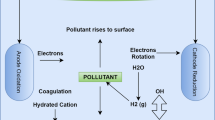
Abstract
Metal plating industry wastewater is a highly toxic wastewater due to its heavy metal and cyanide. This characteristic of wastewater is due to different types of processes used in the metal plating industry. In order to meet discharge limits for the receiving environment, classical chemical treatment methods are widely applied in this type of industry. Consequently, high treatment chemicals are required, resulting in excessive amounts of hazardous sludge. For these reasons, in this study, a pilot scale electrocoagulation (EC) process was developed as an alternative to the conventional chemical treatment currently applied in a metal plating plant. In this study, the effect of pH adjustment on the removal efficiency of the EC process was investigated before and after EC processing in a pilot scale reactor. Particularly, two heavy metals such as copper (Cu) and nickel (Ni), which are problematic to be eliminated in the current treatment, removals were invesigated. With the optimization studies, it was observed that Cu and Ni removals were over 93.75%. Similarly, Cu and Ni removal efficiencies were determined over 95% in the optimization of the EC process after pH adjustment. Indeed, these efficiencies were also achieved in the control study. As a result of the optimization of the study, model analyses were made with response surface methodology and it was observed that the regression coefficients were > 94.00% which were within the 95% confidence interval. This indicated that both the real operating conditions and the results obtained from the model are consistent with each other.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapidly increasing industrialization, it is very important for the environment to ensure the result-oriented treatability of the wastewater generated and to create an industrial model (Fung and Wibowo 2013). Among the industries, especially metal plating industry wastewater treatment is absolutely mandatory due to the toxic components they contain (Al-Shannag et al. 2015). As it is known, metal plating industry wastewaters or sludges are toxic wastewaters and solids for the environment and living life, especially due to their cyanide and acidic nature, as well as many heavy metal species (Poges et al. 2018). Due to their structure, heavy metals can enter the human body through the food chain and cause serious disorders as a result of their bioaccumulation in vital organs (Shaheen et al. 2016).
In metal plating industries, various chemicals, organic solvents, organic or inorganic acids and bases, surfactants, complex organic substances, metal salts such as cadmium, nickel and chromium are frequently used in plating baths (Kobielska et al. 2018; Shao et al. 2014). Metal plating industries are among the industries that use toxic chemicals and constitute a major source of pollutants discharged to all receiving environment such as air, surface waters and soil (Carolin et al. 2017).
As it is known, the removal of metals is mostly achieved by chemical treatment at high pH levels (Aziz et al. 2000). However, because the buffering capacity of metal plating wastewater is very high, high costs are encountered. Considering the need for different pH values for each metal type, there are serious problems in the treatment of water originating from this process (Li et al. 2007).
Electroplating is defined as plating another metal on a metal surface by applying an electric current (electrolysis) (Priya et al. 2009). Electrode plating baths contain metal salts, complexing chemicals, pH buffers, as well as organic and organometallic additives (Sciscenko et al. 2016).
In order to prevent corrosion of many metals (elements) used in natural conditions and similar oxidizing environments, plating them with another more durable element that is not affected by these environments is called "Galvanotechnical" plating (Ghaferi et al. 2017).
Each of the wastewaters generated in metal plating processes has different chemical characteristics and volumes (Al-Shannag et al. 2015; Hosseini et al. 2016). The total liquid wastes generated in metal plating plants are not very bulky, but they are very dangerous and highly toxic wastes (Lee et al. 2017). The most important toxic components are metals, acids, chromium VI and cyanides (Akbal and Camci 2011; Scarazzato et al. 2015; Wijenberg et al. 2015). In addition, wastes with high chromium content come from chrome plating facilities, and cyanide wastes from zinc plating facilities (Hosseini et al. 2016) (Akbal and Camci 2011).
Excessive sludge formed after classical alkali metal precipitation, which is the most common method used during the chemical treatment of wastewater originating from metal plating processes, should also be considered as another important problem(BrbootI et al. 2011; Kabuk et al. 2014; Williams-Beam and Twidwell 2003). Therefore, there is a need to apply new methods to obtain more stable sludge using less chemicals (Rastegar et al. 2014). In order to eliminate these negativities, electrocoagulation process should be considered as an alternative method instead of chemical precipitation (Kobya et al. 2017).
