Abstract
In this study, phosphate and chemical oxygen demand removals of medical waste sterilization plant wastewater were investigated and the Taguchi experimental method was used to determine optimum treatment conditions. In the scope of the study, four important factors influencing the phosphate and chemical oxygen demand removal namely initial pH, current density, initial wastewater concentration, and contact time were optimized. Results show that about 52 % of chemical oxygen demand removal has been obtained at optimum conditions. Also, phosphorus has been completely removed at optimum levels. In addition, analysis of variance shows that the biggest contribution ratio belongs to initial wastewater concentration on the electrocoagulation process efficiency. The contribution percentages of each factor in descending order are as follows: initial wastewater concentration (42.51 %) > pH (32.02 %) > current density (14.56 %) > contact time (6.64 %).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The management of medical waste generally poses a vital problem for countries around the globe, especially for the hazards it can cause to the surrounding environment. Most of the available medical waste treatment methods are not cost effective or environmentally acceptable and do not deal with medical waste in a safe manner. The mismanagement of medical waste can cause catastrophic effects not only to the people involved in clinical facilities, but also to those in the surrounding areas (El-Haggar 2007). The increase in the number of hospitals and patients has also lead to an increase in the amount of medical waste generated from these institutions, which requires proper management to minimize the health and environmental risks associated with such wastes (Abu Qdais et al. 2007). An important part of special or medical waste is that it is potentially dangerous since it may contain pathogenic agents which require special handling, treatment, and disposal (Insa et al. 2010). Medical wastewater contains chlorinated molecules in high concentrations, and heavy metals such as mercury and silver. But major contaminants for medical wastewater can be given as chemical oxygen demand, phosphorus and biological oxygen demand at high concentrations (Özkan et al. 2010). Therefore, this type of wastewater cannot be discharged to sewerage system untreated. A number of alternative treatment technologies have been proposed, including incineration, autoclaving, chemical disinfections, gas disinfections, and such new technologies as microwave and irradiation. However, use of these technologies is still limited for several reasons (Jangsawang et al. 2005). In general, depending upon the type of technology, the residues are transformed from one phase to another. For example, in incineration, the combustible components of the wastes are converted into gaseous byproducts (CO2, H2O, CO, and other gases, some of which are toxic) and non-combustible components remain as a solid byproduct, namely ash (Diaz et al. 2005).
Of all the methods available for sterilization, moist heat in the form of saturated steam under pressure is the most widely used and the most dependable (Rutala and Weber 1999). This method is known to be very efficient when used for infectious waste and can be applied to most types of microorganisms, if the time and temperature of the reaction and contact between steam and waste are sufficiently provided to kill microbial spores (Jang et al. 2006). Autoclaves that are designed to deal with medical waste often operate along with a shredder and a compactor in order to minimize the volume of waste produced (El-Haggar 2007). Steam is generated in a pressure chamber so that it reaches a temperature 121–148 °C (250–300 °F) at 15 p.s.i. The duration in which the items are exposed to the temperature and size of load ranges from 10 to 60 min. (McKeen 2012). Finally, the discharged liquid resulting from the sterilization process is defined as “medical waste sterilization plant wastewater (MSW)”.
Generally, medical wastewater has high toxic and chemical contents, and, it is also known that this type of wastewater causes some problems for biological treatment process related to adverse effects of the contaminants on the community of organisms in charge of the biological decomposition of the organic matter (Kajitvichyanukul and Suntronvipart 2006).
