Abstract
The processing and evaluation of industrial wastes together with other wastes may help material to remain in the system longer within circular economy. This study aimed to evaluate marble processing effluent physicochemical treatment sludge (K1) as catalyst in the waste poly(ethylene terephthalate)-PET pyrolysis. The effects of K1 dose (10–50%) and target temperature (300, 500, 700 °C) on the pyrolysis product yields and char characteristics were investigated. Fixed bed batch pyrolysis system was used under nitrogen atmosphere. The pyrolysis gas, oligomer and char product quantities changed in the range of 10.2–43.9, 2.42–21.9 and 23.5–83.8%, respectively. The increase in the K1 dose increased the char and decreased the liquid amount. Thermal, chemical, mineralogical characterization and proximate analyses were applied to char products. PET + K1 mixtures’ chars mainly contain calcium carbonate (CaCO3), dolomite (CaMg(CO3)2), Ca(OH)2, CaO, calcite (Mg0.03Ca0.97)(CO3). The char humidity and heat values decreased with increasing K1 doses. The residual value at 900 °C for PET waste was 14.2% while for PET + K1 mixtures it varied between 15.2 and 33.9%. Thermal degradation residual percentages of the 500 °C pyrolysis chars varied between 79.2 and 58.8%. K1 increased the thermal stability of the char, and this can provide it new areas of use. For example, when thermally stable char is needed with low pore volume and low surface area, K1 dose between 30 and 50% and the pyrolysis temperature of 500 °C can be suggested. By using K1, the variety of chars having different characteristics was obtained which transformed K1 and chars economic value as second-generation solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics, as nonbiodegradable materials, are one of the biggest contributors to the waste problem, and plastic consumption has been increasing dramatically (Xue et al. 2017; Diaz-Silvarrey et al. 2018). In European plastic consumption, poly (ethylene terephthalate)-PET is third with a share of about 16% among the most preferred polymers in the packaging industry (Nistico 2020). In Turkey, PET packaging materials occupied 23% of the eight million tons of total plastic production in 2020 (PAGEV 2020). New perspectives are required for the management of this waste in line with the principles of circular economy.
PET is completely recyclable plastic, and different routes have been followed for it: mechanical recycling (Nait-Ali et al. 2011), chemical recycling to the monomers (Crippa and Morico 2019), using it as additive (Bachtiar et al. 2020) and incinerating for energy recovery (Brems et al. 2011). Mechanical recycling is the process in which waste plastics are transformed into the lower-quality products than the original previous case (Panda et al. 2010; Sikdar et al. 2020); therefore, after a few cycles, they eventually become non-usable and nonrecyclable. Hydrolysis, glycolysis, methanolysis, and aminolysis are some examples of chemical recycling processes reported by Khoonkari et al. (2015), but initial and operating costs of these processes are higher than thermochemical conversion and needs the proper design (Norena et al. 2012). Incineration causes hazardous emissions and loose valuable material (Brems et al. 2011). Other promising technologies, such as pyrolysis or gasification of plastics, in the management of such end-of-life plastic waste should still be reviewed and re-evaluated (Dogu et al. 2021). Pyrolysis involves the thermal processing in inert atmosphere, which allows it to transform into small chain hydrocarbons, monomers and so on. A variety of useful, valuable chemicals can be recovered by pyrolysis gas, liquid and solid products. Unless recovered, these products are severe pollutants; when recovered, they are economical values; moreover, gas product has a significant calorific value (Fakhrhoseini and Dastanian 2013). When pyrolysis is applied directly to the collected unwashed waste plastics, it does not consume water and cleaning reagent, and so, it is considered as green technology.
Plastics pyrolysis can be conducted as non-catalytic (Dhahak et al. 2020; Singh et al. 2019; Al-Salem et al. 2017) and catalytic (Diaz-Silvarrey et al. 2018; Kumagai et al. 2015). Non-catalytic or thermal pyrolysis is the degradation of plastics at 350–900 °C. Plastic exhibits very low thermal conductivity, and plastic pyrolysis is highly complex since macromolecules require large amounts of energy (Yoshioka et al. 2004). During catalytic pyrolysis, a catalyst is used to change the product composition, to reduce the pyrolysis reaction temperature by lowering the reaction activation energy and/or to reduce the reaction time. The use of catalysts has shown increased selectivity towards the pyrolysis products which may then be converted to some feedstocks or fuel (Hafeez et al. 2019). The catalytic pyrolysis products characteristics are affected by the quantity and the structure of catalyst, characteristics of pyrolysed material, pyrolysis temperature, etc. Approximately 60 different types of catalysts have been proposed in the literature for the catalytic pyrolysis of plastics. These include activated alumina, carbonate-derived, zeolite, mesophore, fluid catalytic cracking (FCC) catalysts; each has different structure and characteristics (Alp, 2005; Koutinas et al.2011; Jeon et al.2012). For PET depolymerization, base catalysts, such as calcium oxide (CaO) (Kumagai et al. 2015), magnesium oxide (MgO) (Morishita et al. 2006), sodium carbonate (Na2CO3) (Shukla and Kulkarni 2002), nickel oxide (NiO), iron(III) oxide (Fe2O3) or titanium dioxide (TiO2) (Yoshioka et al. 2005), and acid catalysts, such as HZSM-5 zeolite (Xue et al. 2017) and zirconia (Diaz-Silvarrey et al. 2018), had been studied. These catalysts are produced mainly from raw materials, and there has not been any study producing catalyst directly from the waste material.
Different catalysts have different effects on the degradation mechanism. An increase in the pyrolysis char yield with some metal oxides has been reported due to the strengthening of the crosslinking of polyene structures compared to the nonmetal oxide state (Terakado and Hirasawa 2005). In the formation of pyrolysis tar and char, covalent bonds are broken at elevated temperature, and free radical species are formed by thermal cleavege reactions. The main mechanism of these reactions is free radical mechanism in which hydrogen atom is extracted from the radical chain during chain transfer, then the formed radical joins a different chain, and all these steps also affect pyrolysis product distribution (Savage 2000; Norena et al.2012). Yoshioka et al. (2005) reported that PET was firstly hydrolyzed by the effect of calcium hydroxide (Ca(OH)2) and then around 31% benzene by weight formed by decarboxylation of terephthalic acid. During the steam pyrolysis of PET with CaO and Ca(OH)2 catalysts, benzene was selectively produced with 74% yield (Grause et al. 2011a) and mixed plastics containing PET were also decomposed by using CaO and Ca(OH)2; 30–50% by weight oil was produced (Grause et al. 2011b). Another study was conducted by MgO catalyst, 22–27% pyrolysis char yield was achieved, and it was stated that char yield changed with MgO/PET ratio (Morishita et al. 2006).
The condition of the pyrolyzed PET material influences the pyrolysis product yields; for example, carbon dioxide/acetaldehyde concentration was determined as 43.93, 33.61 and 20.73% for contaminated PET flakes, recycled PET and unprocessed PET, respectively (Dimitrov et al. 2013).
Another important factor is the pyrolysis target temperature. The characteristics and quantities of each pyrolysis product fraction change with changing pyrolysis target temperature. For instance, the highest pyrolysis liquid quantity was achieved at 500 °C for the pyrolysis of mixture of plastic wastes and the increment in the temperature resulted in aromatic compound increment in the liquid (Maqsood et al. 2021). In plastic pyrolysis, optimum oil yield is reported to be obtained at 500–550 °C for non-catalytic pyrolysis, and this temperature can be reduced to 450 °C by using a catalyst (Sharuddin et al. 2016).
Char is a fraction of the pyrolysed waste material, and it can also be considered as a by-product of pyrolysis. Then new second- or third-generation solutions are needed to evaluate this product in waste management. The potential uses of char depend on the char structure which is directly related to the pyrolyzed substance and the catalyst (Lopez et al. 2011). Conventionally, it can be used as filler in construction works (Sharuddin et al. 2016), adsorbent in water treatment (Tan et al. 2015) and filler in composite matrices (Sogancioglu et al. 2017a, 2020). Recently, char is a promising substitute for traditional cathodic oxygen reduction reaction (ORR) electrocatalysts as low-cost carbon-based cathode in both actual and industrial future applications (Li et al. 2020). In air cathode production for microbial fuel cells (MFCs), chars were used in catalytic mixtures with activated carbon and these MFCs were being used as sustainable bioelectrochemical systems in wastewater treatment (Kang et al. 2017; Koo et al, 2019; Koo and Jung, 2021). Furthermore, in microbial electrolysis cells (MECs) hydrogen is produced in cathode while organic matter is decomposed in anode, and here, hydrogen is the main site for hydrogen evolution reaction which is the rate-limiting step for the MECs (Pawar et al. 2022). Pyrolysis chars are used in the catalyst layer of the cathode of MECs as conductive powder together with activated carbon (Son et al. 2021; Pawar et al, 2022). These examples indicate that, for finding new evaluation areas, the composition and physicochemical characteristics of a particular char should be investigated first.
In marble processing plants, the cutting is performed with the help of water in order to prevent the cutter from friction heat and cutting powder damage. The process effluent is the water combined with these fine marble particles (mainly CaCO3), additional components coming from the mineral content of the raw marble, other debris and some lubricating oil from the contact of water to the mechanic equipment. In marble processing plants, these effluents are being treated by physicochemical methods. In the treatment sludge, Ca structures coming from marble are adsorbed onto the surface of precipitates coming from the coagulant or buried in the flocs. The sludge is generally being stored onto the land, and besides the land occupation, there are some adverse environmental effects of it both in its wet form and after dried under ambient conditions (Cobo-Ceacero et al, 2019). In order to eliminate the adverse effects of these physicochemical treatment plant sludges, new recycling/recovery alternatives should be introduced to convert these waste materials into a product having economical value, so helping to contribute industrial symbiosis and circular economy.
