Abstract
Tailings from the Zeïda mining region, located in the Middle Atlas Mountains of Morocco, contain high levels of lead and zinc which have many adverse effects, regarding both the environment and the health of the local human population. Finding practical methods to limit heavy metal dispersion and subsequent pollution of ecosystems in this area is therefore critical. This study aims to evaluate lead-tolerant rhizobacteria with an aim of exhibiting multiple plant growth-promoting traits. Thus, the growth of Medicago sativa may be improved and its resistance under lead stress conditions and may be subsequently used for the phytostabilization of lead-contaminated soils. Forty bacteria were isolated from the rhizospheric soil of Astragalus armatus plants growing wildly in the Zeïda mine tailings. After preventing the duplicates of obtained isolates, the resistance to various heavy metals at high levels allowed the selection of two strains (i.e. AaR114 and AaR72). These strains were evaluated in vitro for characteristics that promote plant development, such as the synthesis of 1-aminocyclopropane-1-carboxylic acid deaminase, indoleacetic acid, hydrogen cyanide, siderophore, phosphate solubilization, and antifungal activity. Inoculation of M. sativa plants with rhizobacteria AaR114 and AaR72, in the presence of 100 μg mL−1 of lead-acetate, was shown to significantly improve plant tolerance, increase aerial and root biomass, and diminished the negative impacts of heavy metals on plants. The 16S rRNA sequences analyses of the bacteria revealed that the strains AaR114 and AaR72 were linked to Bacillus subtilis DSM 10 T and Neobacillus niacini NBRC 15566 T, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities, such as manufacturing, industrial procedures and mining, have enormously increased the amount of heavy metals in the environment, which has caused widespread worry, with regards to the health of the environment (Suman et al. 2018; Manoj et al. 2020).
Mining activities remain the most harmful activity and have received increasing amounts of attention in recent years (Dias et al. 2022). Mining only affects a relatively small area at a time but could have a significant impact on the environment, especially after the mine’s closure, after which tailings from the mining are discharged into surrounding soils, destroying them (El Khalil et al. 2008).
Heavy metals from mining tailings are mostly permanent and non-biodegradable, so cannot be easily eliminated. Thus, the existence of heavy metals, from many sources, in soils will consequently transfer down into food chains, terminating in humans and animal bodies via consumption. (Rebello et al. 2021).
Morocco has many metal mines and the Zeïda mine was one of the largest Moroccan Pb–Zn mining districts in the last century. These deposits, currently abandoned, have largely contributed to Moroccan lead production (Hachimi et al. 2014). Between 1972 and 1985, around 630 172 t of lead were produced at Zeïda. Moreover, in fully exposed mining piles, 12 Mt and 70 Mt of tailings and waste were left unattended to (El Hachimi et al. 2007).
Lead (Pb) is perhaps the most perplexing element, as it is widely used, but it has no use in biological systems. It is dangerous to the health of plants, animals and humans (especially children) (Dapul and Laraque 2014; Wani et al. 2015; Schindler et al. 2021; Wani et al. 2015; Zhang et al. 2022). Furthermore, lead poisoning harms plants in various ways, from germination to yield development (Zulfiqar et al. 2019).
In order to eliminate or control heavy metals’ waste removal, the use of metal-resistant plants and their associated rhizobacteria has been proposed as a potential green alternative to standard chemical and physical methods (Chen et al. 2022; Suman et al. 2018). Legumes are one of the plants commonly used to remediate metal-contaminated soils, such as Medicago sativa (alfalfa), which shows strong adaptability to adverse environments, with abundant biomass and extensive root systems. In recent years, many studies have revealed that alfalfa has the ability to adsorb and accumulate various heavy metals (e.g. V, Pb, Cd, Cu, Ni, and Zn) (Helaoui et al. 2020; Raklami et al. 2021a; Xiong 2018; Chen et al. 2022). For these reasons, the alfalfa plant is considered one of the most studied species for phytoremediation of heavy metal-contaminated sites and is also commonly used in practice (Noori 2018; Yahaghi et al. 2019; Gan et al. 2020; Chen et al. 2022).
Considering its significance in phytoremediation, rhizobacteria can reduce plant toxicity from metals in the soil (Ma et al. 2016). Moreover, numerous studies have highlighted that plant growth-promoting rhizobacteria (PGPR) can stimulate plant development in metal-contaminated soils in many ways. Through either the accumulation or biosorption processes, or other plant growth-promoting properties, such as the synthesis of phytohormones, ACC deaminase activity, solubilization of phosphate and siderophores production (Glick 2010; Rajkumar et al. 2010; Babu et al. 2015; Ma et al. 2016; Kong and Glick 2017; Manoj et al. 2020; Suman et al. 2018; Tirry et al. 2018).
Thus, the objectives of this study were as follows: (i) the isolation and characterization of heavy metal-tolerant bacteria from Astragalus armatus rhizosphere, assembled from lead mine tailing, (ii) the selection of the heavy metal tolerance and PGP trait of the bacteria, regarding both boosting plant nutrition and stress resistance, and (iii) the evaluation of selected bacteria capacity to enhance M. sativa growth under Pb-acetate stress and to enhance the efficiency of phytoremediation in metal-contaminated soils.
This study was carried out since 2019 in Morocco at Biotechnology and Biomolecular Engineering Research Team, FST Tangier, Abdelmalek Essaadi University, Morocco.
Materials and methods
Soil sampling and isolation of lead-resistant bacteria
The samples were taken from A. armatus rhizospheric soil: plants that have grown wildly in the abandoned lead mine tailing of Zeïda, in the High Moulouya, west of Midelt city in the Northeastern region of Morocco (Fig. 1). According to Hachimi 2016, this area is characterized by a cold and dry climate and a mountainous inclination. It is also a highly lead-contaminated area (5547 ppm), making it the largest lead deposit in Morocco (El Hachimi et al. 2013).