The basic principle of electrocoagulation is the use of iron electrodes instead of iron sulfate, which is used as a coagulant by means of electric current (Chen 2004). Metals dissolved by electric current can act as coagulants (Ahmad et al. 2020). One of the most important advantages of the electrocoagulation process is that as a result of the electrolytic reactions that occur during the process, adsorption, oxidation and flotation (air stripping in waters containing ammonia) processes are carried out simultaneously due to the mechanism in addition to chemical coagulation (Emamjomeh and Sivakumar 2009; Moussa et al. 2017). At the same time, since pure coagulant (iron) passes into the water during the reaction, the anions (Cl-, SO4 2- etc.) in the chemical coagulation process do not pass into the water (Akbal and Camci 2010). The only disadvantage of the process, as the name suggests, is the energy cost. Compared to chemical coagulation alone, as the chemical cost and sludge cost are lower, it seems to be more economical (Yuksel et al. 2012). Due to all these advantages of electrocoagulation, it is widely used in the treatment of domestic and industrial wastewater(Kurt et al. 2008; Lu et al. 2023; Mollah et al. 2001; Moussa et al. 2017). One of the most important operating conditions in the electrocoagulation process is pH (Bayramoglu et al. 2007; Canizares et al. 2009). Generally, while the EC process is applied after pH adjustment, if the EC process is applied beforehand, the pH of the sample will also change due to the reaction (a small increase), and the cost will also decrease (Kabuk et al. 2014).
For all these reasons, it is seen that the electrocoagulation process can be a very efficient method for metal plating industrial wastewater (Al-Shannag et al. 2015; Kabuk et al. 2014; Piri et al. 2020). When the iron electrode is used in the electrocoagulation process, the electro-dissolution resulting from the electrical voltage will lead to the formation of metal hydroxides such as the dissolving iron electrode Fe(OH)3. The purification in the system starts after that. This treatment method, which is based on the principle that metal hydroxides with very high adsorption capacity adsorb various pollutant parameters in water and remove them from the water by precipitation, has been used in many places today (Mollah et al. 2001). During this process, as a result of the electrochemical processes occurring in the electrodes, tiny gas bubbles begin to emerge from the water. Considering that these gas bubbles form the basis of electroflotation, it will be seen that some pollutants are also removed from the water by electroflotation during electrocoagulation (Harif et al. 2012). In addition, the electrooxidation mechanism can be realized, albeit partially, by activity in the anode region (Yavuz and Ogutveren 2018). In other words, as a result of these common reactions in the electrocoagulation process, a high removal efficiency can be obtained depending on the pollutant concentration and pollutant parameters in the wastewater.
The aim of this study is to investigate the removal of Cu and Ni, which are two important heavy metals that are problematic in the removal of metal industry wastewater. In the study, yields close to 100% were obtained at both low pH and high pH values.
Materials and methods
Analyses
All analyses in the study were carried out in accordance with Standard Methods (Eaton et al. 2005). Heavy metal analyses were performed with Perkin-Elmer AAS 400 atomic absorption spectrophotometer device according to the Standard Method 3110 method. Conductivity and pH analysis was performed with the conductivity and pH probes with the Thermo Scientific brand Orion 5-Star Plus multimeter according to Standard Methods 4500 and 2510, respectively.
The wastewater used in the experimental study was taken from the inlet channel of İkitelli Galvanoteknik Metal Plating Industry Wastewater Treatment Plant in Istanbul. Wastewaters were kept in the waste water tank at + 4 °C in the laboratory. As a result of the analyses made, the characteristics of the wastewater were determined as given in Table 1.
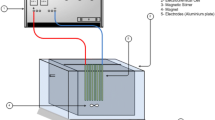
Reactors (pilot scale EC)
The power supply of the pilot plant operates between 100 and 300 A, and there is an electrical component that continuously measures voltage, current and pH values. The filter installed at the EC outlet, on the other hand, can separate the coagulated wastewater after EC from the sludge phase and collect the filtrate and sludge in separate tanks.