The inorganic content of marble processing effluent physicochemical treatment sludges (such as Al, Fe, Mg, CaCO3) has catalytic effect on the pyrolysis of synthetic organics. It can be seen from the literature information above that oxides or carbonates of metals can be used as catalysts in PET pyrolysis. Most of them were chemically prepared under laboratory conditions either no or partially including some waste materials (Sanjeev 2017; Yadagiri et al. 2017), but there are not any studies that use the waste material itself directly as catalyst. It has been emphasized that the cost of the catalyst is as important as the time and temperature advantages. Inexpensive, even cost-free materials are strongly preferred. Physicochemical treatment sludge and especially marble processing plant sludge have not been studied as catalyst in the thermal decomposition of any plastic. Use of this sludge as catalyst in PET pyrolysis will be both economic and environmentally friendly approach, and the resulting pyrolysis products will find use in many areas. Therefore, char characteristics are important in determining the second- or third-generation use of the material in the system as a feedstock.
In this study, marble processing effluent treatment sludge was used as catalyst in the PET pyrolysis process for the first time and the effects of the catalyst dose and pyrolysis target temperature on the PET waste pyrolysis product yields and on the pyrolysis char characteristics were investigated. Effects of marble sludge on pyrolysis liquids and gas products will be presented in the second article of this series.
The remaining sections were organized as follows: Chemical, marble sludge, PET wastes, utilized pyrolysis system, the experimental approach and analytical methods were explained in the second section. The findings were presented and are discussed in Sect. 3 where first the physicochemical treatment performance and K1 production conditions were investigated. This was followed by discussion of thermal properties of PET waste and K1. Then solid, liquid and gas fraction distributions as a result of pyrolysis were compared. In the final subsection, the characteristics of obtained pyrolysis chars were investigated as char surface properties, chemical structures, mineralogical contents, thermal properties, moisture/ash content and heat values. The paper was finished with the drawn conclusions.
Materials and methods
Materials
Chemical
Alum (Al2(SO4)3.18H2O) (Merck:1.01102) was used as the coagulant in the physicochemical treatment of marble processing effluent.
Marble processing effluent physicochemical treatment sludge (K1)
Marble processing effluent samples were taken from the marble processing plant of REMAR Inc. Co., operating in Konya (Turkey). In the plant, the processing effluents (cooling water sprayed to the marble cutting equipment) are collected in a channel and transferred to the inlet of the wastewater treatment plant. The working period of the plant is 8 h per day, and average effluent flowrate of the plant is about 6.5 m3/h. The effluent sample was collected from the channel and the identical treatment was applied both in the laboratory and on the plant site to obtain the sludge sample of the same quality. The characteristics of this industrial effluent slightly vary according to the type of marble that is being processed. The collected sample was the effluent generated during travertine-type marble processing. Alum was used for physicochemical treatment of the turbid effluent. Sequential coagulation (1.5 min rapid mixing at 200 rpm), flocculation (25 min slow mixing at 15 rpm) and sedimentation (60 min under quiescent conditions) were applied under the dosage, mixing time, mixing speed, pH conditions reported in the previous study (Onen et al. 2018). At the end of the settling period, the supernatant samples were withdrawn and the final turbidity and pH were measured to investigate the physicochemical treatment performance for the marble processing effluent. The settled sludges were dried at 40–50 °C, sieved through 300-micron sieve and powdered form of marble processing effluent physicochemical treatment sludge which was prepared, and its name was abbreviated as K1. The particle size distribution of the dried and sieved K1 was determined.
Poly(ethylene)terephthalate (PET) waste plastic
PET waste used in the study consists of packaging for food, beverages, etc., disposed of as a result of domestic use. The collected wastes were separated only from the impurities and liquids, if any. Another operation has not been applied such as prewash and cleaning. The main expectation is the success of recycle products without the need for washing, in terms of being environmentally friendly and economical. Waste plastics were shredded to less than 8 mm in a laboratory scale plastic crusher with 3 rotary 2 fixed blades.
Pyrolysis system
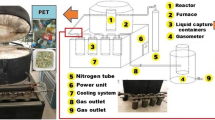
Pyrolysis was applied in laboratory-scale fixed bed batch pyrolysis system which consists of power control, heating, condensation pots and gas storage parts (Fig. 1). Nitrogen gas was used with a pressure of 150–200 bar to provide inert atmosphere. The stainless-steel reactor that is 11 cm in diameter, and 24 cm in length was placed vertically in the ceramic furnace. As the pyrolytic reaction proceeds, the fluid portion leaves the reactor and flows through the piping. There are four liquid condensation pots connected serially to capture the condensed liquids from the hot fluid phase. The gaseous part of the fluid that cannot be condensed in the pots was collected in the cylindrical gasometer with an inner diameter of 36 cm, outer cylinder diameter of 40 cm and a height of 51 cm. Pyrolysis system operating conditions were controlled with the help of the PLC control panel (Fig. 1).
Experimental
Waste PET samples were pyrolyzed in the laboratory-scale fixed bed batch pyrolysis system with and without K1 catalyst. In catalytic pyrolysis experiments, K1 powder and shredded PET wastes were mixed homogeneously in varying proportions to obtain total mass of mixture as 100 g. In each experiment, K1 and PET plastic waste samples were placed in the reactor. Pyrolysis of PET waste was studied at varying conditions as 0, 10, 20, 30 and 50% K1 dose and 300, 500 and 700 °C pyrolysis temperature with at least two replicates. In case of inconsistent results, more replicates were performed. For each test condition, the heating rate was set at 5 °C/min and the heating process was terminated after the system reached the target temperature and then the reactor was taken out from the system. The char, which is the carbon-black residue remained in the reactor, was weighed and stored in glass bottles. The liquid portions (pyrolysis oils) were collected in liquid capture pots placed in a water-cooled chamber and were weighed and then stored in glass bottles. In addition, oligomer structures accumulated in the pipes of the pyrolysis system were also collected. At the end of pyrolysis processes, product (solid, liquid, gas) yields were determined and solid, liquid and gas fractions were characterized. Within the scope of this first study in a binary article series, pyrolysis product yields were examined and the change of char characteristics were interpreted based on different pyrolysis conditions and K1 dose. The compositions and characteristic differences of liquid and gas products will be presented in the second article of this series.
Analytical methods for K1 and char characterization
Turbidity values of the marble processing effluent and treated effluent were determined at HACH DR4000 model direct reading spectrophotometer. pH measurements were performed with ThermoScientific A111 model pH meter. Dynamic light scattering (DLS) technique was applied for the characterization of particle sizes of the dried K1 powder. For the structural characterization of K1 and pyrolysis chars obtained from PET + K1 pyrolysis, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), Brunauer–Emmet–Teller (BET) surface area, moisture and ash content, thermal value analyzes were performed and the changes of the char structure with K1 dose and pyrolysis temperature were investigated by comparing the findings.
Perkin-Elmer TGA4000 Model instrument was used in TGA analysis. During the analysis, nitrogen gas was continuously fed into the device at a rate of 20 mL/min to swept the oxygen in the environment. TGA temperature and the heating rate studied in TGA analysis were 900 and 20 °C/min, respectively. SEM imaging and EDS was performed by JEOL JSM-6610 model at 15 kV acceleration voltage, SEI signal, standard filament current and probe current conditions. FTIR spectrometer (Thermo Scientific Nicolet iS5) was used to determine the chemical structures of pyrolysis chars. Spectrums were obtained with the help of KBr pellets. By using Rigaku SmartLab Model XRD instrument, XRD patterns were obtained. BET analyses were performed via Quantachrome® ASiQwin™ ©, Quantachrome Instruments v5.2 multi-point surface area and pore measurement system. Moisture and ash content was determined according to ASTM standards D3173-85 and D3174-82. Leco AC-350 model calorimeter was used to measure the calorific values of K1 and pyrolysis chars.
Results and discussion
Marble processing effluent treatment and physicochemical sludge (K1)
In the marble processing plants, the generated wastewater quantities and qualities may not be uniform and identical because of variabilities in the (a) origin of the extracted marble sample, (b) operational speed of the system, (c) properties of the cut block and (d) daily cut blocks quantity. Therefore, the initial turbidity of the sample taken for each experiment may differ. In order to prevent impacts of the factors affecting the turbidity in the wastewater, coagulation–flocculation trials were repeated with 1550 L wastewater and standard deviations of the average values of each data were calculated. In K1 production, the alum dosage was verified through several trials that were carried out with marble processing effluent (initial turbidity 4283 ± 1801 NTU) by applying 10, 20, 30, 40 and 50 mg/L alum at original pH (8.1 ± 0.1) of the wastewater which was consistent in all samples and this assures the alkali property of the sludge. At the end of coagulation–flocculation–settling, the supernatant turbidity values of wastewater were obtained as 10.5, 7.0, 8.8, 7.7 and 6.5 NTU for each alum dosage. This efficiency was consistent with the efficiencies obtained in the previous studies, which performed with the wastewater of the same plant and that referred for coagulant dose selection in this study (Onen et al. 2018). According to the studied treatment conditions, the final turbidity fixed in the range of approximately 6.5–8.8 NTU between 20 and 50 mg/L coagulant dosage. Therefore, 20 mg/L was selected as the optimum coagulant dosage in this study. This coagulant dose was applied to high volume of wastewater in the marble processing plant and treatment sludges that were used as catalyst in this study were obtained under the same conditions. Approximately 6.5 kg of dry, powdered K1 material was obtained from the physicochemical treatment of 1500 L of travertine processing wastewater. In other words, approximately 4.2 kg sludge can be obtained per m3 of travertine processing wastewater. If a medium-sized marble processing plant generates 100 m3 wastewater per day, the daily production of K1 will be around 420 kg.