Suspensions of soil samples were prepared by immersing 1 g of rhizospheric soil in 9 mL of physiological water at a 0.9% concentration of NaCl. After 1 h of agitation, a 0,1 mL suspension of each dilution (10–1–10–7) was plated on tryptone-soybean agar (TSA) medium, amended with 500 mg L−1 of lead-acetate. The incubation of the plates was at 30 °C for 72 h. Colonies with different morphologies were selected.
Determination of metal-resistant bacteria
The resistance to lead and heavy metals of the bacteria was tested using tryptic soy agar (TSA) mediums supplemented with increasing metal concentrations, lead-acetate (1000–2000-2250 mg L−1), CuCl2 (600–800–1000 mg L−1), ZnCl2 (500–600–700 mg L−1) and Cd-nitrate (25–50 mg L−1). The incubation of the plates was at 28 °C for 7 days. Any development of the bacterial strains was considered as a favorable response.
Screening for PGP traits
The quantitative estimation of Tri-Calcium Phosphate solubilization was conducted in Pikovskaya’s liquid medium (Pikovskaya 1948). The bacterial suspension (0.5 mL) was inoculated in 100 mL flasks containing 50 mL of PVK broth. The control consisted of uninoculated medium. After 7 days of incubation under 180 rpm at 28 °C, bacterial cultures were centrifuged at 13 000 rpm for 20 min. The supernatant was used to determine soluble phosphorus content using the Ames colorimetric method by comparing with the standard curve of KH2PO4 (Ames 1966).
To evaluate siderophores production by bacterial isolates, the Chrome-azurol S (CAS) was used (Schwyn and Neilands 1987). The supernatant of each isolate grown in tryptone-soybean broth (TSB) was mixed with CAS solution (1:1; v:v) and then incubated for 20 min in darkness. The optical density of the test solutions was measured at 630 nm (OD630). The production of siderophores was calculated using the following formula:
where Ar = reference solution absorbance and As = sample absorbance (Gokarn 2010).
Indol-3-acetic acid production (IAA, Auxin) was tested according to the Gordon and Weber (1951) method. The bacterial strains were grown for 48 h at 28 °C in sucrose-minimal salt (SMS) medium supplemented with 0,05% of L-Tryptophane. Centrifugation of the cultures was at 13 000 rpm for 10 min; then, the Salkowski reagent [ 10 mM of FeCl3; 35% of perchloric acid] was blended with the supernatant (1:2 v:v) and incubated for 20 min at ambient temperature. The optical density determined at 535 nm (OD535) and IAA concentration was then estimated with the help of a standard curve created from numerous solution dilutions of 50 μg mL−1 in SMS medium.
Quantitative estimation of ACC-deaminase was done following the method prescribed by Jacobson et al. 2011. In a plate of 96 wells, 120 μL of the minimum DF salt medium (Dworkin and Foster 1958) was added to each well. For each of the four columns, 15 μL of MgSO4 (0.1 M), (NH4)2SO4 (0.1 M) and ACC-solution (3 mM), previously sterilized, was introduced, respectively. For the inoculation of each well, 15 μL of bacterial culture was used. In the untreated control wells, 15 μL of MgSO4 (0.1 M) was used instead of inoculation. The optical density was measured after 48 h at 600 nm. DO values of ACC and (NH4)2SO4 were compared with those of MgSO4 to determine the bacteria ability to use ACC for their growth.
The Hydrogen cyanide (HCN) production of the bacteria was qualitatively evaluated by adapting the Bakker method (Bakker 1987). The bacterial cultures were streaked on a TSA medium supplemented with glycine (4,4 g L−1). In the lid of each plate, a Whatman paper impregnated with a yellow reagent [2% of sodium carbonate, 0,5% of picric acid] was placed. The plates were closed perfectly with parafilm and held for 96 h at 28 °C. Discoloration of Whatman paper to an orange/brown coloration indicated the production of HCN.
The isolates were examined for their capacity to produce ammonia (NH3) using the method described by (Cappuccino and Welsh 2017). 10 mL of Peptone water broth was inoculated by each bacterial culture and incubated at 28 °C. After 72 h of incubation, Nessler’s reagent was added. The appearance of a brown coloration indicated a positive test for ammonia production.
Screening of antagonism
The ability of isolated bacteria to inhibit the phytopathogen fungus Fusarium oxysporum’s growth was tested on a Potato dextrose agar (PDA) medium. A small fungal agar disk from fresh cultures was centrally placed on the plates filled with the PDA medium, which had been previously inoculated with each strain at approx. 3 cm away from the mycelium disk. Plates without bacteria were reserved for control. The plates were then incubated for 7 days at 28 °C (Rabindran and Vidhyasekaran 1996). The percentage of inhibition of fungal growth was determined following the formula:
where R is the fungal mycelium’s greatest growth on control plates, and r the radius of fungal that grew in the presence of bacteria (Kumar et al. 2002).
Antagonism among strains
Two estimating approaches were employed to prevent detrimental impacts between strains. First, streak as a strip at one end of the plate and incubate at 30 °C for four days to see whether there is any diffusible material in the media (Anandaraj and Delapierre 2010). Secondly, to determine volatiles, 0.1 mL of bacterial suspension was placed on separated plates using TSA medium. The plates were stacked on top of each other and closed perfectly with parafilm then incubated at 28 °C for 72 h (Bennis et al. 2022).
DNA preparation, 16S rDNA gene amplification and sequencing
Extraction of DNA and PCR reactions was effectuated as reported by Lamin et al. 2019. The BOX A1R primer was used for BOX-PCR (Versalovic et al. 1994). For amplification of the 16S rDNA gene, the bacterial universal primers fD1 (5’AGAGTTTGATCCTGGCTCAG-3’) and rD1 (5’AAGGAGGTGATCCAGCC GCA-3’) were used. For PCR reactions, the MyTaq Mix was utilized, as indicated by the producer. The PCR products were verified by electrophoresis in 1% agarose gel amended with ethidium bromide in TAE buffer at 70 V. The Qiagen (PCR products purification Kit) was utilized to purify the amplifier. For sequencing, the primers used were the same as for the amplification by PCR, using the chemistry of ABI prism dyes, and analyzed with a 3130xl automatic sequencer at the National Centre for Scientific and Technical Research (CNRST) in Rabat (Morocco).