The EC system used in the pilot plant application consists of 4 reactors of the same size, each with a volume of 30 L, operating in series with each other. The reactors, made of cylindrical stainless steel, have internal dimensions of 0.15 m in diameter and 2.2 m in height. The interior of these reactors also acts as a cathode. Inside, there are circular rod-shaped anodes. The thickness of these anode rods is 0.075 m. In each series of EC applications, a total of 120 L water was electrocoagulated. There are anode electrodes inside the cathode outside of the reactors, and depending on the EC working principle, the inner cylinder can be easily replaced when the anode is worn. Schematic representation and general information about the system are given in Fig. 1 and Table 2, respectively.
Optimization
The operating conditions determined by the preliminary study (Ithan et al. 2019; Karunakaran et al. 2022) were selected as 3 factor 5 level with Taguchi design, taking into account the suitability of the pilot scale system. For ease of comparison between studies, only pH was changed before and after EC. Operating conditions and levels are given in Table 3. Optimization studies were carried out using Design Expert 12.0 software.
Results and discussion
In the study, it was investigated whether the pH adjustment process should be done before or after the EC process considering the removal efficiencies. After that, the optimum conditions were determined.
Application of EC process after pH adjustment
In the study, EC application was made after the pH value of the original wastewater, which was pH 1.5, was increased. For this purpose, pre-EC pH values were studied at levels between 6 and 10, and data were created according to the results obtained under 100–300 A-current, and these values are presented in Table 4.
When the results obtained were examined, the values of approximately 87.5% for Cu and 98.4% for Ni were obtained as the maximum removal efficiency. Moreover, these values are the values realized under different operating conditions. In order to determine the optimum operating condition, a model study related to these values is needed (Sun et al. 2013).
Optimization studies
Treatment studies give results for certain operating conditions. However, optimum operating conditions are often different from these selected operating conditions. For this purpose, one of the most important stages is the optimization studies to be carried out after the experimental design selection. For this purpose, the results of the optimization made using the Design Expert 12.0 software and the visually expressed figures are given in the following headings.
Copper (Cu) removal
One of the most important parameters expressing the consistency of this regression equation is the R2 value. Another is the adjusted R2 value. The following is the regression equation for the optimization of Cu removal when the EC process is run at the original pH and then the pH is increased.
Model summary
The equation obtained as a result of the regression studies was obtained depending on 3 different variables, current (A), time (min) and the actual values of pH, and is given as follows:
Regression equation:
Analysis of variance is a statistical analysis model used to express the consistency of the data and the consistency of the model results. For this purpose, the results of analysis of variance performed in this study (EC application after pH adjustment) are given in Table 5.
The Model F value of 43.54 implies that the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise. P values less than 0.0500 indicate model terms are significant. In this case, B, C, and C2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant.
The graphic of the model, which is consistent according to the ANOVA test evaluations, is given in Fig. 2.
As can be seen from Fig. 2, it is seen that the experimental results of Cu removal in both graphs are consistent with the model results. It is seen that the results are more consistent especially in high removal efficiencies where the yields are concentrated. As a matter of fact, consistency in the desired region is more important as seen in the literature (Nizamuddin et al. 2016). Moreover, when examined for Cu removal, it is seen that similar results were obtained in laboratory-scale studies (Akbal and Camci 2011).
Nickel (Ni) removal
One of the most important parameters expressing the consistency of this regression equation is the R2 value. Another is the adjusted R2 value. The following is the regression equation for the optimization of Ni removal when the EC process is run at the original pH and then the pH is increased.
Model summary
The equation obtained as a result of the regression studies was obtained depending on 3 different variables, current (A), time (min) and the actual values of pH, and is given as follows:
Regression equation
As can be seen, the model result R2 value is 95.43% and is extremely close to Adjusted R2. This is one of the most important parameters showing the consistency of the results of the equation.
Analysis of variance is a statistical analysis model used to express the consistency of the data and the consistency of the model results. For this purpose, the results of analysis of variance performed in this study (EC application after pH adjustment) are given in Table 6.
The Model F value of 34.83 implies that the model is significant. There is only a 0.01% chance that a F value this large could occur due to noise. P values less than 0.0500 indicate that model terms are significant. In this case, C and C2 are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. The consistency of the experimental data and the model results in the pH adjustment study applied before EC is as given in Fig. 3.