Particle size distribution of the settled, dried and sieved treatment sludge samples, named K1 and marble processing effluent, is indicated in Fig. 2 based on DLS analysis. The average values of three replications for each sample indicated that particle size of both samples varies predominantly in the range of 1000–3000 nm (95–98%) and also in the range of 80–120 nm (2–5%) (Fig. 2). The particle size range of the K1 sample and the raw wastewater sample are similar, due to the dispersion of agglomerates of the K1 sample after drying and sieving.
PET waste, K1 and the PET-K1 mixture thermal properties
The thermogravimetric behavior of waste PET, K1 and PET + K1 mixtures has been investigated as it will help in interpreting the possible effect of K1 in the pyrolysis process (Fig. 3). PET is a plastic with a high melting temperature of 250 °C and begins to decompose at a temperature of approximately 350 °C. Approximately 81% of the PET degradation occurs in the range of 357–474 °C in one step and 50% of it is completed at 432 °C. When the TGA temperature reached 700 °C, the structure of the waste PET was completely degraded and the weight become constant. The detected temperatures with no mass loss for PET + 10–50% K1 were 800–870 °C. The highest mass loss was detected in the temperature range of 400–500 °C. Due to the intense aromatic structure of PET, 14.23% residue remained at 900 °C (Fig. 3).
K1 started to decompose after 557 °C, but this was very slow decomposition (mass loss does not exceed 1–2%) until 613.4 °C and only 10% of it decayed at about 750 °C. It showed decomposition in a single stage up to 850 °C, and 54.9% residual remained at 900 °C (Fig. 3). In this thermogram, a typical CaCO3 degradation profile was observed; K1 can remain stable up to 557 °C and it is thermally stable (Fig. 3). Therefore, pyrolysis temperatures up to 600 °C are the temperatures at which thermal degradation of the K1 is not expected. These indicated that when K1 will not be degraded unless it interacts with the PET waste. From the TGA findings of PET plastic and K1 mixtures, as the K1 ratio in the mixture was increased, an increase was observed in both IDT and SDT values as well as the T5, T10 and T50 temperatures due to the high thermal stability of K1. The third degradation step in the TGA curves of the mixtures of powdered K1 and waste PET samples is thought to be due to the degradation of K1 and PET residue (Fig. 3). TDT levels were slightly higher than degradation temperature of K1. There was also an increase in final residual percentages at 900 °C, and the residual value for PET was 14.2% while for PET-K1 mixtures the residue values varied between 15.2 and 33.9%. Polyesters may be subject to degradation during heating due to temperature, heating time, presence of catalysts and oxidizing agents. PET undergoes only chain end fragmentation around 300 °C, and small molecule volatile compounds are formed from this fragmentation. According to Venkatachalam et al. (2012), thermal degradation of PET occurs by intramolecular thermal degradation and chain end thermal degradation mechanisms. Thermal degradation of PET in intramolecular thermal degradation starts with the breakage of the ester link in the chain, and smaller chains are formed that terminate with carboxyl and vinyl ester groups (Fig. 4, Reaction 1). Small molecule volatile compounds, such as acetaldehyde, are formed by chain end degradation and the other mechanisms (Fig. 4, Reaction 2). At about 440 °C, 60% of the PET decomposes (Girija et al. 2005), possibly by both Reaction 1 and Reaction 2.
Holland and Hay (2002) examined PET degradation products by selecting the 350–370 °C pyrolysis temperature range and reported that the residue product contained conjugated aromatic rings (Fig. 4, Reaction 3), and aliphatic part of the PET structure was broken down into volatile compounds and the important decomposition products of PET are CO2, linear dimers, aromatic oligomers containing 2–3 monomer units, vinyl benzoate, acetaldehyde, terephthalic acid. Small cleavage products of PET include ethylene, methane, CO, benzaldehyde, toluene, 1,4-dioxane, benzoic acid and divinyl terephthalate. In addition, it was determined that aliphatic groups rather than aromatic groups degraded during thermal degradation. The degradation of PET plastic in different temperature ranges has also been investigated in the literature with some other studies. It has been stated that more than 50% of PET degrades by random chain breakage in the temperature range of 377–427 °C (Gullon et al. 2001). According to Fig. 3, PET plastic itself degrades in the range of 350–450 °C. PET plastic completes degradation above 500 °C while PET + K1 mixtures complete their degradation at about 800 °C. Therefore, thermal degradation of the plastic has been affected from the presence of K1 such that K1 in the mixture increased the thermal stability of the PET. The higher thermal resistance of K1 and lower mobility of the matrix chains may lead to this during the interaction between K1 and the plastic matrix (Awad et al. 2019). The following sections will indicate what will change in the pyrolysis products when this mixture is pyrolyzed.
PET + K1 pyrolysis product fractions
The quantities of products obtained as a result of the pyrolysis of PET plastic wastes with K1 in different proportions with a temperature range of 300–700 °C and a heating rate of 5 °C/min are given in Fig. 5. The main products of the pyrolysis process are the pyrolytic oil (tar, wax or oil), gases and a carbonaceous char (solid product) (Diaz-Silvarrey et al. 2018). The quantity of char obtained from the pyrolysis of PET without K1 was around 19–20% at 300–700 °C pyrolysis temperatures. This quantity can vary between 18 and 25% according to Shah and Jan (2014) so char quantity obtained in the study is compatible with the literature. As the pyrolysis trials were performed with a total mass of 100 g at each run, the units in grams in Fig. 5 also represent the percentages of those liquid and solid products. In our previous study, it was obtained that while there was a 20% char yield at 300 °C pyrolysis of waste PET itself, a small decrement was observed in the char product yield as the pyrolysis temperature increases and it was 18.5% at 700 °C (Sogancioglu et al. 2017b).
In the PET pyrolysis, primary degradation of the PET structure can be explained by the random breakage of ester bonds in the main chain, which create carboxylic acid and olefinic groups (Jenekhe et al. 1983). Increment in the temperature leads to increment of the bond breakage, so that the radicals become saturated with the released hydrogen and the tar yield increases; however, secondary polymerization reactions at higher temperatures can decrease the tar yields and increase the coke yields (Cit et al. 2010). The mechanisms of pyrolysis reported to be very complicated due to the existence of oxygen atom in the structure (Fig. 4) (Li 2019).
In the pyrolysis of PET + K1 mixtures, the increase in the K1 ratio in the pyrolyzed mixture mostly increases the pyrolysis char and decreases the liquid amount, but a systematic effect on the gas product volume cannot be defined. The amount of char fraction increased from 300 to 700 °C pyrolysis temperature with 10, 20 and 30% K1 (Fig. 5a). When the K1 dosage was added at 50%, the percentage of char fraction did not change significantly with the increment of the temperature due to the completed depolymerization reactions occurring. 500 °C can be considered as a breakpoint for the PET pyrolysis since the quantity of char gets higher at 500 °C and higher pyrolysis temperatures. The PET pyrolysis char is weakly acidic up to 400 °C and therefore does not completely decompose (Yucel et al. 2015). Accordingly, as can be seen from Fig. 3, PET and PET + K1 mixtures have not decomposed yet at 300 °C pyrolysis temperature. Therefore, it can be said that the pyrolysis solid products are consisting of K1 and non-depolimerized PET at 300 °C. Jouhara et al (2018) claimed that further research is required for low temperature pyrolysis from the point of view of process economy.
As K1 doses increase, the increase in the quantity of char can be associated with the presence of K1 in the pyrolysis environment. However, at 700 °C pyrolysis, since K1 itself started to decompose, proportionally less char was obtained. PET fragmentation reactions accelerate due to the both K1 amount and pyrolysis temperature at 50% K1 dose; on the other hand, K1 degradation also begins as from 613 °C. Accordingly, although the char product yield at 50% K1 dose did not change with the pyrolysis temperature, it was still higher than the other K1 doses (Fig. 5a). As temperature increased, K1 dose higher than 30% increased PET degradation. As for 50% K1 dose, catalyst activity increased and the degradation reactions accelerated.
In the pyrolysis of PET, solid products and gas products formed, pyrolysis liquid products are not expected (Sogancioglu et al. 2017b). These findings were also supported by pyrolysis studies made with a mixture of different plastic types, and it was stated that the increase in PET ratio in the mixture decreased the amount of pyrolysis liquid, while pyrolysis oil has not been observed in the pyrolysis of PET alone (Shah and Jan 2014). Similarly, in this study, pyrolysis oil was not observed in the system, but a wax-type oligomer product formed. Cyclic oligomers are the primary products in the pyrolysis of aliphatic or aromatic polyesters. However, oil formation was observed at all pyrolysis temperatures and different K1 doses in the study (Fig. 5b). It can be said that the catalytic effect of K1 on the liquid product yield is higher at low K1 doses, because as the K1 dose increases, a decrease in the total liquid product yields has been observed. While a very high quantity of liquid product was observed at a dose of 10% K1, the decrement in the quantity of pyrolysis liquids with increasing K1 dose indicates that more different decomposition reactions occurred as a result of the catalytic effect of K1.
