Abstract
The Zoige alpine wetland is one of the most sensitive ecosystems to global climate change. It is the largest alpine wetland in the world and currently suffers from serious drought and degradation. In this study, soil microbial communities of five soils with different moisture content were investigated by Illumina MiSeq high-throughput sequencing of 16S rRNA. The results showed that soil acidity and the content of soil nutrients decreased with the decrease in soil moisture. The microbial richness indices (Chao1 and ACE) and diversity index (Shannon) were highest in the flooded wetland (FW) and lowest in the unflooded mound near the flooded wetland (UW). The relative abundance showed four dominated phyla among all the soil microbes in five soils: Proteobacteria (36.5%), Acidobacteria (26.1%), Actinobacteria (9.4%) and Bacteroidetes (5.8%). Moreover, Proteobacteria (51.4%) of UW was significantly (P < 0.05) higher than that of other soils, while Actinobacteria (1.6%), Gemmatimonadetes (0.9%) and Nitrospirae (0.03%) of UW were significantly (P < 0.05) lower than those of other soils. Principal component analysis (PCA) and redundancy analysis (RDA) revealed that soil samples of UW differed most from samples of other soils. Proteobacteria was positively correlated with water-soluble phosphorus, while Actinobacteria and Bacteroidetes were negatively correlated with total organic carbon and total nitrogen. Our findings revealed that soils in transitional unstable state from flooded to aridification had the lowest microbial diversity, and drought led to long-term changes in the microbial community in the Zoige wetland, which may cause further degradation of alpine wetland ecosystem functions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Zoige alpine wetland is the most typical high-altitude and low-latitude wetland in the world, and it is sensitive to climate change (Cui et al. 2015a; Huo et al. 2013). The soil organic carbon density in the Zoige alpine wetland is three times greater than the wetland average in China and six times greater than the national average (Ma et al. 2016). It has the biggest peat deposition in China (Iqbal et al. 2019) and is an important global carbon storage reservoir for water conservation and biodiversity maintenance (Xiang et al. 2009). In recent years, due to the influence of natural and human activities, the trend of fragmentation and aridification of the Zoige alpine wetland has become increasingly serious (Pang et al. 2010). Wetland degradation promotes soil organic matter mineralization and carbon dioxide emission (Huo et al. 2013), accelerating carbon transfer from the soil to atmosphere and surface water. At present, research on the Zoige Wetland focuses on climate change (Bai et al. 2013; Li et al. 2014; Liang et al. 2015; Zhang et al. 2016), wetland degradation and restoration (Gao et al. 2014b; Jiang et al. 2017; Tang et al. 2012), carbon mineralization and carbon quality (Gao et al. 2011b; Gavazov et al. 2018; Luan et al. 2014), and specific microbial communities (Cui et al. 2015a; Fu et al. 2015; Tian et al. 2015; Wu et al. 2015; Zhang et al. 2008).
Soil microbes are the most active components in the soil. They play an important role in soil nutrient cycling, the maintenance of soil fertility and ecosystem function stability (Fierer 2017), and are important in exploring natural life mechanisms and the response of ecosystems to natural and anthropogenic disturbances such as global warming (Andersen et al. 2013). Microbial abundance and diversity in soil are mainly dependent on the physiochemical properties of the soil (e.g., pH, texture and nutrient status), rather than many macro-biogeographic factors which predict plant and animal diversity (Dequiedt et al. 2011; Fierer and Jackson 2006; Lauber et al. 2008). Research showed that moisture availability is the primary driver of microbial biomass carbon (it accounts for 34% of the global variance) (Serna-Chavez et al. 2013). Recently developed molecular biology techniques have the advantages of high data output and have been used to study the response of soil microbial communities to wetland degradation. Zhong et al. (2017) studied the depth-dependent response of microbial communities to water table drawdown. Other research focused on the different degradation stages of the Zoige wetland and analyzed the response of nitrogen-cycling microbial communities (Gu et al. 2019; Wu et al. 2016) and the association between microbial community changes and soil properties (Gu et al. 2018; Tang et al. 2012).