As can be seen in Fig. 3, it is seen that the experimental results are consistent with the model results in both graphs. The fact that the predictive line and the actual data are concentrated on the line is extremely important in terms of showing the consistency of the model (Ilo et al. 2022). Moreover, when examined for Cu removal, it is seen that similar results were obtained in laboratory-scale studies (Akbal and Camcı 2011).
Figure 4 shows the shapes created by the equations obtained for both Cu and Ni removal efficiencies obtained in the EC process after the pH adjustment.
As can be seen from the surface graphs in Fig. 4, pH is the most effective parameter in both processes. As expected, a removal efficiency of the order of 95% was achieved in studies carried out at high pH after treatment. The studies carried out by putting the EC process forward and then increasing the pH are below. As can be seen from the graphics, it is seen that the most effective parameter in this process is pH. Therefore, the importance of the study (whether the pH should be adjusted before or after) has emerged. Although pH is not seen as the most effective parameter in the electrocoagulation process (Coskun et al. 2012), this situation changes when the parameters to be removed are heavy metals (Al-Qodah and Al-Shannag 2017).
Application of EC process before pH adjustment (EC + pH)
At this stage of the study, the EC process was first applied to the wastewater and then the pH was increased. Moreover, the OH−ions formed as a result of the EC process allowed a partial pH increase and reduced the possible base cost. The results obtained are given in Table 7.
According to Table 7, when the results obtained are examined, it is seen that Cu removal has reached to 89% and Ni removal has reached to 98%. Optimum operating conditions for both values can be found by model studies.
Optimization studies
Determining what kind of result will be obtained under which operating conditions by modeling the experimental data allows to determine both the consistency of the studies and the approximate removal efficiencies with the help of the obtained equations.
Cu removal
The following is the regression equation obtained as the first step in the optimization of Cu removal after electrocoagulation was studied at the original pH and then pH increased.
Model summary
As can be seen, the model result R2 value is 91.9% and is extremely close to adjusted R2. This is one of the most important parameters showing the consistency of the results of the equation.
Regression equation
The equation in terms of actual factors can be used to make predictions about the response for given levels of each factor. Here, the levels should be specified in the original units for each factor. This equation should not be used to determine the relative impact of each factor because the coefficients are scaled to accommodate the units of each factor and the intercept is not at the center of the design space.
One of the most important parameters expressing the consistency of this regression equation is the R2 value. Another is the adjusted R2 value. The following is the regression equation for the optimization of Cu removal when the EC process is run at the original pH and then the pH is increased.
Analysis of variance (ANOVA) tests the hypothesis that the means of two or more populations are equal. ANOVAs assess the importance of one or more factors by comparing the response variable means at the different factor levels. The null hypothesis states that all population means (factor level means) are equal while the alternative hypothesis states that at least one is different.
Analysis of variance is a statistical analysis model used to express the consistency of the data and the consistency of the model results. For this purpose, the results of analysis of variance performed in this study (pH adjustment after EC) are given in Table 8.
The Model F value of 15.93 implies that the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise. P values less than 0.0500 indicate that model terms are significant. In this case, B and C are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. The consistency of the experimental data and the model results in the pH adjustment study applied before EC is as given in Fig. 5.
As can be seen in Fig. 5, it is seen that the experimental results are consistent with the model results in both graphs. The fact that the predict line and the actual data are concentrated on the line is important in terms of showing the consistency of the model (Batista and Graczyk 2013) Considering this situation, it is seen that the model results reflect the experimental results (Vigneswaran et al. 2012).
Nickel (Ni) removal
One of the most important parameters expressing the consistency of this regression equation is the R2 value. Another is the adjusted R2 value. The following is the regression equation for the optimization of Cu removal when the EC process is run at the original pH and then the pH is increased.
Model summary
The regression equation obtained for Ni removal after pH increase after electrocoagulation process at original pH is given as follows:
Regression equation
The equation in terms of actual factors can be used to make predictions about the response for given levels of each factor. Here, the levels should be specified in the original units for each factor. This equation should not be used to determine the relative impact of each factor because the coefficients are scaled to accommodate the units of each factor and the intercept is not at the center of the design space.
The model result is very close with the R2 value of 94.24% and Adjusted R2 as seen. This is one of the most important parameters showing the consistency of the results of the equation.
Another important analysis while examining the model results is the analysis of variance, and the analysis of variance table of this model is given in Table 9.