As compared to non-catalyzed (0% K1) conditions, higher oil and oligomer products were obtained by using K1. The effect of pyrolysis temperatures on these liquid products did not give a systematic trend, but it can be followed from Fig. 5b that the liquid product quantity is the highest with 10% K1 and decreased as K1 dosage increased. The condensation temperature of the pyrolysis gases obtained at high pyrolysis temperatures also increased, and these gases could not condense at the ambient temperature of the liquid capture vessels and thereby decreasing the liquid product yields. These findings pointed out significant changes in liquid and gas product compositions, which will be presented in the second paper of this serial paper. Similar to the findings in char yields, it can be said that the 500 °C pyrolysis temperature is a change point for the liquid products as well. Although the structural deterioration starts between 300 and 500 °C, the main structure of PET remains in the char structure since the side groups attached to the main structure are broken down first (Venkatachalam et al. 2012); but, at a temperature of 500 °C, these also degrade and convert to the liquid products.
In the pyrolysis of PET + K1, it is seen that the increment in the temperature mostly increased the gas volume at all K1 doses (Fig. 5c). This increment in gases with temperature is an expected result as the increase in temperature accelerates the decomposition reactions in pyrolysis (Dwivedi et al. 2019). It can be said that mostly less gas volume was obtained when high K1 dose was used (30 and 50%). This situation can be attributed to the formation of more char at these doses due to the aromatic nature of PET and the pyrolysis reactions with the catalytic effect of K1. Within the scope of this paper, the characteristics of the char obtained from pyrolysis of waste PET with K1 are investigated in the following sections.
PET + K1 pyrolysis chars characteristics
Surface properties
SEM images of the pyrolysis chars obtained from the pyrolysis of K1, PET plastic waste and waste PET + K1 mixtures at different temperatures are indicated in Fig. 6. K1 particles are in white color as CaCO3 is the main content in the physicochemical treatment sludge of marble processing effluent, and they are irregularly shaped and non-formed particles (Fig. 6). The particles gathered loosely and form agglomerations with an irregular structure. When released into water, the aluminum sulfate (Al2(SO4)3) hydrolyzes and aluminum hydroxide (Al(OH)3) precipitates as a result of reaction with water alkalinity. For this reason, it is thought that clumping is caused by Al(OH)3 in the structure of marble processing effluent treatment sludges. EDS analysis of these particles indicated that Ca (19.25%), C (13.63%), O (65.25%) are the main components for K1 structure while Mg and Al are also observed in K1 structure coming from the other components of marble and from the coagulant dose, respectively (Table 1). On the other hand, in addition to the C (28.89%) O (26.88%) and Na (16.42%), Al, Si, S, P are the elements observed in the waste PET structure. The minor components can be associated with the contaminant residues on the waste, dyes used during production, etc. In PET pyrolysis, while there was PET fibers that did not decompose at 300 °C, these fibers were completely lost at 700 °C (Fig. 6). As the pyrolysis temperature increases, the length of the PET fibers becomes shorter, and more fragmentation occurs. There were distinct differences in the char structures of waste PET chars obtained with and without K1 according to SEM images.
In particular, although a fibrous structure is seen in the 500 °C pyrolysis chars of waste PET, the pyrolysis char of PET with 10–20% K1 at the same pyrolysis temperature has less and much smaller fibrous parts, and the char structures are generally less rough and porous. At 30–50% K1 ratios and at all pyrolysis temperatures, a fiber-like appearance has not been encountered, which reveals the catalyst effect of K1 on PET decomposition and indicates that PET is subjected to deeper fragmentation.
All the chars obtained at 300 °C pyrolysis temperature are in fact the molten state of PET with K1 whose chain end structures undergo further changes due to the catalyst effect in terms of chemical structure (Fig. 4, Reaction 2). Therefore, it would be more correct to call the solid product of 300 °C pyrolysis temperature as ‘residue’ instead of ‘char’ similar to ‘solid coal fuel’ in torrefaction of biomass. On the surface of the chars, well-developed pores with circular and irregular shapes, and even large cavities described as 'caves' in most of them, are quite high, but these varied according to the pyrolysis temperature and K1 dose (Fig. 6). It is also seen that K1 is dispersed as fine grains on the main char structure. The surface roughness of the pyrolysis chars obtained at different temperatures of PET + 10% K1 mixture was low. As the pyrolysis temperature increased, first the large pores formed, and then the char grains were fragmented by collapsing the walls between them. K1 particles were proportionally higher in 700 °C pyrolysis char, which was associated with the higher degradation of PET at high temperature. The surface roughness of PET + 20% K1 pyrolysis chars was still low, but larger pores were formed and a noticeable change in pore size was observed as the pyrolysis temperature increased (Fig. 6). PET components, which passed from plastic structure to fluid phase with pyrolysis reactions, and the presence of 20% K1 caused the formation of macro-pores on the char surface at 700 °C pyrolysis temperature. This is simply because of the removal of the material that was blocking the pore by clogging the pore entrance and thermal cracking with increasing pyrolysis temperature (Tomczyk et al.,2020). As the temperature increases, the aromatic structure becomes a graphite-like and random cross-links decrease, which cause a decrease in natural pores (Jouhara et al. 2018). The radius of the pores, especially at 20–30% K1 doses, showed a great increase at 700 °C pyrolysis temperature and was measured in the interval of 414 and 497 Å, while in the range of 27–65 Å in other pyrolysis temperatures. The surface area of the chars obtained at 500 (135.8 ± 19.86 m2/g) and 700 °C (102 ± 9.84 m2/g) pyrolysis temperatures with 20% K1 have higher surface area as compared to other studied K1 doses. With the increasing K1 doses, the char surface area values were all remained below 44 ± 5.46 m2/g at 30% K1 dose and 500 °C pyrolysis temperature. Likewise to SEM images, these results also showed that the surface area was significantly reduced compared to pyrolysis chars of PET + 20% K1. As the K1 percentage in the mixture increases, the micropore volume in chars reduced and almost nonexistent. This can be attributed that the K1 particles settling in the char structure at high temperatures may block the pores. Surface images of pyrolysis chars obtained at different temperatures of PET + 50% K1 mixtures gave similar findings and differences were observed from the observations at lower K1 doses (Fig. 6). Although the roughness at this dose was higher than that observed at previous low doses, it was found that there was a change in both the visual size of the rough pieces and the number and size of the pores observed on the SEM images. This observation can be interpreted as even if the K1 ratio is high at 300 °C, K1 has not decomposed and some of it is trapped between the pores opened by the decomposition of PET at high temperature in the pyrolysis reactor. Some portion of K1 remained on the char surface of PET + K1 chars of the 500 °C pyrolysis. This situation may reduce the catalytic effect of K1 in the fixed bed system at pyrolysis temperatures 500 °C and above. Especially, the detail in Fig. 6, which was from the SEM image of the chars obtained at 700 °C with 50% K1 dose, showed this situation quite clearly. Depending on this situation, since the mass in the reactor at constant temperature reacts from outside to inside with the increasing pyrolysis temperature, the gas formation is not linear and the buried K1 affects the amount of gas collected. This might be the reason that as the K1 dose increases, the amount of gas and liquid product decreases unlike the increasing char product.
Chemical bond structures
FTIR spectra of the samples were used to determine the functional groups and the aromatic/aliphatic status of the structures of char products obtained from PET + K1 pyrolysis (Fig. 7). The bands at 712, 876, 1423–1425, 1796 and 2516 cm− 1 in FTIR spectra are the bands belonging to carbonate (calcite) and other components belonging to travertine structure in K1. In addition, in the region of 3434–3447 cm− 1, O–H stretching infrared water bands due to moisture has been seen (Ross 1974).
Al(OH)3, which is present in the K1, transforms to partial alumina, than to boehmite and finally to χ-Al2O3. Boehmite (γ-AlO(OH)) phase forms between 250 and 400 °C (Bhattacharya et al. 2004). The FTIR spectra show characteristic vibrations for boehmite at 405, 505, 755, 875, 1095–1097, 1149–1150, 1369–1371, 3056 cm− 1 (Boumaza et al. 2009) for 300 °C pyrolysis temperature. At 542 °C, the γ-AlO(OH) turned into γ-Al2O3 (Redaoui et al. 2016). Absorption peaks between 430 and 860 cm− 1 wave numbers are related to the stretching vibrations of Al–O (Naayi et al. 2018). Bands at 712, 876, 1793 and 2516 cm− 1, which are common in FTIR spectra of all chars obtained from pyrolysis of PET + K1 mixtures, proved that K1 is completely or partially taken back in the char. Similar bands were detected for all pyrolysis temperatures at 10–50% K1 doses (Fig. 7).
