sealing, dishes were maintained in the dark at high humidity at 25–26°C for 72 hr, and root and shoot elongation were measured. Upon evaluation of the seedling bioassay, ether and ethyl acetate fractions were combined and subjected to flash column chromatography. Silica gel flash column chromatography was performed by using a chloroform/methanol solvent gradient, starting with 100% chloroform and terminating with 100% methanol, to further separate the active extracts. Fraction number 4 out of 25 total fractions collected was analyzed by GC-MS, based on activity-guided fractionation with the same cress seedling bioassay.
Identification of Volatiles by SPME Headspace GC-MS
Clear vials (2 ml, Supelco) with PTFE rubber seals (11 mm, Supelco) were used to obtain equilibrium of sample solids (groundcover foliage) with their respective gaseous phase (volatile constituents). After weighing fresh foliar tissues (1 g) of N. × faassenii for analysis, volatile adsorption to SPME fiber (polyacrylate, 85 μm) was conducted at room temperature for 30 min. In the injection port of the GC, temperatures of 260°C resulted in desorption of volatiles, which were continuously injected into the GC for 5 min. GC-MS (Agilent 6890 + Agilent 5973) was used to analyze all volatile constituents present in each sample. A DB-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm) was used for the separation of analytes. The column was temperature-programmed from 50°C (held for 3 min) to 200°C (held for 2 min) at a rate of 10°C/min, and then to 300°C (held for 5 min) at a rate of 15°C/min. Helium was used as a carrier gas with a constant flow rate of 1.0 ml/min. For the mass selective detector (MSD), the electron energy was 70 eV, ion source temperature was 230°C, quadruple temperature was 150°C, and interface temperature was 280°C. MSD was used in SCAN mode over a mass scan range at m/z 30–400, with every analysis verified with air as a blank control for injection between samples to reduce contamination.
Retention indices for all compounds on the DB-5MS capillary column were determined according to the Van Den Dool approach, with n-alkanes (especially n-hexane) as standards (Kovats 1958). Identification of the components was based on comparison of their mass spectra with those of Wiley Libraries and those described by R. Adams, as well as by comparison of their retention indices with published values (Van Den Dool and Kratz, 1963).
Results
Effects of Groundcover Foliar Volatiles on the Growth of Curly Cress Seedlings
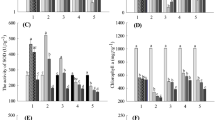
Of the groundcovers evaluated for volatile production, foliar tissue of most, with the exception of S. acre, inhibited the shoot growth of curly cress seedlings, with increasing inhibition observed with increasing rates of fresh foliage placed in the enclosed environment (Figure 1). In contrast, the volatiles of tomato leaves did not inhibit the growth of curly cress seedlings (Figure 2). Overall, N. × faassenii volatiles produced the greatest inhibitory effects by consistently reducing both shoot and root elongation. N. × faassenii volatiles inhibited shoot growth by 21% and 48% relative to the untreated controls at 5 and 10 g of foliage, respectively. Root elongation was inhibited by 7% and 44%, respectively (Figure 2), in comparison to controls. Furthermore, radicle elongation and shoot growth were completely inhibited at the 20 g foliage treatment (Figure 2). Foliar volatiles of A. mollis also significantly inhibited shoot growth, but volatiles had little impact on root elongation. Interestingly, radical elongation of curly cress exposed to 5 g of P. subulata and S. cutleri foliage was increased by 179% and 164%, respectively, indicating some stimulatory activity of certain collected volatile constituents. In comparison, root elongation, when exposed to 5 g of S. acre foliage, was not significantly different from the control, but increased with exposure to 10 g of foliage, indicating that S. acre foliage also possessed stimulatory potential (Figure 2).