The Model F value of 22.91 implies that the model is significant. There is only a 0.01% chance that an F value this large could occur due to noise.
P values less than 0.0500 indicate that model terms are significant. In this case, B, C, and ABC are significant model terms. Values greater than 0.1000 indicate that the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model.
The consistency of the experimental data and the model results in the pH adjustment study applied after EC is as given in Fig. 6.
As can be seen from Fig. 6, it is understood that the experimental results in both graphs are consistent with the model results. Predict and actual graphics are very important in optimization studies. The figures obtained in this study are the most important expression of this consistency (Ilo et al. 2022). When compared with the literature data, it is seen that the optimization study is successful (Abdel-Shafy et al. 2022).
Surface Plots: Optimization results are plotted with the response surface methodology. In this context, the graphs prepared for the said equation are given in Fig. 7. Part a and b show Cu and Ni removals, respectively.
When Fig. 7 is examined, the effects of each operating conditions on the removal efficiency and the efficiencies obtained as a result of the model according to these operating conditions are seen. As can be seen from the graphics, it is seen that the most effective parameter in this process is pH. It is seen from both the graphs and the coefficients in the equations that the time is also effective after pH. Considering the importance of pH, the importance of the study (whether pH should be adjusted before or after) has emerged. Although pH is not seen as the most effective parameter in the electrocoagulation process (Coskun et al. 2012), it is seen how this situation changes when the parameters to be removed are heavy metals (Al-Qodah and Al-Shannag 2017). This reveals the importance of this study.
Figures 8 and 9 show desirability graphs showing what kind of removal will occur under which operating conditions. Desirability is an objective function that ranges from zero outside of the limits to one at the goal. The numerical optimization finds a point that maximizes the desirability function. The characteristics of a goal may be altered by adjusting the weight or importance. For several responses and factors, all goals get combined into one desirability function. Figure 8 shows the removal efficiencies obtained under different operating conditions.
As can be seen in Fig. 8, removal efficiency of over 93% and 93,7% was achieved for Cu and Ni, respectively, in a period of 11.75 min under 220 A (current density of 31.15 mA/cm2) and at pH 10. And again, as can be seen in Fig. 8, it is seen that the most effective parameter is pH (Ahmad Tajudin, Azim, Syakeera, Jefferson, and Rogers 2017). This shows that this is due to the precipitation of heavy metals in the form of metal hydroxide (Likon and Zemljič 2020).
As can be seen from Fig. 9, removal efficiency of over 95.6% for Cu and 95.2% for Ni is achieved under 300 A (current density of 45 mA/cm2) in a period of 11.75 min and when pH adjustment is made before EC (around 10) has been obtained. And again, when we pay attention to Figs. 8 and 9, it is clearly seen that the most effective parameter is pH. As a matter of fact, these results show similarities with the literature data (Akbal and Camci 2011; Beyazit 2014; Kabuk et al. 2014; Lu et al. 2023).
Conclusion
In this study, it has been studied that wastewater with highly variable character originating from the metal plating industry can be treated with a pilot scale EC process. High removal efficiencies have been achieved even in very low times such as 3 min, and the necessity of pH adjustment has come to the fore. It was observed that whether the pH adjustment was before or after did not have a major effect. As a result, it is seen that the precipitation of metal complexes in the form of metal hydroxides is also related to pH and that the outlet pH is also important in the EC process. As a matter of fact, pH was the most effective parameter in the modeling studies, as expected.
With the EC process proposed in this study, it was determined that discharge limits below 5 mg/l for Cu and Ni were achieved in cases where discharge limits could not be met with the classical chemical treatment method available in the existing wastewater treatment plant.
The average removal efficiency for two different heavy metals (Cu and Ni) obtained in this study using iron electrodes in the EC process was at the level of 95%. The regression coefficients for these two different heavy metal types were determined between 90.15 and 96.31% in two different optimization results according to the pH adjustment status. When these data are taken into consideration, the results are quite consistent with the model study.