In the FTIR spectrum of the residue obtained at 300 °C pyrolysis temperature, outside the bands seen for K1, disubstituted benzenes with carbonyl groups at 756 cm− 1, C(O)–O stretch of the ester group at 1281 cm− 1, trans-ethylene glycol segment at 1365 cm− 1, aromatic CH2 streching band at 1423 cm− 1, C=C stretching of the aromatic phenyl core at 1599 cm− 1, aromatic acid C=O of terephthalic acid at 1693 cm− 1, acid O–H stretching at 2526 cm− 1, symmetrical C–H stretch at 3051 cm− 1 and diethylene glycol O–H bands around 3450 cm− 1 were observed. This indicates that most of the PET chain structure remains unbroken at 300 °C pyrolysis. When the pyrolysis temperature increased, the bands at 754, 1284, 1365, 1693 and 2546 cm− 1 shrunk, the band at 1705 cm− 1 almost disappeared, and no other new bands have been seen. This indicates that more volatile compounds were formed by the breakage of the PET chain, but there were very few compounds containing some terephthalic acid-based groups in the pyrolysis solid product. Catalytic degradation reactions occur with two mechanisms according to Norena et al. (2012). The first one is carbon atom protonation in the polymer chain with Brønsted acid protons, while the second mechanism is withdrawal of hydride ion from the polymer chain by Lewis acid sites (Norena et al.2012). In the FTIR spectra of chars obtained at 500–700 °C pyrolysis temperatures, the bands at 1705, 1693, 1365 and 758 cm− 1 disappeared, and the other bands remained the same. These results showed that the chars have an aromatic structure. In general, the aromatic structure is the char forming group and the more aromatic content, the more compact or larger amount of char (Wu et al. 2018). K1 contains large amount of CaCO3 which is a base compound and it can absorb acids, which can finally produce aromatic hydrocarbons throuch decarboxylation reaction (Lim and Kim 2019). The bands belonging to groups, such as ester and carbonyl, thoroughly downsizing at 500 °C and above pyrolysis temperatures, indicated that the chain structure of PET plastic deteriorates more as the pyrolysis temperature increases at these temperatures in the presence of K1.
Mineralogical contents of the chars
While PET wastes are being pyrolyzed with K1, it was expected that not only organic carbon bonded structures but also K1 would be completely or partially present in the structures of char products. Therefore, after determining the chemical structures with FTIR, the presence of K1 was confirmed in char structures with another analysis. Depending on the crystallographic properties of the solid materials and the specific atomic sequences of the contained phases, XRD patterns were obtained (Fig. 8). In the literature the following diffraction peaks at 2θ degrees were assigned to PET: (0–11) = 16.3°, (1–11) = 27.65°, (010) = 17.5°, (100) = 26.1°, (110) = 22.7° and (− 111) = 21.5° (Mayoral et al. 2013; Alexiou et al. 2020). In the XRD patterns of PET + 10% K1, approximately 2θ = 18.8° (NiSeO4.6H2O) at 300 °C pyrolysis char, the peaks around 21.7° (C3H4O)n and 25.7° (C6H6N2S2) thought to be caused by the chemicals in the waste PET, were not observed, probably due to the degradation of these compounds with the increasing pyrolysis temperature (Fig. 8a). It was determined from the TGA curve of PET that most of the degradation takes place between 357 and 474 °C, and these findings are also supported by XRD results. In the other studied K1 ratios, the peak observed at approximately 21.7° (2θ) (CH2CH2CH2O) for the 300 °C pyrolysis char was not observed with the increasing pyrolysis temperature (Fig. 8b–d). For all studied K1 percentages, CaCO3 and CaMg (CO3)2 peaks were observed in the XRD pattern of the char structure obtained at 300 °C pyrolysis temperature, while only CaCO3 peaks were observed in 500 °C pyrolysis chars due to the degradation of MgCO3. When the pyrolysis temperature increased to 700 °C, CaCO3 began to decompose. Therefore, Ca(OH)2 peaks were determined around 18°-29°-34°-47°-51°(2θ) and CaO peaks around 32°–37,5°–54°(2θ) (Fig. 8a–d). Park et al. (2019) indicated that at higher temperatures (> 650°) in the presence of CaO, phenols, acids, carbonyls are converted to stable aromatic hydrocarbons, ketones and CaCO3. Kumagai et al (2018) performed the catalytic pyrolysis of PET over CaO catalysts with different basicity. CaO with strong basicity provides higher aromatic hydrocarbons formation during the catalytic pyrolysis of PET; it also decreases the reaction temperature and enhances decarboxylation reaction (Kumagai et al. 2018). Therefore, CaCO3 decomposes to CaO and CO2 at the temperatures higher than 650°. It can be inferred that K1-catalyzed PET pyrolysis at the temperature > 600 °C can produce aromatic hydrocarbons and may increase the overall lifetime of K1 (Park et al, 2019; Lim and Kim, 2019).
As a result of matching the XRD patterns with the library scanning of the instrument, the possible components of each pattern were determined and compared in Table 2. The XRD pattern of K1 basically represents calcite and dolomite structures. Pyrolysis chars of PET + K1 mixtures mainly contain calcium carbonate (CaCO3), Ca(OH)2, dolomite (CaMg(CO3)2), CaO, calcite (Mg0.03Ca0.97) (CO3) (Table 2). CaCO3 starts to decompose at 700 °C, and it completely decomposes above 850 °C. MgCO3 and KNO2 degradation occurs at temperatures of approximately 500 °C. For this reason, the presence of (Mg0.03Ca0.97) (CO3) disappeared at pyrolysis temperatures of 500 °C, and above, and KNO2 was not found any. On the other hand, minor structures likely to be in the structure are mostly organic components (Table 2). The number of these is higher in all K1 doses at 300 °C pyrolysis chars, whereas the possible minor compounds were identified only in pyrolysis chars obtained at 500 °C and higher pyrolysis temperatures with 10% K1 dose. These observations support the explanation that as the K1 dose and pyrolysis temperature increased, the organic structure had gradually broken down and passed into the fluid phase from char structures.
Thermal properties of the chars
In the findings presented so far, surface properties, chemical bond structures, and mineralogical contents of pyrolysis chars have been explained, and the thermogravimetric properties of the chars were determined by TGA (Fig. 9). Although the thermal stability of the PET waste + 10% K1 dose mixture is slightly higher than PET waste in the range of 400–700 °C degradation temperature (Fig. 3), it was observed that the difference between the thermal stability of PET + 10% K1 and PET waste chars decreases as the pyrolysis temperature increases (Fig. 9). In addition, the degradation of PET + 10% K1 char increased above 600 °C and the residue percentage at 900 °C was close to waste PET char (Figs. 3 and 9). This indicates that K1 has a catalytic effect on the degradation of PET. The char obtained from pyrolysis at 300 °C had the lowest thermal stability, the initial degradation phase started at approximately 300 °C, and the secondary degradation phase continued at approximately 590 °C and completed at 800 °C. Thermal stability of 300 °C pyrolysis chars was much lower than other pyrolysis temperatures for all K1 doses. At 20 and 30% K1 doses, the residual percentage of 300 °C pyrolysis chars was close to that of K1, and at 50% dose it was found below the K1 residue (Fig. 9). At the same pyrolysis temperature, the decrease in the char residue percentages with the increase in the K1 dose showed that the catalyst effect still continued in the chars. The higher pyrolysis temperature resulted in increased aromaticity through the separation of non-aromatic fraction depending on the increased relative portion of the K1 in the char (Fig. 4, Reaction 3). This can be the reason for higher percentage of final TGA residues of chars with increasing pyrolysis temperature (Fig. 9). A remarkable situation for the 300 °C pyrolysis temperature is that the 900 °C residual percentage of PET pyrolysis char without K1 was 69.9%. Thermal degradation residual percentages of 300 °C pyrolysis temperature PET waste pyrolysis chars which varied between 68.5 and 47.8%, decreased with increasing K1 dose from 10 to 50% (Fig. 9). This situation may be explained as follows: All solid products obtained at different K1 doses at 300 °C pyrolysis temperature are actually K1 and molten or partially degraded form of PET (PET chain structure has changed at its ends, probably under the influence of catalyst), and thermal decomposition of char obtained at this pyrolysis temperature continues with K1 effect. Accordingly, the inference of the previous discussions is further strengthened with TGA findings, that is, (a) complete pyrolysis does not occur in PET and char does not form at 300 °C pyrolysis temperature, and (b) PET is only exposed to some chain end degradation according to Reaction 2 (Fig. 4); therefore, the process at 300 °C is not actually complete pyrolysis for PET.