Top: Inhibition of seedling growth of curly cress (Lepidium sativum L.) by volatiles produced by groundcover and tomato foliage (5 and 10 g fresh weight) as compared to an untreated control. Values presented are means of curly cress seedlings (N = 80) with standard errors. Nepeta × faassenii volatile effect: overall Pr >F of curly cress shoot is <0.001 and LSD = 1.336, and overall Pr >F of curly cress root is <0.001 and LSD = 1.831. Solidago cutleri volatile effect: overall Pr >F of curly cress shoot is <0.001 and LSD = 0.784, and overall Pr >F of curly cress root is 0.083 and LSD = 2.0389. Phlox subulata volatile effect: overall Pr >F of curly cress shoot is >0.001 and LSD = 1.042, and overall Pr >F of curly cress root is 0.001 and LSD = 1.837. Alchemilla mollis volatile effect: overall Pr >F of curly cress shoot is >0.001 and LSD = 1.183, and overall Pr >F of curly cress root is 0.795 and LSD = 2.046. Thymus praecox volatile effect: overall Pr >F of curly cress shoot is >0.001 and LSD = 1.078, and overall Pr >F of root is 0.023 and LSD = 2.044. Sedum acre volatile effect: overall Pr >F of curly cress shoot is 0.009 and LSD = 1.117, and overall Pr >F of curly cress root is 0.006 and LSD = 2.476. Solanum lycopersicum volatile effect: overall Pr >F of curly cress shoot is >0.001 and LSD = 1.012, and overall Pr > F of curly cress root is 0.423, LSD = 2.118. Bottom: Seedling growth of curly cress after exposure to Nepeta × faassenii foliage. Activity of fresh foliage of Nepeta × faassenii was assessed at 0, 5, 10, and 20 g fresh weight in enclosed volatile bioassays.
Identification of Volatiles in N. × faassenii by SPME-Headspace GC-MS
A total of 22 compounds were identified in N. × faassenii volatile headspace by GC-MS (Figure 3 and Table 1). Seventeen compounds were detected directly within the volatile cocktail collected from N. × faassenii leaf tissue (Table 1). The components included sabinene, β-pinene, β-myrcene, 2-(2-ethoxyethoxy)-ethanol, 1,8-cineole, ocimene, neryl acetate, 4aα,7α,7aα-nepetalactone, α-copaene, trans-caryophyllene, alloaromadendrene, 4aβ,7α,7aβ-nepetalactone, germacrene D, β-farnesene, χ-cadinene, germacrene B, and β-sesquiphellandrene. Many of the volatiles we observed were similar to those reported previously in the essential oils of N. crassifolia, N. cataria L., and N. macrosiphon Boiss. (Baranauskiene et al., 2003; Dabiri and Sefidkon, 2003; Javidnia et al., 2004).
As root elongation of curly cress seedlings were most affected by aqueous extracts of crude N. × faassenii foliar tissues, polyacrylate fibers (85 m) were also utilized to detect polar or semivolatile materials within one fraction (number 4) that was collected and further purified by column chromatography after aqueous extraction. Three components were detected, including 2-(2-ethoxyethoxy)-ethanol, alloaromadendrene, and γ-cadinene, at the detection limit (≤0.04% of GC peak area). These constituents were detected within the volatile cocktail collected from N. × faassenii foliage.
Methanolic extraction of N. × faassenii foliage produced similar results, with 10 similar constituents observed at similar detectable levels as above. Five of the 10 components, 5,5-dimethyl-2(5H)-furanone, methyl benzoate, 2,4-decadienal, isodihydronepetalactone, and caryophyllene oxide were only detected in the methanolic extract.
Leaf and Stem Structures of N. × faassenii
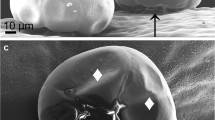
N. × faassenii, commonly known as catmint, is an attractive ornamental groundcover used in the landscape for mass plantings. It forms a dense canopy of pubescent foliage, and produces attractive blue inflorescences for most of the summer season (Armitage 1997). Trichomes of N. × faassenii were observed in great density on the surface of both leaf and stem tissues (Figure 4). Trichomes were denser on the underside of the leaf in comparison to the upper surface, and were less numerous on the stem surface in comparison to leaf. Glandular exudates were also observed on both catmint leaf surfaces and stems.
Seedling Growth of Curly Cress on Either Aqueous or Methanolic Extracts
Seedling growth of curly cress was measured to compare the influence of extraction method, with water or methanol upon extract phytotoxicity (Figure 5). Inhibitory effects upon the seedling growth were more significant with water than with methanolic extracts, when similar dosages of extracts were evaluated (Figure 5). However, total yield of extracts was 26-fold higher in methanolic extracts (17.20 g/100 g DW foliar tissues) than in water extracts (0.77 g/100 g DW). Further fractionation with ether provided greater yield in comparison to other organic solvents (14.09 g). Cress seeds did not germinate when exposed to concentrations of 0.5 mg/ml aqueous extracts or above. When methanolic extracts were further partitioned, the hexane fraction at 1.0 mg/ml dosage showed the strongest inhibitory activity among all fractions evaluated, indicating that less polar constituents were associated with inhibitory activity; curly cress seed germination was completely inhibited at 1.0 mg/ml concentrations.