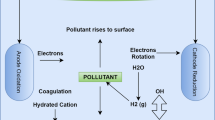
Nowadays, a large amount of research has been conducted inthe application of electrochemical methods to environment protection (Dimoglo et al. 2004). Electrocoagulation (EC) is an electrochemical technique whereby anodes (aluminum or iron electrodes) corrode to release active coagulants into the solution (Phalakornkule et al. 2010). This results in the dissolution of the electrode to yield metal ions (Fe2+, Fe3+ or Al3+) which are active coagulant precursors involved in the neutralization of the negative charges on the colloids of the effluent. These metal ions then react with the hydroxyl ions generated at the cathode to give metal hydroxides which favor the formation of flocks; these flocks are easily separated by decantation or filtration, depending upon their density (Tchamango et al. 2010). Basically, electrocoagulation takes place at several steps such as formation of coagulants in aqueous phase, adsorption of soluble or colloidal pollutants on coagulants. When aluminum is used as electrode, the following anode and cathode reactions take place;
Al3+ and OH− ions generated by electrode reactions (1) and (2) react to form various monomeric species such as Al(OH)+2, Al(OH) +2 , Al2(OH) 4+2 , Al(OH)4− and polymeric species such as Al6(OH) 3+15 , Al7(OH) 4+17 , Al8(OH) 4+20 , Al13O4(OH) 7+24 , Al13(OH) 5+34 , which transform finally into Al(OH)3(s) according to complex precipitation kinetics (Yildiz et al. 2013)
Formation rates of the different species play an important role in treatment process. For electrocoagulation process, there are two interaction mechanisms between contaminant and hydrolysis products. These are precipitation and adsorption, each one being proposed for a separate pH range. Flocculation in the low pH range is explained as precipitation while the higher pH range (>6.5) as adsorption (Gürses et al. 2002).
EC can be considered a powerful pollution control technique because of more stringent environmental regulations on the discharge of industrial and municipal wastewater (Song et al. 2010). When compared to traditional methods, EF has great advantages such as high efficiency, a fast reaction rate, cost-effectiveness, simple equipment and compact size of EC systems (allowing decentralized treatment), avoidance of chemical additions, and ease of automation (Palahouane et al. 2014).
Meuiner et al. studied recovery and treatability of heavy metals (Cd, Cr, Cu, Ni, Pb, or Zn) in order to evaluate the performance of EC process and according to their results, the residual metal concentrations after the EC process were below the acceptable level of the effluent discharge limits except for Ni and Cd at the end of the process (Meunier et al. 2006). Vepsäläinen et al. also indicate that EC can remove toxic pollutants such as Cr(IV), dyes, olive mill pollutants from wastewater (Vepsäläinen et al. 2011).
In this study, total phosphorus (TP) and chemical oxygen demand (COD) removals of MSW were investigated and the Taguchi experimental method was used to determine optimum treatment conditions. There is a need for experiments to estimate the best process conditions. The experimental design methods like Taguchi offer accurate analysis of process features. This method has proved to be beneficial since it not only reduces the number of experiments but also specifies controllable and uncontrollable factors (Mehrara et al. 2014). Taguchi's experimental design method provides some advantages such as keeping the experimental cost at a minimum level, and thereby, it minimizes the variation in product response while keeping the mean response on target.
In the scope of the study, the four important factors initial pH (pH i ), current density (CD), initial wastewater concentration (C o), and contact time (CT), which influence the TP and COD removal have been optimized.
Materials and methods
Wastewater source and characterization
MSW was obtained from medical waste sterilization plant in Kayseri, Turkey. In this plant, on the average, 5,000 kg/day medical waste is sterilized. Daily wastewater volume which is discharged from the plant is 50 m3/day on average (Özkan et al. 2010). Table 1 shows the characteristics of wastewater used in this study. Deionized water was used to prepare the desired concentration of wastewater. The pH of the sample was adjusted to the desired pH value (5.0–7.0) using 1 N H2SO4 and 1 N NaOH. In electrolysis studies, Na2SO4 (Merck, 99.5 %) was used as the supporting electrode. Wastewater samples were kept at 4 °C until analysis or experiments.