Phylogenetic characterization
The 16S rRNA sequences that were obtained were compared to those from GenBank by searching in the BLASTn software (Altschul et al. 1990). The MEGA 7 program was used to align various sequences (Kumar et al. 2016) and the distances were estimated using Kimura’s 2-parameter model to create a phylogenetic tree using neighbor-joining methodology (Saitou and Nei 1987).
Plant inoculation, growth, and lead accumulation
A preliminary rhizoremediation pot experiment was conducted in the growth room to evaluate the performance of the selected strains in enhancing M. sativa (Alfalfa) growth in the presence of lead-acetate, knowing that Alfalfa was chosen as a plant model.
Alfalfa (M. sativa L.) seed sterilization was conducted by soaking them in 70% Ethanol for 10 min and then in 0.1% of mercury chloride (HgCl2) for 2 min, with sterile distilled water. The seeds were rinsed and placed on plates containing agar/water 0.7% (w/v) to germinate at 26 °C for 3 days.
Each pot was filled up with vermiculite/perlite (2:1) and 100 mL of nutrient solution (Broughton and Dilworth 1971) amended with Pb-acetate at 100 μg mL−1. Every pot was sowed with five seeds. 1 mL of bacterial suspension was introduced into each seedling separately. Uninoculated pots served as negative controls and four repetitions were made for each treatment. Pots were deposited in the growth room under pre-determined conditions (16/8 h light/dark photoperiod). Throughout the experiment, plants were irrigated four times a week with 50 mL of nutrient solution.
The plants were later collected using tap water after two months of growth and dried for 48 h at 70 °C. The dry weight and length of shoot and root parts of plants were measured.
To assess the effect of inoculated rhizobacteria on accumulation of lead by alfalfa, the concentration of lead in shoots of plants was measured using Inductively Coupled Plasma Optical Emission Spectrometry (Agilent 5110 ICP-OES, USA) in Water, Soil and Agriculture Analysis Laboratory within the Mohammed VI Polytechnic University (UM6P) of Ben Guerir.
Statistical analysis
For three replicates, the data are presented as means + SD (standard deviation). Using Statgraphics Plus version 4.0, the findings were compared using analysis of variance (ANOVA) and Fisher protected LSD test (p < 0.05).
Results and discussion
Sample site
Forty bacteria were successfully isolated from A. armatus rhizospheric soil based on the difference in their morphological appearance on the TSA medium, amended with lead-acetate (500 μg mL−1). To determine diversity and to prevent the duplication of obtained isolates, BOX-PCR was initially used. Isolates' effectiveness was reduced to ten distinct fingerprints. (Fig. 1S).
Resistance to heavy metals
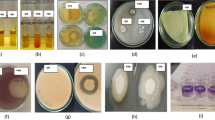
All retained strains were found resistant to the selected heavy metals with varying capabilities. The ten isolates were tolerant to lead-acetate until 1000 μg mL−1, CuCl2 (400 μg mL−1) and ZnCl2 (200 μg mL−1). 60% of isolates resisted to Cd-nitrate at 25 μg mL−1 while only two strains (AaR114 and AaR115) could grow at 50 μg mL−1, 80% were able to grow on the TSA medium amended with 1700–400 μg mL−1 of Pb-acetate and ZnCl2, respectively, and 70% grew in presence of 600 μg mL−1 of CuCl2, whereas only two strains grew in 2250 μg mL−1 of Pb-acetate (AaR1 and AaR72) (Fig. 2).
Plant growth-promoter potential
The obtained results of PGP proprieties of the selected strains are presented in Table 1. Inorganic tri-calcium phosphate solubilization was detected in both bacteria with amounts that were significantly different (P < 0.05). The highest concentration of P soluble was observed in AaR114 strain (186.4 mg L−1), while AaR72 solubilized 140.8 mg L−1. A significant drop of pH was observed during the solubilization of P in PVK liquid medium, principally in the presence of AaR114 strain as compared to control (pH 7.00). Potential of isolates to produce siderophores was found to be positive. The percentage of siderophores production was 62,7% and 66,4% by AaR114 and AaR72, respectively. In the presence of L-Tryptophane, quantitative measurement of IAA indicated that only the AaR114 strain was able to synthesis a low amount of IAA (2,7 μg mL−1). Additionally, all bacterial strains were capable of producing ACC-deaminase and ammonia, while the AaR72 strain did not show any HCN production. The antifungal activity of the strains was tested toward F.oxysporum and they were shown to inhibit the fungal growth to differing extents. Moreover, there was no antagonism between the two strains. They were able to grow simultaneously without any inhibition in growth.
Analysis of 16S rRNA gene
The 16S rRNA sequences of AaR114 and AaR72 strains showed a link to the genera of Bacillus and Neobacillus. Phylogenetic analysis indicated that the two strains assembled in separated clusters and presented a 98,05% and 98,91% of similarity with Bacillus subtilis and Neobacillus niacini, respectively. The nucleotide sequences assigned to this study were sent to the GenBank and registered with the accession numbers OM049547 and OM084759 for strains AaR114 and AaR72, respectively (Fig. 3).