A comparison of activity observed from aqueous (a) or methanolic (b) extraction of dried N. × faassenii shoot tissue using a curly cress seedling elongation bioassay. Each extract was fractionated with organic solvents using stepwise fractionation. Each fraction was dissolved in distilled water and bioassayed with solutions of 0, 5, and 10 mg/ml. Shoot length of curly cress was measured at 4 d after germination initiation. Values presented are means (N = 80) with standard errors.
Discussion
The same volatile bioassays we designed to assess in vitro inhibitory activity could be considered a potential means to assess the impact of leaf detachment upon volatile production from living plants. Leaf detachment may trigger the production of abscisic acid and ethylene that is caused by change in water status or availability in the leaf (Taiz and Zeiger, 2002). The presence of these hormones may further impact volatile production by inducing metabolic changes in higher plant systems (Taiz and Zeiger, 2002; Feng et al., 2004; LeNoble et al., 2004). We utilized foliar tissues from tomato (S. lycopersicum L.) for comparison with groundcover tissues in this volatile bioassay as a positive control. It is well known that excised tomato leaves can produce significant levels of ethylene over time, besides other scented volatiles (Smith et al., 1996; Farag and Paré, 2002; Feng et al., 2004). In these studies, foliar volatiles of tomato did not greatly inhibit seedling growth of curly cress when compared to groundcover foliage (Figure 2), and neither groundcovers nor tomato produced significant levels of detectable ethylene in this closed assay system.
Given the large numbers of surface glands or trichomes observed on the abaxial surface of N. × faassenii leaves, it is likely that significant quantities of volatiles may be emitted by foliage under laboratory or field conditions. In past studies, we have shown that volatiles produced by foliage of mugwort (A. vulgaris) successfully bind to soil particles, and render soil in an enclosed environment inhibitory to subsequent seedling germination and growth (Barney 2004). Although we did not perform groundcover volatile assays in the presence of field soil in the laboratory or conduct field-based collections of volatiles under actual field conditions, few—if any—weed seedlings emerged directly around or beneath established N. × faassenii plants over a 3-yr period in two separate locations across New York state (Eom et al., 2005). Our findings indicate that foliarly produced allelochemicals may play a role in reduction of weed seedling growth, besides that of resource competition in plant interference. The volatiles of N. × faassenii could act as allelochemicals in field settings, if significant concentrations occur under field conditions. Our laboratory assays indicate that in an enclosed environment with detached tissues, foliar volatiles emitted from small quantities of N. × faassenii foliage are quite active as seedling growth inhibitors.
Plants of the genus Nepeta, commonly called catmints, synthesize and emit various volatiles through foliar trichomes and glands (Plepys et al., 2002). Trichomes were highly abundant in catmint foliage, especially along the abaxial surface of N. × faassenii leaves (Figure 4). Circular glands containing volatiles produced by N. × faassenii were also observed to be numerous and were located on the leaf surface itself. Of the volatiles detected, isomers of 7S-nepetalactone, which are monoterpenoids, were the principal constituents of the essential oil accumulated by the genus Nepeta (Hallahan et al., 1998). In our experiment, three forms of nepetalactone were detected in N. × faassenii foliar volatiles. Of the three forms, 4α,7,7α-nepetalactone was the most abundant component, representing 73% of all detected components in a direct crude sample analysis using GC-MS (Table 1). Nepetalactones are believed to attract insects for insect plant pollination (Plepys et al., 2002). However, they have not yet been associated with inhibition of plant growth. Because of their complex structure and our inability to purchase or synthesize these compounds, we have yet to assess their biological activity. Many of the other volatile components detected are also reported allelochemicals, influencing either plant–plant or plant–microbial interactions, including β-myrcene (Ward et al., 1997), ocimene, β-farnesene (McAuslane and Alborn, 1998), and caryophyllene (Stipanovic et al., 1990). Subjected to volatile emission, curly cress was completely inhibited when 20 g or more of fresh foliar tissues of N. × faassenii were suspended in closed bioassay system in 500 ml glass bottles (Figure 2).