References
Abdel-Shafy HI, Morsy RMM, Hewehy MAI, Razek TMA, Hamid MMA (2022) Treatment of industrial electroplating wastewater for metals removal via electrocoagulation continous flow reactors. Water Pract Technol 17(2):555–566. https://doi.org/10.2166/wpt.2022.001
Ahmad MK, Mohammed MA, Barbooti MM (2020) Electrocoagulation for the removal of copper and zinc ions from water using iron electrodes. Open Chem J. https://doi.org/10.2174/1874842202007010037
Ahmad Tajudin SA, Azim MAM, Syakeera NN, Jefferson I, Rogers C (2017) Application of electrokinetic stabilisation (EKS) method for soft soil: a review. IOP Conf Ser Mater Sci Eng 226:012075. https://doi.org/10.1088/1757-899X/226/1/012075
Akbal F, Camci S (2010) Comparison of electrocoagulation and chemical coagulation for heavy metal removal. Chem Eng Technol 33(10):1655–1664. https://doi.org/10.1002/ceat.201000091
Akbal F, Camci S (2011) Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 269(1–3):214–222. https://doi.org/10.1016/j.desal.2010.11.001
Akbal F, Camcı S (2011) Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 269(1):214–222. https://doi.org/10.1016/j.desal.2010.11.001
Al-Qodah Z, Al-Shannag M (2017) Heavy metal ions removal from wastewater using electrocoagulation processes: a comprehensive review. Sep Sci Technol 52(17):2649–2676. https://doi.org/10.1080/01496395.2017.1373677
Al-Shannag M, Al-Qodah Z, Bani-Melhem K, Qtaishat MR, Alkasrawi M (2015) Heavy metal ions removal from metal plating wastewater using electrocoagulation: kinetic study and process performance. Chem Eng J 260:749–756. https://doi.org/10.1016/j.cej.2014.09.035
Aziz NH, Shahin AAM, Abou-Zeid AAM, El-Zeany SA (2000) Correlation of growth and aflatoxin production by Aspergillus flavus with some essential metals in gamma irradiated crushed corn. Nahrung Food 44(5):354–359. https://doi.org/10.1002/1521-3803(20001001)44:5%3c354::aid-food354%3e3.0.co;2-4
Batista HS, Graczyk T (2013) An analysis of the welding-induced deformation of ship panels using a statistical tool. Polish Maritime Res 20(2):74–81
Bayramoglu M, Eyvaz M, Kobya M (2007) Treatment of the textile wastewater by electrocoagulation Economical evaluation. Chem Eng J 128(2–3):155–161. https://doi.org/10.1016/j.cej.2006.10.008
Beyazit N (2014) Copper(II), Chromium(VI) and Nickel(II) Removal from Metal Plating Effluent by Electrocoagulation. Int J Electrochem Sci 9(8):4315–4330
BrbootI MM, AbiD BA, Al-ShuwaikI NM (2011) Removal of heavy metals using chemicals precipitation. Eng Technol J 29(3):595–612
Canizares P, Martinez F, Jimenez C, Saez C, Rodrigo MA (2009) Technical and economic comparison of conventional and electrochemical coagulation processes. J Chem Technol Biotechnol 84(5):702–710. https://doi.org/10.1002/jctb.2102
Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5(3):2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Chen GH (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38(1):11–41. https://doi.org/10.1016/j.seppur.2003.10.006
Coskun T, İlhan F, Demir NM, Debik E, Kurt U (2012) Optimization of energy costs in the pretreatment of olive mill wastewaters by electrocoagulation. Environ Technol 33(7):801–807. https://doi.org/10.1080/09593330.2011.595829
Eaton AD, Clesceri LS, Franson MAH, Association APH, Greenberg AE, Rice EW, Federation WE (2005) Standard methods for the examination of water & wastewater: American public health association
Emamjomeh MM, Sivakumar M (2009) Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J Environ Manag 90(5):1663–1679. https://doi.org/10.1016/j.jenvman.2008.12.011
Fung KY, Wibowo C (2013) Design of industrial wastewater treatment plants: a multi-faceted problem. Curr Opin Chem Eng 2(4):455–460. https://doi.org/10.1016/j.coche.2013.09.003