Analytical methods
COD and TP were measured spectrophotometrically according to the Standard Methods [2120 C - 4,500-P C (APHA 1998) spectrophotometric method] by APHA, AWWA, and WEF (APHA 1998). Turbidity was measured by a turbidimeter (WTW TURB 430 IR) with a range of 0–1000 NTU (Nefolometric turbidity unit). Solids were measured utilizing standard methods (APHA 1998). The apparatus used for biological oxygen demand (BOD) measurements was the OxiTop Control system (WTW). All of the nitrogen species analyses were performed with test kit (MERCK). Sulfate and chloride analyses were determined according to standard methods (APHA 1998). The pH and conductivity measurements were carried out with a multiparameter (Hach-Lange HQ40D). Deionized water (18 mΩ cm) was obtained from Elga Option-Q system and used in all the experiments. All experiments were performed in duplicate.
Experimental setup and procedure
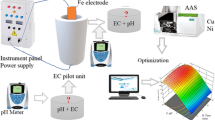
The electrochemical cell used in the experimental studies is shown in Fig. 1. The cell is made of polyethylene with the total capacity of 950 ml. EC studies were carried out in batch mode with a volume of 850 ml wastewater. Eight parallel connected aluminum electrodes were used as both anode and cathode with effective surface area of 73.15 cm2. The electrode distance between anode and cathode was kept constant of 0.5 cm during electrolysis. Direct current was applied by power supply (Shenzhen Mastech HY3005-3, 0–5 A and 0–30 V). Continuous mixing was provided in the cell by magnetic stirrer (IkaRCT basic). All the experiments were carried out under constant current conditions at room temperature. The samples were taken at every 5 min interval from the cell, and the solution was filtered for COD and TP analyses.
Taguchi’s design of experiments methodology
In this work, all of the parameters affecting the EC process performance were optimized with Taguchi's design of experiment (DOE) methodology. Taguchi experimental design method has proved to be beneficial since it not only reduces the number of experiments but also specifies controllable and uncontrollable factors (Mehrara et al. 2014). Experimental design was created in the form of four factors (pH i , CD, C o, and CT) and three levels (L 9) for each factor. This orthogonal array was employed due to no interaction among the factors (Madaeni and Koocheki 2006). Both COD and TP analysis were repeated twice at the same conditions to ensure the accuracy of data. Table 2 shows L9 OA with four parameters and three levels and Table 3 shows their values.
The performance characteristics (Signal to Noise (S/N) ratios) were chosen as the optimization criteria. There are three different performance characteristics. These are the larger the better, smaller the better, and the nominal the better. In this study, larger the better is chosen as performance characteristic. The performance characteristics (removal percentages of the COD and TP) are evaluated by using Eqs. (4) and (5) (Gökkuş et al. 2012).
Larger is better
Smaller is better
Here, SNL and SNS represent performance characteristics, “n” is the number of repetitions done for an experimental combination, and “Y i ” is the performance value of the ith experiment (Gökkuş et al. 2012).In both cases, the main purpose is to reach higher S/N ratio which means better treatment results (Aber et al. 2010).
Analysis of variance (ANOVA)
To determine the effect of each factor on COD and TP removal by EC process, ANOVA was applied. Statistical significance of the model equation and model terms was evaluated by F-test and ANOVA (Jadhav et al. 2014). Sum of squares (SS), DOF, mean of square (MS), and associated F-test of importance (F) can be calculated as follows (Gönder et al. 2010):
Description of abbreviations are as follows: “k A” is the number of the levels of factor A, “n Ai ” is the number of all observations at level “i” of factor A, A i is the sum of all observations of level “i” of factor A and T is the sum of all observations. SS of error is computed using the following equation (Gönder et al. 2010):
where SST is the total SS:
where “y i ” is the observation of “i”. MS is calculated by dividing the sum of squares by the degrees of freedom. DOFA is estimated by DOFA = k A − 1. F value is calculated as follows (Gönder et al. 2010):
MSe is the variance of error (Gönder et al. 2010).