Plant inoculation, growth, and lead accumulation
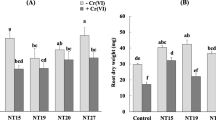
After 2 months of growth, the results of inoculation demonstrated a significant effect of the strains on alfalfa growth (P < 0,05). Inoculation with strains AaR114 and AaR72 increased shoots and roots lengths compared to uninoculated plants. However, the most significant length stimulation was attributed to the double combination of AaR114 + AaR72, achieving a 39% increase for shoots and 36% increase for roots (Fig. 4). Plants inoculated with strains AaR114, AaR72 and AaR114 + AaR72 had dry root weights that were 26%, 43% and 58% greater than the control, respectively. Also, inoculation of plants with AaR114 and double-inoculated increased shoots dry weight by 17% and 12%, respectively, while the AaR72 strain showed a insignificant reduction of shoots dry weight (Fig. 4).
A root and shoot length (cm); B dry weight of shoot and root of plants (mg plant−1) of M. sativa inoculated with single and dual combination of the AaR114 and AaR72 strains. The nutrient solution was amended with 100 µg Ml–1 of lead-acetate. Plants are grown in growth chamber for 60d. The data presented are the average of 4 replicates. Column with the same letter at the top are not significantly different
Lead accumulation in shoot parts of M. sativa plants was determined and is shown in Fig. 5. The finding showed that lead accumulation in shoots was significantly influenced by bacterial inoculation. In fact, the inoculation with the strain AaR72 was found to significantly (P < 0,05) enhance the concentration of lead from 115.6 mg Kg−1 of dry weight to 145.7 mg Kg−1 compared to the uninoculated control. Moreover, a significant increase in lead concentration of 10% was observed as a result of dual inoculation with AaR114 and AaR72. Upon inoculation of M. sativa with AaR114 strain, metal accumulation in shoots was non-significantly lower than that of uninoculated plants (Fig. 5).
Discussion
Mining, metal smelting and associated activities have been known to be the main sources of ecosystems pollution with heavy metals (Liu et al. 2013; Rodríguez et al. 2009). Usually, the tailings are stored in soils without any environmental management, which leads to heavy metal dispersion into neighboring surfaces, ground water and agricultural soils, negatively impacting human and animal health (El Khalil et al. 2008; Liu et al. 2013; Nagajyoti et al. 2010).
To remediate mining sites, various strategies have been developed. However, phytoremediation is a new ecologically beneficial method and cost-effective technology to remediate soils contaminated with heavy metals (Raklami et al. 2021b; Tirry et al. 2018). It implies the use of plants to limit bio-mobility and bio-availability of metals in soils (Koptsik 2014; Ma et al. 2011).
The use of plant-associated rhizobacteria may directly improve plant performance for phytoremediation (Yan et al. 2020). Plant growth-promoting rhizobacteria played a crucial role in enhancing plant growth and tolerance to heavy metals, as well as in biomass production (Etesami and Maheshwari 2018; Tirry et al. 2018).
Forty bacteria were isolated from the rhizospheric soil of wild-growing A. armatus in metal-contaminated soil of the Zeïda mining area in northeastern Morocco. Based on their PGP properties, genotypic and phenotypic characteristics, strains AaR114 and AaR72 were selected for further M. sativa inoculation. Both strains were grown on medium containing Pb and Zn, which are present in high concentrations in plant-growing soil.
The two selected rhizobacteria, AaR114 and AaR72, were identified as B. subtilis and N. niacini (basionym: B. niacini), respectively (Fig. 3). Generally, the bacillus genus represents an important proportion of soil microbial communities. Their ability to form spores to promote plant growth and survival under various stress conditions gives them a real advantage in the rhizosphere (Agarwal et al. 2017; Chrouqi et al. 2017; Rosier et al. 2018).
ACC deaminase-containing bacteria can convert the immediate ethylene precursor ACC into α-ketobutyrate and ammonia, which can be considered as an indirect bacterial source of N and carbon. Moreover, by decreasing the levels of plant ethylene, ACC deaminase may facilitate plants growth by protecting them from the inhibitory effects of certain environmental stresses (Kong and Glick 2017; Tak et al. 2013; Yan et al. 2020). In this study, two strains were screened for ACC utilization ability (Table 1). Deaminase activity was revealed in other strains of B. subtilis and B. niacini (Cedeño-García et al. 2018; Mohamed and Gomaa 2012).
Some bacterial activities can enhance plant’s mineral nutrient uptake and facilitate their growth under different conditions. These activities include phosphate solubilization and siderophores production (Kong and Glick 2017).
Phosphorus (P) is an important macro-element for plant growth and development although it is frequently immobilized and has limited bio-availability in soils (Beneduzi et al. 2012). Furthermore, the use of phosphate-solubilizing bacteria (PSB) may be an important alternative method to overcome this deficiency (Shin et al. 2015). The strains AaR114 and AaR72 demonstrated the ability to solubilize inorganic phosphate in different concentrations. In this regard, several studies have shown that Bacillus strains are able to solubilize phosphorus through the production of various organic acids (Borriss 2015; Saeid et al. 2018), which could explain the acidification of the mediums observed during the P-solubilization of the two strains.
Furthermore, metal ions, such as iron, are often a limiting factor for plant development. In response to low Fe levels in the rhizosphere, most PGPRs produce a low molecular mass iron chelator called siderophores. Bacterial siderophores can enhance plant growth by improving plant Fe nutrition, and/or by preventing the proliferation of pathogens by decreasing the amount of available iron (Ma et al. 2011). The bacteria selected in this study were able to synthetize siderophores (Table 1). This is unsurprising because the strain AaR114 was identified as B. subtilis, a species well known to have this capacity in previous studies (Mohamed and Gomaa 2012; Zhang et al. 2009).
The plant hormones produced by bacteria, indole-3-acetic acid (IAA), are of great importance. IAA is a key regulator of plant growth, as it is involved in numerous developmental processes, such as stimulation of cell division and root elongation (Beneduzi et al. 2012; Spaepen et al. 2007). In this study, the strain AaR114 was positive for IAA production (Table 1), which is phylogenetically linked to B. subtilis (Fig. 3), a well-known species for IAA production (Blake et al. 2021; Walia et al. 2014).