Ghaferi Z, Sharafi S, Bahrololoom ME (2017) Characterization of Co–Fe magnetic films fabricated by galvano-static electrodeposition. Iran J Mater Sci Eng 14(2):60–70. https://doi.org/10.22068/ijmse.14.2.60
Harif T, Khai M, Adin A (2012) Electrocoagulation versus chemical coagulation: coagulation/flocculation mechanisms and resulting floc characteristics. Water Res 46(10):3177–3188. https://doi.org/10.1016/j.watres.2012.03.034
Hosseini SS, Bringas E, Tan NR, Ortiz I, Ghahramani M, Shahmirzadi MAA (2016) Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: a review. J Water Process Eng 9:78–110. https://doi.org/10.1016/j.jwpe.2015.11.005
Ilo OP, Nkomo SPL, Mkhize NM, Mutanga O, Simatele MD (2022) Optimisation of process parameters using response surface methodology to improve the liquid fraction yield from pyrolysis of water hyacinth. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-22639-z
Ithan F, Ulucan-Altuntas K, Avsar Y, Kurt U, Saral A (2019) Electrocoagulation process for the treatment of metal-plating wastewater: kinetic modeling and energy consumption. Front Environ Sci Eng. https://doi.org/10.1007/s11783-019-1152-1
Kabuk HA, Avsar Y, Ilhan F, Ulucan K (2014) Comparison of pH adjustment and electrocoagulation processes on treatability of metal plating wastewater. Sep Sci Technol 49(4):613–618. https://doi.org/10.1080/01496395.2013.839705
Karunakaran A, Chaturvedi A, Ali J, Singh R, Agarwal S, Garg MC (2022) Response surface methodology-based modeling and optimization of chromium removal using spiral-wound reverse-osmosis membrane setup. Int J Environ Sci Technol 19(7):5999–6010. https://doi.org/10.1007/s13762-021-03422-y
Kobielska PA, Howarth AJ, Farha OK, Nayak S (2018) Metal-organic frameworks for heavy metal removal from water. Coord Chem Rev 358:92–107. https://doi.org/10.1016/j.ccr.2017.12.010
Kobya M, Demirbas E, Ozyonar F, Sirtbas G, Gengec E (2017) Treatments of alkaline non-cyanide, alkaline cyanide and acidic zinc electroplating wastewaters by electrocoagulation. Process Saf Environ Prot 105:373–385. https://doi.org/10.1016/j.psep.2016.11.020
Kurt U, Gonullu MT, Ilhan F, Varinca K (2008) Treatment of domestic wastewater by electrocoagulation in a cell with Fe–Fe electrodes. Environ Eng Sci 25(2):153–161. https://doi.org/10.1089/ees.2006.0132
Lee CG, Lee S, Park JA, Park C, Lee SJ, Kim SB, Choi JW (2017) Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere 166:203–211. https://doi.org/10.1016/j.chemosphere.2016.09.093
Li F, Du P, Chen W, Zhang SS (2007) Preparation of silica-supported porous sorbent for heavy metal ions removal in wastewater treatment by organic-inorganic hybridization combined with sucrose and polyethylene glycol imprinting. Anal Chim Acta 585(2):211–218. https://doi.org/10.1016/j.aca.2006.12.047
Likon M, Zemljič M (2020) Does sustainable management of biodegradable sludge exist at all? A BACOM project case
Lu X, Xu T, Zhou Y, Peng Q, Ou J, Hu B, Yu G (2023) Effect of iron ion configurations on Ni2+ removal in electrocoagulation. J Environ Sci 124:823–834. https://doi.org/10.1016/j.jes.2022.01.037
Mollah MYA, Schennach R, Parga JR, Cocke DL (2001) Electrocoagulation (EC)-science and applications. J Hazard Mater 84(1):29–41. https://doi.org/10.1016/s0304-3894(01)00176-5
Moussa DT, El-Naas MH, Nasser M, Al-Marri MJ (2017) A comprehensive review of electrocoagulation for water treatment: potentials and challenges. J Environ Manag 186:24–41. https://doi.org/10.1016/j.jenvman.2016.10.032
Nizamuddin S, Mubarak NM, Tiripathi M, Jayakumar NS, Sahu JN, Ganesan P (2016) Chemical, dielectric and structural characterization of optimized hydrochar produced from hydrothermal carbonization of palm shell. Fuel 163:88–97. https://doi.org/10.1016/j.fuel.2015.08.057