Results and discussions
Table 4 shows the obtained removal rate results of the EC experiments performed according to Taguchi DOE. The obtained data were analyzed using Minitab 15.0 software to determine the effects of each parameter on COD and TP removals.
It can be seen from the Table 4 that TP was removed in all EC trials carried out according to Taguchi DOE. Therefore, TP removal, which is one of the performance criteria, was out of assessment due to complete removal obtained in all Taguchi experiments. ANOVA was performed to determine the effect of each parameter on EC process and confidence intervals of the removal result. Thus, it can be seen which parameters are statistically important on the EC process performance.
Determination of optimum conditions
In the Taguchi experimental matrix, a total of 18 trials (with replications) were carried out according to L 9 orthogonal array with 4 factors and three levels. The S/N ratios of each parameter are depicted in Figs. 2, 3, 4, and 5. The higher S/N ratio means a better removal result, and it is explained as optimum removal efficiency. In this study, experimental analyses are expressed as percentage of removal (%) for COD and TP in terms of the following equation.
a Effect of CT on process performance, b the solubility diagram of aluminum hydroxide (Holt 2006)
In Figs. 2, 3, 4, and 5, the points in the circles refer to the highest value of the S/N ratios among three levels, and so, it means the optimum parametric conditions for EC process. According to figures, the optimum parametric conditions for removal of COD and TP are as follows: (1) First level (5) for pH; (2) third level (1.5 mA/cm2 for CD; (3) third level (C o/4) for C o; and (4) second level (40 min) for CT. Finally, a verification test was performed at the peak points to check the accuracy of the values.
Optimum pH
Solution pH is one of the most important parameters for EC process (Daneshvar et al. 2007). So, it was adjusted in the range from 5 to 7 by adding sodium hydroxide or sulfuric acid solution to investigate the effect of pH on process performance. Figure 2a shows the variation of EC process performance as a function of the solution pH.
It is seen in Fig. 2a, the process efficiency depends strongly on pH value of the solution. Especially, low pH i value increases the removal efficiencies, because pH values of the solution rise during electrolysis process (Fig. 2b) and after a certain point it reaches favorable pH value for Al(OH)3 precipitation (Mouedhen et al. 2008). According to Bayramoglu et al. (2004), the best COD and turbidity removals could be obtained in acidic pH i value with aluminum electrodes (Bayramoglu et al. 2004). In addition, they reported that when solution pH was increased to 6, removal rates decreased dramatically.
When aluminum is used as the electrode material, acidic pH i values are more convenient for the removal kinetics due to predominant form of AlPO4(s) (Attour et al. 2014). For this study, optimum pH i value was determined as 5 (first parametric level) for the treatment of MSW by EC. The result is in agreement with similar studies in literature (Attour et al. 2014; İrdemez et al. 2006)
Optimum CD
The second investigated parameter is the effect of CD on the EC process performance. CD is another important parameter for all of the electrochemical processes because variations in the CD value highly affects coagulant dosage and bubble production in EC process (Akyol 2012). As seen clearly in Fig. 3, the third parametric level (3.5 mA/cm2) provides the best removal results in terms of COD.
It can be seen that when the CD is increased from 1.5 to 3.5 mA/cm2, the COD removal increases. This is because of the formation of Al+3 species at the anode by increasing CD according to Faraday’s law (Mollah et al. 2004). According to Faraday’s law (Eq. (11)), the charge passed to the solution is directly proportional to the amount of aluminum electrode dissolved.
where C electrode is electrode consumption (kg/m3), i is the current (A), t EC is the operating time (min), M Fe is the molecular weight of Al (26.98 g/mol), F is the Faraday’s constant (96,487 C/mol), z is the number of electrons involved in the oxidation/reduction reaction, and v is the volume of the treated solution (m3).