To investigate another possibility for the release of allelopathic constituents, we evaluated both the aqueous and methanolic extracts from foliar tissues of groundcovers for inhibitory potential in order to assess possibilities for production of non-volatile inhibitors. By using a standard seed germination/radical elongation bioassay, both aqueous and methanolic extracts of dried foliar tissues of N. × faassenii (Figure 5) and S. cutleri inhibited seedling growth (data not shown). Although the use of solvent extraction for increasing yield of allelochemicals from extracts of plant tissues is common in the literature, certain extracted chemicals may not be typically released into a natural environment under average field conditions (Lovett and Jessor, 1982). Instead of the relatively harsh solvent extraction technique with methanol, we also decided to use an aqueous method to extract volatile and non-volatile constituents from the foliar tissues of N. × faassenii. In an activity comparison between aqueous and methanol extracts, the aqueous extracts of foliage exhibited greater specific activity in terms of seedling growth reduction on a per-weight basis (Figure 5). Not surprisingly, the actual amount of dried residue obtained after aqueous extraction was 26-fold less than that obtained by methanolic extraction. Volatile components were evaluated in both aqueous and methanolic extracts, even though nonvolatile allelopathic components may also be present in considerable quantities in catmint foliage.
From the GC-MS results, fractionation of the aqueous extracts by column chromatography resulted in only three chemicals being detected in purified active fraction number 4 including 2-(2-ethoxyethoxy)-ethanol, alloaromadendrene, and γ-cadinene, at the detected level (≤0.04% of GC peak area). Methanolic extraction yielded 10 chemical constituents including the key components also present in aqueous extracts (Table 1). Volatile analysis yielded 17 chemical constituents present in the crude sample of N. × faassenii foliage. As we were not able to evaluate all of these components individually or in mixtures in separate plant growth bioassays, it is unknown which constituents are associated with plant growth inhibition. Further analysis and separation of aqueous N. × faassenii extracts by HPLC would likely result in isolation of other active non-volatile constituents.
In summary, previous field experiments showed that A. mollis and N. × faassenii were highly suppressive of weed growth, as were several other groundcovers that were moderately suppressive. Laboratory experiments indicate that only one of these, N. × faassenii, may show strong potential to suppress curly cress growth by volatile allelopathic interference. It evidently produces allelochemicals that are active both as volatile and non-volatile forms from its foliar tissues. Although we examined the effects of allelopathic traits in an in vitro volatile assay, our fieldwork mainly focused on examination of weed suppression and competition for resources. Although we have no proof of allelopathy under field conditions, we have evidence to support the concept that catmint tissues could potentially interfere with weed seedling growth due to allelochemical production.
References
Armitage, A. M. 1997. Herbaceous Perennial Plants, 2nd edition. Stipes Publishing, Champaign, IL.
Baranauskiene, R., Venskutonis R. P., and Demyttenaere Jan, C. R. 2003. Sensory and instrumental evaluation of catnip (Nepeta cataria L.) aroma. J. Agric. Food Chem. 51:3840–3848.
Barney, J. 2004. Characterization of allelopathic and invasive potential of mugwort (Artemisia vulgaris L.), pp. 34–63. MS thesis, Cornell University, Ithaca.
Barney, J., Hays, A., and Weston, L. A. 2005. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 31:247–265.
Bradow, J. M. and Connick, W. J. Jr. 1990. Volatile seed germination inhibitors from plant residues. J. Chem. Ecol. 16:645–666.
Dabiri, M. and Sefidkon, F. 2003. Chemical composition of Nepeta crassifolia Boiss. & Buhse oil from Iran. Flavour Fragr. J. 18:225–227.
Eom, S. H. 2004. Herbaceous perennials for use along N.Y. roadsides: Groundcover suppression of weeds, tolerance of environmental stress and allelochemical content, pp. 7–34. PhD dissertation, Cornell University, Ithaca.
Eom, S. H., Senesac, A. F., Tsontakis-Bradley, I., and Weston, L. A. 2005. Evaluation of herbaceous perennials as weed suppressive groundcovers for use along roadsides or in landscapes. J. Environ. Hortic. 23:198–203.
Farag, M. A. and ParÉ, P. W. 2002. C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61:545–554.