The strains AaR114 and AaR72 produced Hydrogen cyanide (HCN), a volatile secondary metabolite, and because of its toxicity toward plant diseases, it is considered a biocontrol agent. (Sehrawat et al. 2022). Ammonia emission is another significant PGPR process that promotes plant development indirectly (Joseph et al. 2007); all the isolates were able to synthetize ammonia. These activities were detected in other strains of B. subtilis (Ahmad et al. 2008; Etesami and Maheshwari 2018) and numerous strains of B. niacini (Kisiel and Kępczyńska 2016).
Fusarium oxysporum is a serious pest, affecting many crops through fusarium wilt, the most destructive disease affecting a wide variety of plants leading to huge loses around the world (Joshi, 2018). Therefore, it was selected to evaluate the antagonistic activity of the selected bacterial strains. The results of the antagonism test showed that both strains inhibited the growth of F. oxysporum. Among the plant growth-promoting rhizobacteria, Bacillus spp. strains have been commonly used as biocontrol agents against several plant diseases (Vassilev et al. 2006; Kumar et al. 2018).
Alfalfa (M. sativa L.) is a perennial plant of Papilionoidea. It is an important forage crop with extensive taproot system as well as being the most widely cultivated herb in the world. Alfalfa plants can easily tolerate and absorb various heavy metals through various defense mechanisms (Chen et al. 2022) and are considered as a lead hyperaccumulator species (López et al. 2005). Inoculation of rhizosphere microorganisms may be a feasible method to increase resistance and accumulation in M. sativa plants (Gan et al. 2020).
The positive effects of PGPR on plant growth grown under metal stress have been well documented (El Faiz et al. 2015; Navarro-Torre et al. 2017; Raklami et al. 2019). In this current study, inoculation with B. subtilis strain AaR114 and N. niacini strain AaR72 alone, or in double combination, improved several growth parameters, including root length and dry biomass of M. sativa cultivated under Pb-contaminated conditions (Fig. 4).
In addition, the present study showed that the two strains could affect metal accumulation in plants shoot (Fig. 5).
The inoculation of M. sativa with B. subtilis strain AaR114 decreased the amount of lead in shoots by 17%, which could indicate less metal translocation to shoots. This is an important fact for legume plants to be utilized in metal phytostabilization, thus restricting the amount of metal that may enter the food chain and spread across the ecosystem. Different plant parts have been observed to accumulate lead in a similar trend (Ahsan et al. 2017; He et al. 2013; Wu et al. 2010).
The finding suggests also that alfalfa combined with N. niacini strain AaR72 has potential for lead-uptake. The production of siderophores by soil microorganisms, one of the processes through which metal uptake is improved, which might be a reason for the high metal absorption by the inoculated plants with the AaR72 strain.
Metal complexation by organic acids produced by bacteria, which improve metals absorption and their transfer from root to shoot, has also been suggested as a method for enhancing metal intake of plant inoculated with PGPR (He et al. 2010).
The obtained results are in accord with those suggesting that the inoculation of alfalfa with different species of Bacillus has positively affected plant growth parameters and increased lead accumulation in plant roots and shoots (Yahaghi et al. (2019).
Felici et al. (2008) found that the inoculation with B. subtilis strains improved the dry weight of tomato plants shoots and roots, and also improved the growth and cd-accumulation in alfalfa plants (Li et al. 2021).
Additionally, Shah et al. (2021) reported that B. subtilis promotes the growth of Solanum melongena under lead-contaminated conditions. Other scientists have found that a Bacillus niacini strain isolated from the M. sativa rhizosphere promoted Medicago truncatula growth under controlled conditions (Kisiel and Kępczyńska 2016).
Conclusion
In conclusion, two rhizobacteria were chosen based on their performance, regarding their high tolerance to various heavy metals, phosphate solubilization and siderophores production. Both the B. subtilis strain AaR114 and N. niacini strain AaR72 were metal-tolerant and exhibited different plant growth-promoting activities. Single and/or combined inoculation of M. sativa with AaR114 and AaR72 likely promoted growth and decreased lead toxicity. The findings point to the possibility to use M. sativa, in association with PGPR, for the remediation of lead abandoned mine sites in Morocco.