Optimum C o
In the study, another investigated parameter is initial wastewater concentration because wastewater properties continuously change daily or even hourly. Therefore, C o was chosen as one of the parameters affecting the treatment efficiency of the system. It is clear from Fig. 4 that the third parametric level (C o/4) has given the best result for COD removal. According to the result, high initial wastewater concentration decreases COD removal efficiencies, and this could be explained by the high soluble COD content of the wastewater. In addition, this is possibly due to the formation of insufficient number of aluminum hydroxide complexes by the electrode at a given conductivity and cell voltage to coagulate the excessive number of pollutant molecules at higher concentration. Soluble and miscible compounds that may not react with produced metal ions will not be removed by EC and they will remain in solution. Just a small amount can be adsorbed or absorbed on the floc and may consequently be removed incidentally. In brief, it can be said that COD removal efficiency and its variability will depend on the formation of floc, which usually occurs at appropriate values of solution pH (Moreno et al. 2006)
As can be seen in Table 1, the soluble COD of the wastewater constitutes approximately 72 % of its total COD content. Although EC process can easily remove particulate COD, it may remove just a small amount of soluble COD via sorption mechanism on the floc surface (Hu et al. 2002).
Optimum CT
During EC, anodic reactions take place at anode while cathodic reactions occur on the cathode. The released ions neutralize the particle charges and thereby initiate coagulation. Increase of the electrolysis time increases the ionic concentration in the reaction medium (Daneshvar et al. 2007). Figure 5a depicts that the removal efficiency was not improved continuously with increasing contact time.
The best removal efficiency was obtained at the second parametric level (40 min) for CT. One possible explanation of this could be as follows: When the electrolysis time is increased, pH of the solution continuously increases, and if the aluminum electrodes are used as electrode material for EC, treatment efficiency will reduce depending on the pH value of the solution. Figure 5b clearly indicates that the minimum solubility of aluminum hydroxide is recorded at about pH 6 (Aoudj et al. 2010). Similarly, Millar et al. reported that increasing the contact time generally enhanced the degree of suspended solids removal from solution (Millar et al. 2014). On the other hand, when CT is increased to more than an optimum value, the removal efficiency decreases because the amount of sludge in the cell becomes saturated and the removed contaminant in the EC process is released from the sludge (Daneshvar et al. 2003).
Statistical analysis
In this section of the study, ANOVA results of COD analysis are given. Table 5 shows the contribution ratios (C r) of the each factor which was calculated by ANOVA and the C r values represent the impact of parameters on the system performance numerically.
Equation (12) shows the C r values that can be calculated by dividing the source’s net variation by SStotal, which is given as follows (Gökkuş et al. 2012):
From the calculated variance ratios, F value can be inferred that all factors and interactions considered in the experimental design have statistically significant effects at 95 % confidence limit (in all cases calculated F value is greater than the critical value), and indicates that the variability of experimental data must be explained in terms of significant effects (Barrado et al. 1996). In order to determine whether the results of the verification experiments are suitable or not, a confidence interval (CI) is calculated. CI can be evaluated as follows (Gunes et al. 2011):
Here, Se is the two-standard deviation confidence limit, n is the number of rows in the matrix experiment, “n r ” is the number of repetitions in the verification experiment, and nA i , nB i , nC i ,… are the replication numbers for the parameter levels A i , B i , C i ,… If the prediction error is outside these limits, it is possible that the additive model is not adequate (İrdemez et al. 2006).
The obtained verification experiment results according to Taguchi model are given in Table 6. The verification experiment shows that results are within a ‘‘5 % error”.
When the C r values of the variables are looked into, it is seen that the most effective parameter for EC process efficiency is C o with 42.51 % contribution ratio of the total effect. On the other hand, CT has very little effect on system performance (about 6 %). Based on the above comments and explanations, the results proved that the Taguchi method is a suitable and easily adaptive optimization method for electrochemical treatment applications. Thus, it is possible to reach good results within a short time period with fewer experiments.