For all chars obtained at 300–700 °C pyrolysis temperatures, the residual percentage at 900 °C varied between 68.5 and 81.0% in the presence of 10% K1, and the increase in these values with increasing pyrolysis temperature indicates the formation of char with higher thermal resistance at high pyrolysis temperature. The residual percentages of chars were lower than the PET pyrolysis char without K1. It can be seen from the TGA thermograms that the K1 dose has almost the same effect on char degradation at the same temperature. Regardless of 300 °C pyrolysis temperature, the thermal stability data of chars obtained at 500–700 °C pyrolysis temperatures indicated that the main thermal degradation of the chars generally started after 600 °C and T50 temperatures did not observed in TGA data for all K1 doses (Fig. 9). Thermograms are very close to each other. The systematic decrease in the sample mass loss at TGA temperatures between 600 and 900 °C belongs to the K1 in the system. The T5 and T10 values of 300 °C chars showed a decrease as the K1 dose increased. This can be attributed to the catalytic effect of K1 during pyrolytic degradation of waste PET, and this effect resulted in the changed char structure. On the contrary, the T5 and T10 values of chars obtained over 300 °C a slightly increased with the K1 dose. In addition, these values for the 500 and 700 °C pyrolysis chars were found to be close to each other, indicating that PET pyrolysis was completed at 500 °C. At the pyrolysis temperature of 700 °C, the bands of some groups, such as ester, alcohol and acids, determined for chars shrink as it was observed from FTIR spectra (Fig. 7). This was resulted in separation of volatile fraction from the char structure during pyrolysis and dependingly increased the thermal stability of the char as higher T5 and T10 temperatures (Fig. 9). In other words, pyrolysis chars obtained at high pyrolysis temperatures became more inert, and richer aromatic in structure (Fig. 7), and thus the amount of degradable groups decreased further and the thermal stability increased. This was the case for all K1 doses. Change of T5 and T10 temperatures obtained with the increase in K1 dose was similar. Non-detection of 50% degradation (T50) in chars showed that chars have very high thermal stability. There was no SDT observed for TGA analyses of the chars obtained at 500 and 700 °C pyrolysis temperatures, and higher IDT levels were observed for K1 doses 20% and above. Therefore, it can be concluded that the catalyst dose between 30 and 50% and the pyrolysis temperatures between 500 and 700 °C are convenient in terms of thermal stability for chars, but considering the energy costs for higher pyrolysis temperatures, 500 °C can be suggested as more appropriate pyrolysis temperature for catalytic pyrolysis of PET with K1. Moreover, as the K1 dose increased, there was a general decrease in the final residual values consisting of chars again due to the same reason, and PET hereby creates pyrolysis gas and liquid products, whose compositions are discussed in the second paper of this serial.
Moisture/ash content and heat values of the chars
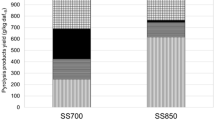
Finally, the moisture, ash content and the heat values of the chars were characterized (Table 3 and Fig. 10, respectively). The moisture content of K1 itself is almost zero. Accordingly all the pyrolysis solid products contain moisture below 1% which is an expected result. The moisture content of the pyrolysis chars of PET + K1 mixtures was lower compared to the chars obtained without K1 for all pyrolysis temperatures (Table 3). A systematic moisture trend between increasing K1 dose and char moisture content cannot be said for 300 °C pyrolysis products, as this product is not considered as char due to the incomplete pyrolysis reaction at 300 °C for PET + K1. For 500 and 700 °C pyrolysis conditions, the char humidity decreased with increasing K1 doses.
The pre-pyrolysis calorific value of the raw pet sample was determined as 6070 cal/g, and the ash content was determined as 0.1%. The ash content and thermal values of the char patterns were in conformity with each other (Fig. 10). The non-pyrolysed samples in Fig. 10 represent the PET samples mixed with K1 at 0 to 50% doses. The same ash and heat value analyses were performed on these samples. As expected, while ash content increased with increasing K1 dose, the thermal value decreased in the char. The ash content of the patterns was analyzed at 750 °C which was decided by looking at the degradation temperatures of the chars from the TGA thermograms. This temperature was also the temperature at which K1 structure is in pyrolytic degradation. For all studied K1 doses, as pyrolysis temperature increased, the higher char ash content was obtained and all ash contents were higher than that of non-pyrolysed samples (Fig. 10a). This finding was similar to the final residue data of the TGA results (Fig. 9), but the quantities of residues were higher than ash quantities (Fig. 10a). The ash analysis was performed in the muffled furnace in which the organic phases in the sample structure combusts and the residue were obtained in an oxygenated environment, whereas a kind of pyrolysis takes place under the nitrogen atmosphere to degrade the components pyrolytically in the TGA. This difference affects the final product quality and quantity and ensures that the structure of the residues mentioned in Fig. 9 and the ash structures in Fig. 10a are not identical. The variation in ash contents and the residual amounts with the K1 dose results from this situation.
The thermal values of the pyrolysis chars of waste PET without K1 were above 6000 cal/g, which was the highest of all studied conditions. On the other hand, the heat value of K1 was 7 ± 0.8 cal/g, which can be considered as no heat value for the catalyst. Therefore, heat values of the pyrolysis chars of PET + K1 decrease with the increasing K1 dose (Fig. 10b). Comparison of heat values of non-pyrolyzed and 300 °C pyrolysed samples confirmed the incomplete pyrolysis reactions of PET + K1 at 300 °C. The heat values of raw mixture were close but slightly different from non-pyrolysed samples, especially which contain K1. The pyrolysis temperature had slight effects on the thermal values of char samples (Fig. 10b). During the pyrolysis process, the organic structures are separated from the initially pyrolysed sample by pyrolytic reactions and enter the fluid phase. These will increase as the pyrolysis temperature increases, so there will be fewer organic structures in the remaining char. This is the reason of lower thermal values. The thermal values of pyrolysis chars obtained with 30 and 50% K1 dose were close to each other, indicating that the increasing effect of K1 doses above 30% will slow down. Similarly, the increase in ash content of chars at K1 doses from 10 to 30% is higher than the ash content difference between 30 and 50% chars. Accordingly, it can be said that the effect of K1 dose up to 30% can be more differentiated as compared to the effect of 30 and 50% K1. Generally, pyrolysis char is considered as the process waste while pyrolysis liquid and gas products were mainly targeted for energy and feedstock recovery. Apart from the energy recovery, the char can be evaluated for number of other purposes, and when it will be used as, for example, a filler material, high ash content and low heat value will be an advantage. From this point of view, the pyrolysis chars obtained with 30 to 50% K1, and at 500 °C pyrolysis temperature can be preferred for such second-generation purposes within the scope of waste recovery and circular economy.
Conclusion
Recent approaches through circular economy suggest the symbiotic utilization of two or more different wastes in one common solution on the production of new generation recycled materials and/or the recovery of valuable components. Accordingly, in this study, marble processing effluent physicochemical treatment sludge (K1) was used for the first time as a catalyst in PET waste pyrolysis process. The motivation of the study was that K1 contain inorganics, such as Al, Fe, Mg and CaCO3, coming from both the marble rock structure and the physicochemical treatment chemicals, and these inorganics would have catalytic effect on the pyrolysis of synthetic organics. Moreover, using K1 as a catalyst in PET pyrolysis process will contribute not only solving waste problem but also serving economic and environmentally friendly approach. Marble processing process produce about 52 m3/day effluent and about 4.2 kg sludge is produced from the treatment of each cubic meter of the effluent. Therefore, daily sludge production is about 218.4 kg. This indicates that when this sludge is disposed to the environment, it will have land occupation problem as well as environmental pollution. However, when evaluated as a second-generation solution, it will become an economical value. This study indicated that that sludge can be used in plastic pyrolysis as catalyst. Generally, pyrolysis char is considered as the process waste while pyrolysis liquid and gas products were mainly targeted for energy and feedstock recovery.
In catalytic pyrolysis process, both quality and quantity of solid, liquid and gas end products change based on the catalyst ratio and the pyrolysis conditions. Therefore, 10–50% K1 doses were applied at different pyrolysis target temperatures (300–700 °C). PET plastic itself degrades in the range of 350–450 °C. K1 increased the thermal stability of PET as it did not decompose up to 613.4 °C and only 10% of it decayed at 750 °C. As a thermally stable material, some of the K1 is trapped between the char pores opened by the decomposition of PET at high pyrolysis temperature. With the increasing pyrolysis temperature, the gas formation is not linear and the buried K1 affects the amount of gas collected. As the K1 dose increases the char product increases. At 300 °C pyrolysis temperature, PET is only exposed to some chain end degradation complete pyrolysis does not occur and char does not form; therefore, this study indicated that the process at 300 °C cannot be considered as complete pyrolysis for PET.
PET pyrolysis alone had not produce pyrolysis liquid, whereas liquid product formation was observed during the pyrolysis of PET with K1. Increment of pyrolysis temperature resulted in gas volume increment for the most K1 doses. PET fibers did not decompose at 300 °C, whereas they were completely lost at 700 °C indicating that PET was subjected to deeper fragmentation especially with 30–50% K1 doses. K1 is completely or partially present in char structures and the K1 containing chars obtained at 500 and 700 °C pyrolysis temperatures were thermally more stable. 500 °C can be suggested as more appropriate pyrolysis temperature for catalytic pyrolysis of PET with K1.
Moisture content and the calorific value of the pyrolysis chars decreased while the ash content increased with increasing K1 doses. Generally, pyrolysis char is considered as the process waste while pyrolysis liquid and gas products were mainly targeted for energy and feedstock recovery and the evaluation of the chars are comparatively rare. Commonly their adsorbent properties, fuel values, additive effects, utilization in cathodic electrode production, utilization in compost or composite had all been studied. The char obtained in this study can be evaluated for number of purposes considering the high ash content and low heat value as an advantage. As a recommendation for further studies from this point of view, the pyrolysis chars obtained with 30–50% K1 and at 500 °C pyrolysis temperature can be preferred for such second generation purposes within the scope of waste recovery and circular economy. This study introduced marble processing effluent physicochemical treatment sludge as catalyst for PET-type plastic pyrolysis. The investigated product fractions and char properties were those obtained in fixed bed batch pyrolysis system. Further study may be performed with PET + K1 pyrolysis char obtained in fluidized bed and/or continuous pyrolysis system.
It is obvious that K1 influenced the yields of PET pyrolysis products. This study discussed the effects on pyrolysis char structures, and these findings revealed that K1 may also have effects on pyrolysis liquid and gas products compositions which are reported at the second paper of this series.