References
Agarwal M, Dheeman S, Dubey RC, Kumar P, Maheshwari D, Bajpai VK (2017) Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum moench. Microbiol Res. https://doi.org/10.1016/j.micres.2017.08.012
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181. https://doi.org/10.1016/j.micres.2006.04.001
Ahsan MT, Najam-ul-haq M, Idrees M, Ullah I, Afzal M (2017) Bacterial endophytes enhance phytostabilization in soils contaminated with uranium and lead. Int J Phytoremediation 19:937–946. https://doi.org/10.1080/15226514.2017.1303813
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Ames, B.N., 1966, Assay of inorganic phosphate, total phosphate and phosphatases, In: Methods in enzymology, complex carbohydrates. Academic Press, https://doi.org/10.1016/0076-6879(66)08014-5
Anandaraj B, Delapierre LRA (2010) Studies on influence of bioinoculants (Pseudomonas fluorescens, Rhizobium sp., Bacillus megaterium) in green gram. J Biosci Technol 1:95–99
Babu AG, Shea PJ, Sudhakar D, Jung I-B, Oh B-T (2015) Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J Environ Manage 151:160–166. https://doi.org/10.1016/j.jenvman.2014.12.045
Bakker AW, Schippers BOB (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol Biochem 19(4):451–457
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Bennis M, Perez-Tapia V, Alami S, Bouhnik O, Lamin H, Abdelmoumen H, Bedmar EJ, Missbah El Idrissi M (2022) Characterization of plant growth-promoting bacteria isolated from the rhizosphere of Robinia pseudoacacia growing in metal-contaminated mine tailings in eastern Morocco. J Environ Manage 304:114321. https://doi.org/10.1016/j.jenvman.2021.114321
Blake C, Christensen MN, Kovács ÁT (2021) Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact® 34:15–25. https://doi.org/10.1094/MPMI-08-20-0225-CR
Borriss R (2015) Bacillus, a plant-beneficial bacterium. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer international publishing, Cham, pp 379–391
Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125:1075–1080. https://doi.org/10.1042/bj1251075
Cappuccino, J.G., Welsh, C.T., 2017. Microbiology: a Laboratory manual, Global Edition. Pearson Education
Cedeño-García GA, Gerding M, Moraga G, Inostroza L, Fischer S, Sepúlveda-Caamaño M, Oyarzúa P, Cedeño-García GA, Gerding M, Moraga G, Inostroza L, Fischer S, Sepúlveda-Caamaño M, Oyarzúa P (2018) Plant growth promoting rhizobacteria with ACC deaminase activity isolated from Mediterranean dryland areas in Chile: effects on early nodulation in alfalfa. Chil J Agric Res 78:360–369. https://doi.org/10.4067/S0718-58392018000300360
Chen L, Beiyuan J, Hu W, Zhang Z, Duan C, Cui Q, Zhu X, He H, Huang X, Fang L (2022) Phytoremediation of potentially toxic elements (PTEs) contaminated soils using alfalfa (Medicago sativa L.): a comprehensive review. Chemosphere 293:133577. https://doi.org/10.1016/j.chemosphere.2022.133577
Chrouqi L, Lahcen O, Jadrane I, Koussa T (2017) Screening of soil rhizobacteria isolated from wheat plants grown in the Marrakech region (Morocco, North Africa) for plant growth promoting activities. JMES 8:3382–3390
Dapul H, Laraque D (2014) Lead Poisoning in children. Adv Pediatr 61:313–333. https://doi.org/10.1016/j.yapd.2014.04.004
Dias YN, da Silveira Pereira WV, da Costa MV, de Souza ES, Ramos SJ, do AmaranteCamposFernandes CBWEOAR (2022) Biochar mitigates bioavailability and environmental risks of arsenic in gold mining tailings from the eastern Amazon. J Environ Manage 311:114840. https://doi.org/10.1016/j.jenvman.2022.114840
Dworkin M, Foster JW (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. https://doi.org/10.1128/jb.75.5.592-603.1958
El Faiz A, Duponnois R, Winterton P, Ouhammou A, Meddich A, Boularbah A, Hafidi M (2015) Effect of different amendments on growing of Canna indica L. Inoculated with AMF on mining substrate. Int J Phytoremediation 17:503–513. https://doi.org/10.1080/15226514.2014.950408
El Hachimi ML, El Founti L, Bouabdli A, Saïdi N, Fekhoui M, Tassé N (2007) Pb et As dans des eaux alcalines minières : contamination, comportement et risques (mine abandonnée de Zeïda, Maroc). Rev Sci Eau J Water Sci 20:1–13. https://doi.org/10.7202/014903ar
El Hachimi ML, Bouabdli A, Fekhaoui M (2013) Les rejets miniers de traitement : caractérisation, capacité polluante et impacts environnementaux, mine Zeïda, mine Mibladen, Haute Moulouya (Maroc). Déchets Sci Tech 63:32–42. https://doi.org/10.4267/dechets-sciences-techniques.2567
El Khalil H, El Hamiani O, Bitton G, Ouazzani N, Boularbah A (2008) Heavy metal contamination from mining sites in South Morocco: Monitoring metal content and toxicity of soil runoff and groundwater. Environ Monit Assess 136:147–160. https://doi.org/10.1007/s10661-007-9671-9
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol Environ Saf 156:225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
Felici C, Vettori L, Giraldi E, Forino LMC, Toffanin A, Tagliasacchi AM, Nuti M (2008) Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: Effects on plant growth and rhizosphere microbial community. Appl Soil Ecol 40:260–270. https://doi.org/10.1016/j.apsoil.2008.05.002
Gan CD, Chen T, Yang JY (2020) Remediation of vanadium contaminated soil by alfalfa (Medicago sativa L.) combined with vanadium-resistant bacterial strain. Environ Technol Innov 20:101090
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374. https://doi.org/10.1016/j.biotechadv.2010.02.001
Gokarn K (2010) Siderophores and pathogenecity of microorganisms. J. Biosci. Tech 1(3):127–134
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195. https://doi.org/10.1104/pp.26.1.192
Hachimi MLE, Fekhaoui M, Abidi AE, Rhoujatti A (2014) Contamination des sols par les métaux lourds à partir de mines abandonnées : le cas des mines Aouli-Mibladen-Zeïda au Maroc. Cah. Agric. 23(1):213–219. https://doi.org/10.1684/agr.2014.0702