Conclusions
In this study, the optimal treatment conditions for MSW were determined by changing the EC process parameters through the Taguchi parameter design method. Since TP was removed in all EC trials carried out according to Taguchi DOE, it was not included in the optimization and statistical procedure. The best operational conditions based on COD removal were 5 for pH i , 3.5 for CD, C o/4 for C o, and 40 for CT.
According to the results, removal efficiency is closely related to the pollution load of wastewater, and EC process performance decreases by an increase in pollution load due to high organic and inorganic content of the wastewater. On the other hand, CD has high importance on EC process because of higher metal hydroxide formation at the cathode by using aluminum electrode at higher CD values. Results showed that 100 % of TP and 52.04 % of COD removals were achieved at optimum parametric conditions. Also, it can be said that the removal efficiency is not improved continuously by increasing the contact time due to the increase in wastewater pH during EC process.
Consequently, EC process can be effectively used for the treatment of this type of wastewater. On the other hand, the problem of electrode fouling or passivation may cause the electrocoagulation process to fail. Fouling on the electrodes occurs within a few hours of processing resulting in reduced efficiency, increased power consumption, reduced bubble formation quantity and size, and eventually resulting in a complete shutdown of the process.
References
Aber S, Salari D, Parsa MR (2010) Employing the Taguchi method to obtain the optimum conditions of coagulation–flocculation process in tannery wastewater treatment. Chem Eng J 162:127–134. doi:10.1016/j.cej.2010.05.012
Abu Qdais H, Rabi A, Abdulla F (2007) Characteristics of the medical waste generated at the Jordanian hospitals. Clean Technol Environ Policy 9:147–152. doi:10.1007/s10098-006-0077-0
Akyol A (2012) Treatment of paint manufacturing wastewater by electrocoagulation. Desalination 285:91–99. doi:10.1016/j.desal.2011.09.039
Aoudj S, Khelifa A, Drouiche N, Hecini M, Hamitouche H (2010) Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem Eng Process 49:1176–1182. doi:10.1016/j.cep.2010.08.019
Apha A (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Attour A, Touati M, Tlili M, Ben Amor M, Lapicque F, Leclerc JP (2014) Influence of operating parameters on phosphate removal from water by electrocoagulation using aluminum electrodes. Sep Purif Technol 123:124–129. doi:10.1016/j.seppur.2013.12.030
Barrado E, Vega M, Pardo R, Grande P, Del Valle JL (1996) Optimisation of a purification method for metal-containing wastewater by use of a Taguchi experimental design. Water Res 30:2309–2314. doi:10.1016/0043-1354(96)00119-4
Bayramoglu M, Kobya M, Can OT, Sozbir M (2004) Operating cost analysis of electrocoagulation of textile dye wastewater. Sep Purif Technol 37:117–125. doi:10.1016/j.seppur.2003.09.002
Daneshvar N, Ashassi-Sorkhabi H, Tizpar A (2003) Decolorization of Orange II by electrocoagulation method. Sep Purif Technol 31:153–162. doi:10.1016/S1383-5866(02)00178-8
Daneshvar N, Khataee A, Amani Ghadim A, Rasoulifard M (2007) Decolorization of CI Acid Yellow 23 solution by electrocoagulation process: investigation of operational parameters and evaluation of specific electrical energy consumption (SEEC). J Hazard Mater 148:566–572
Diaz LF, Savage GM, Eggerth LL (2005) Alternatives for the treatment and disposal of healthcare wastes in developing countries. Waste Manag 25:626–637. doi:10.1016/j.wasman.2005.01.005