References
Alexiou VF, Mathioudakis GN, Andrikopoulos KS, Beobide AS, Voyiatzis GA (2020) Poly(ethylene terephthalate) carbon-based nanocomposites: a crystallization and molecular orientation study. Polymers 12:1–14. https://doi.org/10.3390/polym12112626
Alp E (2005) Transalkylation and Disproportionation of Aromatic Compounds on Zeolite Catalysts. Ankara University, Institute of Science and Technology, Department of Chemical Engineering, Master's Thesis, Ankara, 155s
Al-Salem SM, Antelava A, Constantinou A, Manos G, Dutta A (2017) A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J Environ Manag 197:177–198. https://doi.org/10.1016/j.jenvman.2017.03.084
Awad AH, El-Wahab AAA, El-Gamsy R, Abdel-latif MH (2019) A study of some thermal and mechanical properties of HDPE blend withmarble and granite dust. Ain Shams Eng J 10:353–358. https://doi.org/10.1016/j.asej.2018.08.005
Bachtiar E, Mustaan JF, Artayani M, Tahang RMJ, Setiawan A, Ihsan M (2020) Examining polyethylene terephthalate (PET) as artificial coarse aggregates in concrete. Civil Eng J 6:2416–2424. https://doi.org/10.28991/cej-2020-03091626
Bhattacharya IN, Das SC, Mukherjee PS, Paul S, Mitra PK (2004) Thermal decomposition of precipitated fine aluminium trihydroxide. Scand J Metall 33:211–219. https://doi.org/10.1111/j.1600-0692.2004.00686.x
Boumaza A, Favaro L, Ledion J, Sattonnay G, Brubach JB, Berthet P, Huntz AM, Roy P, Tetot R (2009) Transition alumina phases induced by heat treatment of boehmite: an X-ray diffraction and infrared spectroscopy study. J Solid State Chem 182:1171–1176. https://doi.org/10.1016/j.jssc.2009.02.006
Brems A, Baeyens J, Vandecasteele C, Dewil R (2011) Polymeric cracking of waste polyethylene terephthalate to chemicals and energy. J Air Waste Manag Assoc 61:721–731. https://doi.org/10.3155/1047-3289.61.7.721
Cit I, Sinag A, Yumak T, Ucar S, Misirlioglu Z, Canel M (2010) Comparative pyrolysis of polyolefins (PP and LDPE) and PET. Polym Bull 64(8):817–834. https://doi.org/10.1007/s00289-009-0225-x
Cobo-Ceacero CJ, Cotes-Palomino MT, Martínez-García C et al (2019) Use of marble sludge waste in the manufacture of eco-friendly materials: applying the principles of the Circular Economy. Environ Sci Pollut Res 26:35399–35410. https://doi.org/10.1007/s11356-019-05098-x
Crippa M, Morico B (2019) Chapter 12—PET depolymerization: a novel process for plastic waste chemical recycling. In: Basile A, Centi G, De Falco M, Iaquiniello G (eds) Studies in surface science and catalysis. Catalysis, green chemistry and sustainable energy, vol 179. Elsevier, Amsterdam (The Netherlands), pp 215–229. https://doi.org/10.1016/B978-0-444-64337-7.00012-4
Dhahak A, Grimmer C, Neumann A, Rüger C, Sklorz M, Streibel T, Zimmermann R, Mauviel G, Burkle-Vitzthum V (2020) Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques. Waste Manag 106:226–239. https://doi.org/10.1016/j.wasman.2020.03.028
Diaz-Silvarrey LS, McMahon A, Phan AN (2018) Benzoic acid recovery via waste poly(ethylene terephthalate) (PET) catalytic pyrolysis using sulphated zirconia catalyst. J Anal Appl Pyrolysis 134:621–631. https://doi.org/10.1016/j.jaap.2018.08.014
Dimitrov N, Kratofil Krehula L, Pticek Siroci A, Hrnjak-Murgic Z (2013) Analysis of recycled PET bottles products by pyrolysis-gas chromatography. Polym Degrad Stabil 98:972–979. https://doi.org/10.1016/j.polymdegradstab.2013.02.013
Dogu O, Pelucchi M, Van de Vijver R, Van Steenberge PHM, D’hooge DR, Cuoci A, Mehl M, Frassoldati A, Faravelli T, Van Geem KM (2021) The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog Energy Combust Sci 84:100901/1-100901/69. https://doi.org/10.1016/j.pecs.2020.100901
Dwivedi P, Mishra P, Mondal MK, Srivastava N (2019) Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 5:1–15. https://doi.org/10.1016/j.heliyon.2019.e02198
Fakhrhoseini SM, Dastanian M (2013) Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J Chem 2013:1–5. https://doi.org/10.1155/2013/487676
Girija BG, Sailaja RRN, Madras G (2005) Thermal degradation and mechanical properties of PET blends. Polym Degrad Stabil 90:147–153. https://doi.org/10.1016/j.polymdegradstab.2005.03.003
Grause G, Handa T, Kameda T, Mizoguchi T, Yoshioka T (2011a) Effect of temperature management on the hydrolytic degradation of PET in a calcium oxide filled tube reactor. Chem Eng J 166:523–528. https://doi.org/10.1016/j.cej.2010.11.010
Grause G, Matsumoto S, Kameda T, Yoshioka T (2011b) Pyrolysis of mixed plastics in a fluidized bed of hard burnt lime. Ind Eng Chem Res 50:5459–5466. https://doi.org/10.1021/ie102412h
Gullon IM, Esperanza M, Font RJ (2001) Kinetic model for the pyrolysis and combustion of poly-(ethylene terephthalate) (PET). J Anal Appl Pyrolysis 58–59:635–650. https://doi.org/10.1016/S0165-2370(00)00141-8
Hafeez S, Pallari E, George Manos G, Constantinou A (2019) Catalytic conversion and chemical recovery. In: Al-Salem SM (ed) Plastics to energy. William Andrew Elsevier, Amsterdam, Netherlands, pp 147–172. https://doi.org/10.1016/B978-0-12-813140-4.00006-6
Holland BJ, Hay JN (2002) The thermal degradation of PET and analogous polyesters measured by thermal analysis-fourier transform infrared spectroscopy. Polymer 43:1835–1847. https://doi.org/10.1016/S0032-3861(01)00775-3
Jenekhe SA, Lin JW, Sun B (1983) Kinetics of the thermal degradation of polyethylene terephthalate. Thermochim Acta 61:287–299. https://doi.org/10.1016/0040-6031(83)80283-4
Jeon M-J, Kim S-S, Jeon J-K, Park SH, Kim JM, Sohn JM, Lee S-H, Park Y-K (2012) Catalytic pyrolysis of waste rice husk over mesoporous materials. Nanoscale Res Lett 7(1):18
Jouhara H, Ahmad D, van den Boogaert I, Katsou E, Simons S, Spencer N (2018) Pyrolysis of domestic based feedstock at temperatures up to 300 °C. Therm Sci Eng Prog 5:117–143. https://doi.org/10.1016/j.tsep.2017.11.007
Kang H, Jeong J, Gupta PL, Jung SP (2017) Effects of brush-anode configurations on performance and electrochemistry of microbial fuel cells. Int J Hydrogen Energy 42(45):27693–27700
Khoonkari M, Haghighi AH, Sefidbakht Y, Shekoohi K, Ghaderian A (2015) Chemical recycling of PET wastes with different catalysts. Int J Polym Sci 2015:124524/1-125524/11. https://doi.org/10.1155/2015/124524
Koo B, Jung SP (2021) Improvement of air cathode performance in microbial fuel cells by using catalysts made by binding metal-organic framework and activated carbon through ultrasonication and solution precipitation. Chem Eng J 424:130388
Koo B, Lee SM, Oh SE, Kim EJ, Hwang Y, Seo D, Jung SP (2019) Addition of reduced graphene oxide to an activated-carbon cathode increases electrical power generation of a microbial fuel cell by enhancing cathodic performance. Electrochim Acta 297:613–622
Koutinas A, Papanikolaou S (2011) Handbook of biofuels production–processes and technologies. Woodhead Publishing Limited, Abington Hall, Granta Park, Great Abington
Kumagai S, Hasegawa I, Grause G, Kameda T, Yoshioka T (2015) Thermal decomposition of individual and mixed plastics in the presence of CaO or Ca(OH)2. J Anal Appl Pyrolysis 113:584–590. https://doi.org/10.1016/j.jaap.2015.04.004
Kumagai S, Yamasaki R, Kameda T, Saito Y, Watanabe A, Watanabe C, Teramae N, Yoshioka T (2018) Aromatic hydrocarbon selectivity as a function of CaO basicity and aging during CaO-catalyzed PET pyrolysis using tandem μ-reactor-GC/MS. Chem Eng J 332:169–173
Li S, Ho SH, Hua T, Zhou Q, Li F, Tang J (2020) Sustainable biochar as an electrocatalysts for the oxygen reduction reaction in microbial fuel cells. Green Energy Environ. https://doi.org/10.1016/j.gee.2020.11.010
Li S PET recycling via gasification - Influence of operating conditions on product distribution Master’s thesis in Nordic master in Innovative Sustainable Energy Engineering, Department of Space, Earth and Environment, Chalmers University of Technology, Gothenburg, Sweden 2019, 72 pages (2019)
Lim S, Kim YM (2019) Catalytic pyrolysis of waste polyethylene terephthalate over waste concrete. Appl Chem Eng 30(6):707–711. https://doi.org/10.14478/ace.2019.1084