Hachimi, M., 2016. Impact d’un site minier abandonné sur l’environnement : cas de la mine de Zeïda (Haute Moulouya, Maroc). https://doi.org/10.13140/RG.2.1.4715.6086
He CQ, Tan GE, Liang X, Du W, Chen YL, Zhi GY, Zhu Y (2010) Effect of Zn-tolerant bacterial strains on growth and Zn accumulation in Orychophragmus violaceus. Appl Soil Ecol 44:1–5. https://doi.org/10.1016/j.apsoil.2009.07.003
He H, Ye Z, Yang D, Yan J, Xiao L, Zhong T, Yuan M, Cai X, Fang Z, Jing Y (2013) Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb Zn uptake by Brassica Napus. Chemosphere 90:1960–1965. https://doi.org/10.1016/j.chemosphere.2012.10.057
Helaoui S, Boughattas I, Hattab S, Mkhinini M, Alphonse V, Livet A, Bousserrhine N, Banni M (2020) Physiological, biochemical and transcriptomic responses of Medicago sativa to nickel exposure. Chemosphere 249:126121. https://doi.org/10.1016/j.chemosphere.2020.126121
Jacobson C, Pasternak J, Glick B (2011) Partial purification and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can J Microbiol 40:1019–1025. https://doi.org/10.1139/m94-162
Joseph B, Patra RR, Lawrence R (2007) Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int J Plant Prod 1(2):141–152
Joshi R (2018) A review of Fusarium oxysporum on its plant interaction and industrial use. J Med Plants Stud 6:112–115. https://doi.org/10.22271/plants.2018.v6.i3b.07
Kisiel A, Kępczyńska E (2016) Medicago truncatula Gaertn. as a model for understanding the mechanism of growth promotion by bacteria from rhizosphere and nodules of alfalfa. Planta 243:1169–1189. https://doi.org/10.1007/s00425-016-2469-7
Kong, Z., Glick, B.R., 2017. The role of plant growth-promoting bacteria in metal phytoremediation, In: Advances in microbial physiology. Elsevier, 97–132
Koptsik GN (2014) Problems and prospects concerning the phytoremediation of heavy metal polluted soils: a review. Eurasian Soil Sci 47:923–939. https://doi.org/10.1134/S1064229314090075
Kumar NR, Arasu VT, Gunasekaran P (2002) Genotyping of antifungal compounds producing plant growth-promoting rhizobacteria, Pseudomonas fluorescens. Curr Sci 82:1463–1466
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kumar, A., Singh, V.K., Tripathi, V., Singh, P.P., Singh, A.K., 2018. Plant growth-promoting rhizobacteria (PGPR): perspective in agriculture under biotic and abiotic stress, in: Crop improvement through microbial biotechnology. Elsevier, pp. 333–342. https://doi.org/10.1016/B978-0-444-63987-5.00016-5
Lamin H, Alami S, Bouhnik O, ElFaik S, Abdelmoumen H, Bedmar EJ, Missbah-El Idrissi M (2019) Nodulation of retama monosperma by ensifer aridi in an abandonned lead mine soils in Eastern Morocco. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01456
Li Q, Xing Y, Fu X, Ji L, Li T, Wang J, Chen G, Qi Z, Zhang Q (2021) Biochemical mechanisms of rhizospheric Bacillus subtilis-facilitated phytoextraction by alfalfa under cadmium stress: microbial diversity and metabolomics analyses. Ecotoxicol Environ Saf 212:112016. https://doi.org/10.1016/j.ecoenv.2021.112016
Liu G, Tao L, Liu X, Hou J, Wang A, Li R (2013) Heavy Metal speciation and pollution of agricultural soils along Jishui River in non-ferrous metal mine area in Jiangxi Province. China J Geochem Explor 132:156–163. https://doi.org/10.1016/j.gexplo.2013.06.017
López ML, Peralta-Videa JR, Benitez T, Gardea-Torresdey JL (2005) Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 61:595–598. https://doi.org/10.1016/j.chemosphere.2005.02.028
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258. https://doi.org/10.1016/j.biotechadv.2010.12.001
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage 174:14–25. https://doi.org/10.1016/j.jenvman.2016.02.047
Manoj SR, Karthik C, Kadirvelu K, Arulselvi PI, Shanmugasundaram T, Bruno B, Rajkumar M (2020) Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J Environ Manage 254:109779. https://doi.org/10.1016/j.jenvman.2019.109779
Mohamed HI, Gomaa EZ (2012) Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 50:263–272. https://doi.org/10.1007/s11099-012-0032-8
Muleta D, Assefa F, Granhall U (2007) In vitro antagonism of rhizobacteria isolated from Coffea arabica L. against emerging fungal coffee pathogens. Eng Life Sci 7:577–586. https://doi.org/10.1002/elsc.200700004
Nagajyoti PC, Lee KD, Tvm S (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Navarro-Torre S, Barcia-Piedras JM, Caviedes MA, Pajuelo E, Redondo-Gómez S, Rodríguez-Llorente ID, Mateos-Naranjo E (2017) Bioaugmentation with bacteria selected from the microbiome enhances Arthrocnemum macrostachyum metal accumulation and tolerance. Mar Pollut Bull 117:340–347. https://doi.org/10.1016/j.marpolbul.2017.02.008
Noori F (2018) Mining alfalfa (Medicago sativa L.) nodules for salinity tolerant non-rhizobial bacteria to improve growth of alfalfa under salinity stress. Ecotoxicol Environ Saf 162:129–138
Pikovskaya R (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Rabindran R, Vidhyasekaran P (1996) Development of a formulation of Pseudomonas fluorescens PfALR2 for management of rice sheath blight. Crop Prot 15:715–721. https://doi.org/10.1016/S0261-2194(96)00045-2
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149. https://doi.org/10.1016/j.tibtech.2009.12.002
Raklami A, Oufdou K, Tahiri A-I, Mateos-Naranjo E, Navarro-Torre S, Rodríguez-Llorente ID, Meddich A, Redondo-Gómez S, Pajuelo E (2019) Safe cultivation of Medicago sativa in metal-polluted soils from semi-arid regions assisted by heat- and metallo-resistant PGPR. Microorganisms 7:212. https://doi.org/10.3390/microorganisms7070212
Raklami A, Oubane M, Meddich A, Hafidi M, Marschner B, Heinze S, Oufdou K (2021) Phytotoxicity and genotoxicity as a new approach to assess heavy metals effect on Medicago sativa L.: Role of metallo-resistant rhizobacteria. Environ Technol Innov. https://doi.org/10.1016/j.eti.2021a.101833