Dimoglo A, Akbulut HY, Cihan F, Karpuzcu M (2004) Petrochemical wastewater treatment by means of clean electrochemical technologies. Clean Technol Environ Policy 6:288–295. doi:10.1007/s10098-004-0248-9
El-Haggar DSM (2007) Chapter 9—sustainability of clinical solid waste management. In: El-Haggar DSM (ed) Sustainable industrial design and waste management. Academic Press, Oxford, pp 293–306. doi:10.1016/B978-012373623-9/50011-3
Gökkuş Ö, Yıldız YŞ, Yavuz B (2012) Optimization of chemical coagulation of real textile wastewater using Taguchi experimental design method. Desalin Water Treat 49:263–271
Gönder ZB, Kaya Y, Vergili I, Barlas H (2010) Optimization of filtration conditions for CIP wastewater treatment by nanofiltration process using Taguchi approach. Sep Purif Technol 70:265–273. doi:10.1016/j.seppur.2009.10.001
Gunes S, Manay E, Senyigit E, Ozceyhan V (2011) A Taguchi approach for optimization of design parameters in a tube with coiled wire inserts. Appl Therm Eng 31:2568–2577. doi:10.1016/j.applthermaleng.2011.04.022
Gürses A, Yalçın M, Doğar Ç (2002) Electrocoagulation of some reactive dyes: a statistical investigation of some electrochemical variables. Waste Manag 22(2002):491–499
Holt PK (2006) Electrocoagulation: unravelling and synthesising the mechanisms behind a water treatment process PhD dissertation, University of Sydney, Australia
Hu Z, Chandran K, Smets BF, Grasso D (2002) Evaluation of a rapid physical–chemical method for the determination of extant soluble COD. Water Res 36:617–624
Insa E, Zamorano M, López R (2010) Critical review of medical waste legislation in Spain resources. Conserv Recycl 54:1048–1059. doi:10.1016/j.resconrec.2010.06.005
İrdemez Ş, Demircioğlu N, Yıldız YŞ, Bingül Z (2006) The effects of current density and phosphate concentration on phosphate removal from wastewater by electrocoagulation using aluminum and iron plate electrodes. Sep Purif Technol 52:218–223. doi:10.1016/j.seppur.2006.04.008
Jadhav SB, Chougule AS, Shah DP, Pereira CS, Jadhav JP (2014) Application of response surface methodology for the optimization of textile effluent biodecolorization and its toxicity perspectives using plant toxicity, plasmid nicking assays. Clean Technol Environ Policy 2014:1–12. doi:10.1007/s10098-014-0827-3
Jang Y-C, Lee C, Yoon O-S, Kim H (2006) Medical waste management in Korea. J Environ Manag 80:107–115. doi:10.1016/j.jenvman.2005.08.018
Jangsawang W, Fungtammasan B, Kerdsuwan S (2005) Effects of operating parameters on the combustion of medical waste in a controlled air incinerator. Energy Convers Manag 46:3137–3149. doi:10.1016/j.enconman.2005.03.017
Kajitvichyanukul P, Suntronvipart N (2006) Evaluation of biodegradability and oxidation degree of hospital wastewater using photo-Fenton process as the pretreatment method. J Hazard Mater 138:384–391. doi:10.1016/j.jhazmat.2006.05.064
KASKI (2013) The Directorate of Kayseri metropolitan municipality water and sewerage the regulation of wastewater discharge in sewage system Kayseri in Turkey
Madaeni SS, Koocheki S (2006) Application of Taguchi method in the optimization of wastewater treatment using spiral-wound reverse osmosis element. Chem Eng J 119:37–44. doi:10.1016/j.cej.2006.03.002
McKeen L (2012) Introduction to Food Irradiation and Medical Sterilization. In: McKeen L (ed) The Effect of Sterilization on Plastics and Elastomers, 3rd edn. William Andrew Publishing, Boston, pp 1–40. doi:10.1016/B978-1-4557-2598-4.00001-0