Feng, X., Apelbaum, A., Sisler, E. C., and Goren, R. 2004. Control of ethylene activity in various plant systems by structural analogues of 1-methylcylopropene. Plant Growth Regul. 42:29–38.
Hallahan, D. L., West, J. M., Smiley, D. W. M., and Pickett, J. A. 1998. Nepetalactol oxidoreductase in trichomes of the catmint Nepeta racemosa. Phytochemistry 48:421–42719.
Javidnia, K., Miri, R., Jafari, A., and Rezai H. 2004. Analysis of the volatile constituents of Nepeta macrosiphon Boiss. grown in Iran. Flavour Fragr. J. 19:156–158.
Jose, S. and Gillespie, A. 1998. Allelopathy in black walnut (Juglans nigra L.) alley cropping. I. Spatio-temporal variation in soil juglone in a black walnut-corn (Zea mayz L.) alley cropping system in the Midwestern USA. Plant Soil 203:191–197.
Kobayashi, K. 2004. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manage. 4:1–7.
Kovats, E. 1958. Gas chromatographische Charakteriserung organischer Verbindungen. I. Retentions indices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv. Chim. Acta 41:1915–1932.
Lenoble, M. E., Spollen, W. G., and Sharp, R. E. 2004. Maintenance of shoot growth by endogenous ABA: Genetic assessment of the involvement of ethylene suppression. J. Exp. Bot. 55:237–245.
Lovett, J. V. and Jessor, R. S. 1982. Effects of residues of crop plants on germination and early grown of wheat. Aust. J. Agric. Res. 33:909–91620.
McAuslane, H. J. and Alborn, H. T. 1998. Systemic induction of allelochemicals in glanded and glandless isogenic cotton by Spodoptera exigua feeding. J. Chem. Ecol. 24:399–416.
Plepys, D., Ibarra, F., Francke, W., and L Ö Fstedt, C. 2002. Odour-meditated nectar foraging in the silver Y moth, autographa gamma (Lepidoptera: Noctuidae): Behavioural and electrophysiological responses to floral volatiles. Oikos 99:75–82.
Rai, V. K., Gupta, S. C., and Singh, B. 2003. Volatile monoterpenes from Prinsepia utilis L. leaves inhibit stomatal opening in Vicia faba L. Biol. Plantarum 46:121–124.
Romero-Romero, T., Anaya, A. L., and Cruz-Ortega, R. 2002. Screening for effects of phytochemical variability on cytoplasmic protein synthesis pattern of crop plants. J. Chem. Ecol. 28:617–629.
Shiraishi, S., Watanabe, I., Kuno, K., and Fujii, Y. 2002. Allelopathic activity of leaching from dry leaves and exudates from roots of groundcover plants assayed on agar. Weed Biol. Manage. 2:133–142.
Smith, R. M., Marshall, J. A., Davey, M. R., Lowe, K. C., and Power, J. B. 1996. Comparison of volatiles and waxes in leaves of genetically engineered tomatoes. Phytochemistry 43:753–75821.
Stipanovic, R. D., Elissalde, M. H., and Norman, J. O. 1990. Cell culture bioassay to evaluate allelochemical toxicity to Heliothis virescens lepidoptera noctuidae. J. Econ. Entomol. 83:737–741.
Taiz, L. and Zeiger, E. 2002. Plant Physiology, 3rd edn. Sinauer Associates, Inc., Publishers, pp. 520–533.
UsÓn, A. and Poch, R. M. 2000. Effects of tillage and management practices on soil crust morphology under a Mediterranean environment. Soil Tillage Res. 54:191–196.
Van Den Dool, H. and Kratz, P. D. 1963. A generation of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. 11:463–471.
Ward, B. B., Courtney, K. J., and Langenheim, J. H. 1997. Inhibition of Nitrosomonas europaea by monoterpenes from coastal redwood (Sequoia sempervirens) in whole-cell studies. J. Chem. Ecol. 23:2583–2598.
We like thank Paul A. Weston for review of this manuscript and photos of Nepeta foliage, and Roselee Harmon for assistance in laboratory experimentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eom, S.H., Yang, H.S. & Weston, L.A. An Evaluation of the Allelopathic Potential of Selected Perennial Groundcovers: Foliar Volatiles of Catmint (Nepeta × faassenii) Inhibit Seedling Growth. J Chem Ecol 32, 1835–1848 (2006). https://doi.org/10.1007/s10886-006-9112-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9112-1