Raklami A, Tahiri A, Bechtaoui N, Abdelhay EG, Pajuelo E, Baslam M, Meddich A, Oufdou K (2021b) Restoring the plant productivity of heavy metal-contaminated soil using phosphate sludge, marble waste, and beneficial microorganisms. J Environ Sci 99:210–221. https://doi.org/10.1016/j.jes.2020.06.032
Rebello S, Sivaprasad MS, Anoopkumar AN, Jayakrishnan L, Aneesh EM, Narisetty V, Sindhu R, Binod P, Pugazhendhi A, Pandey A (2021) Cleaner technologies to combat heavy metal toxicity. J Environ Manage 296:113231. https://doi.org/10.1016/j.jenvman.2021.113231
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manage 90:1106–1116. https://doi.org/10.1016/j.jenvman.2008.04.007
Rosier A, Medeiros FHV, Bais HP (2018) Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 428:35–55. https://doi.org/10.1007/s11104-018-3679-5
Saeid A, Prochownik E, Dobrowolska-Iwanek J (2018) Phosphorus solubilization by bacillus species. Molecules 23:2897. https://doi.org/10.3390/molecules23112897
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Schindler M, Santosh M, Dotto G, Silva LFO, Hochella MF (2021) A review on Pb-bearing nanoparticles, particulate matter and colloids released from mining and smelting activities. Gondwana Res. https://doi.org/10.1016/j.gr.2021.07.011
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Sehrawat A, Sindhu SS, Glick BR (2022) Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 32:15–38. https://doi.org/10.1016/S1002-0160(21)60058-9
Shah AA, Yasin NA, Akram K, Ahmad A, Khan WU, Akram W, Akbar M (2021) Ameliorative role of Bacillus subtilis FBL-10 and silicon against lead induced stress in Solanum melongena. Plant Physiol Biochem 158:486–496. https://doi.org/10.1016/j.plaphy.2020.11.037
Shin D, Kim J, Kim B, Jeong J, Lee J (2015) Use of phosphate solubilizing bacteria to leach rare earth elements from monazite-bearing ore. Minerals 5:189–202. https://doi.org/10.3390/min5020189
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448. https://doi.org/10.1111/j.1574-6976.2007.00072.x
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment? Front Plant Sci 9:1476
Tak HI, Ahmad F, Babalola OO (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, vol. 223. Springer, New York, pp 33–52. https://doi.org/10.1007/978-1-4614-5577-6_2
Tirry N, Tahri Joutey N, Sayel H, Kouchou A, Bahafid W, Asri M, El Ghachtouli N (2018) Screening of plant growth promoting traits in heavy metals resistant bacteria: prospects in phytoremediation. J Genet Eng Biotechnol 16:613–619. https://doi.org/10.1016/j.jgeb.2018.06.004
Vassilev N, Vassileva M, Nikolaeva I (2006) Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol 71:137–144. https://doi.org/10.1007/s00253-006-0380-z
Versalovic J, Schneider M, Bruijn FJD, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol 5:25–40
Walia A, Mehta P, Chauhan A, Shirkot CK (2014) Effect of bacillus subtilis strain CKT1 as inoculum on growth of tomato seedlings under net house conditions. Proc Natl Acad Sci India Sect B Biol Sci 84:145–155. https://doi.org/10.1007/s40011-013-0189-3
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8:55–64. https://doi.org/10.1515/intox-2015-0009
Wu FY, Bi YL, Leung HM, Ye ZH, Lin XG, Wong MH (2010) Accumulation of As, Pb, Zn, Cd and Cu and arbuscular mycorrhizal status in populations of Cynodon dactylon grown on metal-contaminated soils. Appl Soil Ecol 44:213–218. https://doi.org/10.1016/j.apsoil.2009.12.008
Xiong PP (2018) Medicago sativa L. enhances the phytoextraction of cadmium and zinc by Ricinus communis L. on contaminated land in situ. Ecolog Eng 116:61–66
Yahaghi Z, Shirvani M, Nourbakhsh F, Pueyo JJ (2019) Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: role of plant growth promoting bacteria. South Afr J Bot 124:573–582
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359. https://doi.org/10.3389/fpls.2020.00359
Zhang H, Sun Y, Xie X, Kim M-S, Dowd SE, Paré PW (2009) A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J 58:568–577. https://doi.org/10.1111/j.1365-313X.2009.03803.x
Zhang M, Shao L, Jones T, Hu Y, Adams R, BéruBé K (2022) Hemolysis of PM10 on RBCs in vitro: an indoor air study in a coal-burning lung cancer epidemic area. Geosci Front 13:101176. https://doi.org/10.1016/j.gsf.2021.101176
Zulfiqar U, Farooq M, Hussain S, Maqsood M, Hussain M, Ishfaq M, Ahmad M, Anjum MZ (2019) Lead toxicity in plants: impacts and remediation. J Environ Manage 250:109557. https://doi.org/10.1016/j.jenvman.2019.109557
Acknowledgements
The authors want to thank all the persons who contributed to the achievement of this work. This work was supported by the Moroccan ministry of Higher Education and Scientific research within the frame of PPR2 2015/5 project “Utilisation des biotechnologies microbiennes et Végétales pour la réhabilitation des sites miniers abandonnés” (BOMIVER).
Funding
Mounia Bakkali Bouarrakia was granted a fellowship from the Moroccan Ministry of National Education, Vocational Training, Higher Education, and Scientific Research within the frame of the “Priority projects for scientific research and technological development, PPR2/2015/5.
Author information
Authors and Affiliations
Contributions
MBB conducted the experimentations, acquired and analyzed the data, wrote the original draft, and contributed to the final version of the paper, AE contributed to software and analyzed the data and methodology, OE contributed to methodology and analyzed the data, MHZ contributed to writing—review and methodology, AL acquired the funding, MB acquired the funding, and AA acquired the funding, supervision, and validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: J. Chen.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakkali Bouarrakia, M., Elyemlahi, A., El Galiou, O. et al. Single and dual inoculation with rhizobacteria on alfalfa (Medicago sativa L.) growth under lead stress conditions. Int. J. Environ. Sci. Technol. 20, 9767–9778 (2023). https://doi.org/10.1007/s13762-022-04669-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04669-9