Mehrara H, Roozbehani B, Shishehsaz MR, Mirdrikvand M, Moqadam SI (2014) Using Taguchi method to determine optimum process conditions for flue gas desulfurization through an amine scrubber. Clean Technol Environ Policy 16:59–67. doi:10.1007/s10098-013-0593-7
Meunier N, Drogui P, Montané C, Hausler R, Mercier G, Blais J-F (2006) Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J Hazard Mater 137:581–590. doi:10.1016/j.jhazmat.2006.02.050
Millar GJ, Lin J, Arshad A, Couperthwaite SJ (2014) Evaluation of electrocoagulation for the pre-treatment of coal seam water. J Water Process Eng 4:166–178. doi:10.1016/j.jwpe.2014.10.002
Mollah MYA, Morkovsky P, Gomes JAG, Kesmez M, Parga J, Cocke DL (2004) Fundamentals, present and future perspectives of electrocoagulation. J Hazard Mater 114:199–210. doi:10.1016/j.jhazmat.2004.08.009
Moreno HA, Cocke DL, Gomes JJA (2006) Electrocoagulation: COD Removal Mechanism. In: AIChE 2006, San Francisco, CA
Mouedhen G, Feki M, Wery MDP, Ayedi HF (2008) Behavior of aluminum electrodes in electrocoagulation process. J Hazard Mater 150:124–135. doi:10.1016/j.jhazmat.2007.04.090
Özkan O, Mıhçıokur H, Azgın ST, Özdemir Ö (2010) Characterisation of medical-waste sterilisation-plant wastewater and a preliminary study of coagulation–flocculation treatment options. Water Sci Technol 62:266–271. doi:10.2166/wst.2010.282
Palahouane B, Drouiche N, Aoudj S, Bensadok K (2014) Cost-effective electrocoagulation process for the remediation of fluoride from pretreated photovoltaic wastewater. J Ind Eng Chem. doi:10.1016/j.jiec.2014.06.033
Phalakornkule C, Sukkasem P, Mutchimsattha C (2010) Hydrogen recovery from the electrocoagulation treatment of dye-containing wastewater. Int J Hydrog Energy 35:10934–10943. doi:10.1016/j.ijhydene.2010.06.100
Rutala WA, Weber DJ (1999) Infection control: the role of disinfection and sterilization. J Hosp Infect 43(Supplement 1):S43–S55. doi:10.1016/S0195-6701(99)90065-8
Song L-J, Zhu N-W, Yuan H-P, Hong Y, Ding J (2010) Enhancement of waste activated sludge aerobic digestion by electrochemical pre-treatment. Water Res 44:4371–4378. doi:10.1016/j.watres.2010.05.052
Tchamango S, Nanseu-Njiki CP, Ngameni E, Hadjiev D, Darchen A (2010) Treatment of dairy effluents by electrocoagulation using aluminium electrodes. Sci Total Environ 408:947–952. doi:10.1016/j.scitotenv.2009.10.026
Vepsäläinen M, Kivisaari H, Pulliainen M, Oikari A, Sillanpää M (2011) Removal of toxic pollutants from pulp mill effluents by electrocoagulation. Sep Purif Technol 81:141–150
Yildiz YŞ, Şenyiğit E, İrdemez Ş (2013) Optimization of specific energy consumption for Bomaplex Red CR-L dye removal from aqueous solution by electrocoagulation using Taguchi-neural method. Neural Comput Appl 23:1061–1069. doi:10.1007/s00521-012-1031-1
Acknowledgments
This research was supported by the FBD-12-4107 doctoral thesis project of the Research Fund of Erciyes University and performed in the laboratories of the Environmental Engineering Department, Engineering Faculty, Erciyes University. Also, special thanks go to Mr. Erkan Ozdogan, for his assistance with proofreading.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gökkuş, Ö., Yıldız, Y.Ş. Application of electrocoagulation for treatment of medical waste sterilization plant wastewater and optimization of the experimental conditions. Clean Techn Environ Policy 17, 1717–1725 (2015). https://doi.org/10.1007/s10098-014-0897-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-014-0897-2