López A, de Marco I, Caballero BM, Laresgoiti MF, Adrados A, Torres A (2011) Pyrolysis of municipal plastic wastes II: influence of raw material composition under catalytic conditions. Waste Manag 31:1973–1983. https://doi.org/10.1016/j.wasman.2011.05.021
Maqsood T, Dai J, Zhang Y, Guang M, Li B (2021) Pyrolysis of plastic species: a review of resources and products. J Anal Appl Pyrolysis 159:105295
Mayoral B, Hornsby PR, McNally T, Schiller TL, Jack K, Martin DJ (2013) Quasi-solid state uniaxial and biaxial deformation of PET/MWCNT composites: structural evolution, electrical and mechanical properties. RSC Adv 3:5162–5183
Morishita T, Soneda Y, Tsumura T, Inagaki M (2006) Preparation of porous carbons from thermoplastic precursors and their performance for electric double layer capacitors. Carbon 44:2360–2367. https://doi.org/10.1016/j.carbon.2006.04.030
Naayi SA, Hassan AI, Salim ET (2018) FTIR and X-ray Diffraction analysis of Al2O3 nanostructured thin film prepared at low temperature using spray pyrolysis method. Int J Nanoelectron Mater 11:1–6
Nait-Ali LK, Colin X, Bergeret A (2011) Kinetic analysis and modelling of PET macromolecular changes during its mechanical recycling by extrusion. Polym Degrad Stabil 96:236–246. https://doi.org/10.1016/j.polymdegradstab.2010.11.004
Nistico R (2020) Polyethylene terephthalate (PET) in the packaging industry. Polym Test 90:106707. https://doi.org/10.1016/j.polymertesting.2020.106707
Noreña L, Aguilar J, Mugica V, Gutiérrez M, Torres M (2012) Materials and methods for the chemical catalytic cracking of plastic waste, material recycling-trends and perspectives, Dr. Dimitris Achilias (Ed.), ISBN: 978-953-51-0327-1, InTech. http://www.intechopen.com/books/materialrecycling-trends-and-perspectives/materials-and-methods-for-the-chemical-catalytic-cracking-of-plastic-waste
Onen V, Beyazyuz P, Yel E (2018) Removal of turbidity from travertine processing wastewaters by coagulants, flocculants and natural materials. Mine Water Environ 37:482–492. https://doi.org/10.1007/s10230-017-0499-4
PAGEV (2020) 3. www.pagev.org
Panda AK, Singh RK, Mishra DK (2010) Thermolysis of waste plastics to liquid fuel: a suitable method for plastic waste management and manufacture of value added products—a world prospective. Renew Sustain Energy Rev 14(1):233–248. https://doi.org/10.1016/j.rser.2009.07.005
Park YK, Jung J, Ryu S, Lee HW, Siddiqui MZ, Jae J, Watanabe A, Kim YM (2019) Catalytic co-pyrolysis of yellow poplar wood and polyethylene terephthalate over two-stage calcium oxide-ZSM-5. Appl Energy 250:1706–1718
Pawar AA, Karthic A, Lee S, Pandit S, Jung SP (2022) Microbial electrolysis cells for electro-methanogenesis: materials, configurations and operations. Environ Eng Res 27(1):53–73
Redaoui D, Sahnoune F, Heraiz M, Raghdi A (2016) Mechanism and kinetic parameters of the thermal decomposition of gibbsite Al(OH)3 by thermogravimetric analysis. Acta Phys Pol A 131:562–565. https://doi.org/10.12693/APhysPolA.131.562
Ross SD (1974) Sulphates and other Oxy-anions of Group VI, Chapter 18 in: Farmer VC (ed) The infrared spectra of minerals, The Mineralogical Society, London. https://doi.org/10.1180/mono-4.17
Sanjeev KC (2017) Understanding the effect of catalytic pyrolysis bio-oil produced using CaO during hydrotreatment, Master Thesis, Auburn University. https://etd.auburn.edu/handle/10415/5962
Savage PE (2000) Mechanisms and kinetics models for hydrocarbon pyrolysis. J Anal Appl Pyrolysis 54(1–2):109–126. https://doi.org/10.1016/S0165-2370(99)00084-4
Shah J, Jan MR (2014) Thermo-catalytic pyrolysis of polystyrene in the presence of zinc bulk catalysts. J Taiwan Inst Chem Eng 45:2494–2500. https://doi.org/10.1016/j.jtice.2014.05.011
Sharuddin SDA, Abnisa F, Daud WMAW, Aroua MK (2016) A review on pyrolysis of plastic wastes. Energy Convers Manag 115:308–326. https://doi.org/10.1016/j.enconman.2016.02.037
Shukla S, Kulkarni K (2002) Depolymerization of poly (ethylene terephthalate) waste. J Appl Polym Sci 85:1765–1770. https://doi.org/10.1002/app.10714
Sikdar S, Siddaiah A, Menezes PL (2020) Conversion of waste plastic to oils for tribological applications. Lubricants 8(8):1–32. https://doi.org/10.3390/lubricants8080078
Singh RK, Ruj B, Sadhukhan AK, Gupta P (2019) Thermal degradation of waste plastics under non-sweeping atmosphere: Part 1: effect of temperature, product optimization, and degradation mechanism. J Environ Manag 239:395–406. https://doi.org/10.1016/j.jenvman.2019.03.067
Sogancioglu M, Yucel A, Yel E, Ahmetli G (2017a) Production of epoxy composite from the pyrolysis char of washed PET chars. Energy Procedia 118:216–220. https://doi.org/10.1016/j.egypro.2017.07.022
Sogancioglu M, Yel E, Ahmetli G (2020) Behaviour of waste polypropylene pyrolysis char-based epoxy composite materials. Environ Sci Pollut Res 27:3871–3884. https://doi.org/10.1007/s11356-019-07028-3
Sogancioglu M, Ozdemir NC, Yel E, Ahmetli G (2017b) Effects of pre-washing on waste PET pyrolysis and use of sulphonated polystyrene (SPS) in physico-chemical treatment of waste pet washing wastewater, 2nd IWA Regional International Symposium on Water, Wastewater and Environment (IWA-PPFW2017b), Proceedings, Izmir-Turkey, 728-735 22-24 March 2017b
Son S, Koo B, Chai H, Tran HVH, Pandit S, Jung SP (2021) Comparison of hydrogen production and system performance in a microbial electrolysis cell containing cathodes made of non-platinum catalysts and binders. J Water Process Eng 40:101844
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85. https://doi.org/10.1016/j.chemosphere.2014.12.058
Terakado O, Hirasawa M (2005) Effect of metal oxides on the pyrolysis residues of poly(ethylene terephthalate): formation of carbonaceous submicron, nano-scale filaments and mesoporous compounds. J Anal Appl Pyrolysis 73:248–256. https://doi.org/10.1016/j.jaap.2005.02.002
Venkatachalam S, Nayak SG, Labde JV, Gharal PR, Rao K, Kelkar AK (2012) Degradation and recyclability of poly(ethylene terephthalate). In: Saleh H (ed) Polyester. IntechOpen. https://doi.org/10.5772/48612
Wu ZZ, Ni YP, Fu T, Liu BW, Wu WS, Chen L, Wang XL, Wang YZ (2018) Effect of biphenyl biimide structure on the thermal stability, flame retardancy and pyrolysis behavior of PET. Polym Degrad Stab 155:162–172. https://doi.org/10.1016/j.polymdegradstab.2018.07.017
Xue Y, Johnston P, Bai X (2017) Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers Manag 142:441–451. https://doi.org/10.1016/j.enconman.2017.03.071
Yadagiri J, Puppala VS, Kannapu HPR, Vakati V, Koppadi KS, Burri DR, Kamaraju SRR (2017) An inexpensive and environmentally friendly activated marble waste as a catalyst for vapour phase dehydration of 1,4-butanediol to tetrahydrofuran. Catal Commun 101:66–70. https://doi.org/10.1016/j.catcom.2017.07.013
Yoshioka T, Grause G, Eger C, Kamınsky W, Okuwaki A (2004) Pyrolysis of poly(ethylene terephthalate) in a fluidised bed plant. Polym Degrad Stab 86:499–504. https://doi.org/10.1016/j.polymdegradstab.2004.06.001
Yoshioka T, Handa T, Grause G, Lei Z, Inomata H, Mizoguchi T (2005) Effects of metal oxides on the pyrolysis of poly(ethylene terephthalate). J Anal Appl Pyrolysis 73:139–144. https://doi.org/10.1016/j.jaap.2005.01.004
Yucel A, Sogancioglu M, Yel E, Ahmetli G (2015) Evaluation of poly(ethylene terephtalate) waste char in epoxy based composites, 2nd International conference on sustainable agriculture and environment, Konya/Turkey, 425–429 30 September-3 October
Acknowledgements
This study was financially supported by The Scientific and Technological Research Council of Turkey – TUBITAK under Grant Code: CAYDAG-118Y475.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in relation to this study.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Onen, V., Ozgan, A., Goktepeli, G. et al. Marble processing effluent treatment sludge in waste PET pyrolysis as catalyst-I: pyrolysis product yields and the char characteristics. Int. J. Environ. Sci. Technol. 20, 3965–3986 (2023). https://doi.org/10.1007/s13762-022-04262-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04262-0