Piri S, Mehranbod N, Moussavi M, Mirsoleimani-azizi SM (2020) Application of response surface method for removal of Cr(VI) from aqueous solutions using foam fractionation process. Int J Environ Sci Technol 17(1):321–332. https://doi.org/10.1007/s13762-019-02349-9
Poges S, Jin J, Guild C, Li WN, Birnkrant M, Suib SL (2018) Preparation and characterization of aluminum coatings via electroless plating onto nickel nanowires using ionic liquid plating solution. Mater Chem Phys 207:303–308. https://doi.org/10.1016/j.matchemphys.2017.12.079
Priya RG, Basha CA, Ramamurthi V, Begum SN (2009) Recovery and reuse of Ni(II) from rinsewater of electroplating industries. J Hazard Mater 163(2–3):899–909. https://doi.org/10.1016/j.jhazmat.2008.07.072
Rastegar SO, Mousavi SM, Shojaosadati SA (2014) Cr and Ni recovery during bioleaching of dewatered metal-plating sludge using acidithiobacillus ferrooxidans. Biores Technol 167:61–68. https://doi.org/10.1016/j.biortech.2014.05.107
Scarazzato T, Buzzi DC, Bernardes AM, Espinosa DCR (2015) Treatment of wastewaters from cyanide-free plating process by electrodialysis. J Clean Prod 91:241–250. https://doi.org/10.1016/j.jclepro.2014.12.046
Sciscenko I, Pedre I, Hunt A, Bogo H, González GA (2016) Determination of a typical additive in zinc electroplating baths. Microchem J 127:226–230. https://doi.org/10.1016/j.microc.2016.03.015
Shaheen N, Irfan NM, Khan IN, Islam S, Islam MS, Ahmed MK (2016) Presence of heavy metals in fruits and vegetables: health risk implications in Bangladesh. Chemosphere 152:431–438. https://doi.org/10.1016/j.chemosphere.2016.02.060
Shao ZC, Cai ZQ, Hu R, Wei SQ (2014) The study of electroless nickel plating directly on magnesium alloy. Surf Coat Technol 249:42–47. https://doi.org/10.1016/j.surfcoat.2014.03.043
Sun J, Yang Y, Wang D (2013) Parametric optimization of selective laser melting for forming Ti6Al4V samples by Taguchi method. Opt Laser Technol 49:118–124. https://doi.org/10.1016/j.optlastec.2012.12.002
Vigneswaran C, Anbumani N, Ananthasubramanian M, Rajendran R (2012) Prediction of optimum process parameters to achieve eco-friendly desizing of organic cotton fabrics with indigenously produced alpha-amylase and their enzyme kinetics. J Textile Inst 103(4):422–433. https://doi.org/10.1080/00405000.2011.581359
Wijenberg J, Steegh M, Aarnts MP, Lammers KR, Mol JMC (2015) Electrodeposition of mixed chromium metal-carbide-oxide coatings from a trivalent chromium-formate electrolyte without a buffering agent. Electrochim Acta 173:819–826. https://doi.org/10.1016/j.electacta.2015.05.121
Williams-Beam C, Twidwell L G (2003, Aug 24–27). Removal of thallium from wastewater. In: Paper presented at the 5th international symposium on hydrometallurgy in honor of Ian M Ritchie, Vancouver, Canada
Yavuz Y, Ogutveren UB (2018) Treatment of industrial estate wastewater by the application of electrocoagulation process using iron electrodes. J Environ Manage 207:151–158. https://doi.org/10.1016/j.jenvman.2017.11.034
Yuksel E, Gurbulak E, Eyvaz M (2012) Decolorization of a reactive dye solution and treatment of a textile wastewater by electrocoagulation and chemical coagulation: techno-economic comparison. Environ Prog Sustain Energy 31(4):524–535. https://doi.org/10.1002/ep.10574
Acknowledgements
The authors received research grants from the Research Fund of the Republic of Turkey Ministry of Industry and Technology with a Project Number 0274.STZ-2013-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Editorial responsibility: Shahid Hussain.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ilhan, F., Avsar, Y., Kurt, U. et al. Optimization and effect of pH on treatability of metal plating wastewater by electrocoagulation process: a pilot study. Int. J. Environ. Sci. Technol. 20, 7671–7688 (2023). https://doi.org/10.1007/s13762-023-04972-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04972-z