Although similar microbial biomass and dominant taxa may exist in various wetlands, different environment conditions and correlations between microbial communities and environmental factors can help us further understand the complex biochemical processes in alpine ecosystems. In this paper, 16S rRNA gene sequencing combined with other data processing and statistical analysis was used to study microbial communities of five soils with different moisture contents in the Zoige alpine wetland of China. Sampling was carried out in June 2016. Our purpose was to (1) identify the specific taxa and microbial community diversity in different soils, (2) compare our results with previous studies, and (3) analyze the association and potential mechanism between soil microbes and environmental factors. This study aims to comprehensively explain the impact of current degradation on the structure and diversity of microbial communities in the Zoige alpine wetland and provide scientific evidence for the protection and restoration of alpine wetland ecosystems.
Materials and methods
Study area and soil sampling
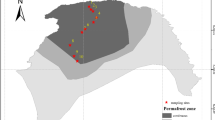
The Zoige alpine wetland (32° 20′–34° 20′ N, 102° 10′–103° 50′ E; 3400–3600 m.a.s.l.), located on the eastern margin of the Qinghai-Tibet Plateau, known as the third pole (Qiu 2008), covers about 31.5% of the whole Zoige plateau (Hao et al. 2011). It is dry in winter and wet in summer, with mean annual precipitation of 650 mm and mean annual temperature of 1.1 °C (Hao et al. 2011). With the current degradation of the Zoige alpine wetland, this area is now continuously distributed with plateau hills, rivers, terraces, lakes, peatlands and meadows. In June 2016, we collected five soils with decreasing moisture levels in the adjacent habitats of Zoige alpine wetland, namely flooded wetland (FW), unflooded mound near the flooded wetland (UW), arid wetland (AW), low meadow (LM) and high meadow (HM) (Fig. 1). There were three replicate samples for each soil, and each replicate was mixed by three points. The sampling depth was 0–10 cm. After removing litter and fine debris, it was ground and passed through a 100 mesh sieve and stored at 4 °C. One part of the soil was used to determine the physiochemical properties of the soil; the other part was used for high-throughput sequencing of 16S rRNA.
Physicochemical properties determination
Soil pH was determined by a pHS-25CW acidity meter; total organic carbon (TOC) and total nitrogen (TN) were determined by Elementar vario MACRO cube elemental analyzer; dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) were extracted and determined by Elementar vario TOC cube total organic carbon analyzer (Jones and Willett 2006); water-soluble phosphorus (WSP) was determined by ICP-AES (US, Thermo Jarrell Ash).
DNA extraction and PCR amplification
Soil genomic DNA was extracted using MP Biomedicals' FastDNA® Spin Kit for Soil kit, performed according to the instructions. The resulting DNA solution was stored at − 20 °C until use. The DNA was thawed and diluted and then subjected to PCR amplification as a template. The dilution factor was determined by the DNA concentration (50 times in this study). The soil microbial 16S rRNA gene (v4–v5 region) was amplified using universal primers 515F (5′-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′). The 50 μL PCR amplification system contained 1 μL of forward and reverse primers, 2 μL of DNA sample template, 25 μL of TaKaRa Taq version 2.0 plus dye (TaKaRa, Dalian, China) and 21 μL of sterile water. The PCR reaction conditions were: 94 °C, 5 min; 32x (94 °C, 45 s; 55 °C, 45 s; 72 °C, 1 min); 72 °C, 10 min.
Illumina MiSeq high-throughput sequencing
The PCR product was purified by Takara Agarose MiniBEST DNA Fragment Purification Kit Ver.2.0 kit and dissolved in 30 μL of DNase-free H2O, and then, the concentration was measured with a Nano Drop® ND-1000 UV spectrophotometer using TruSeq Nano DNA LT Sample Prep Kit. After the kit was built, the sample concentration was calculated, and the soil samples were mixed in equimolar numbers, and high-throughput sequencing was performed using the Illumina MiSeq sequencer. The sequence was submitted to the National Center for Biotechnology Information (NCBI) Genbank repository under BioProject number PRJNA603635.
Data processing and statistical analysis
Pairs of reads were merged by Flash (Magoc and Salzberg 2011). Quality filtering was performed using QIIME as described by Bokulich et al. (2013). Then, the DNA sequences were clustered to the operation taxonomic unit (OTU) under 97% similarity using UPARSE algorithm (Edgar 2013), with 24,386 to 44,381 reads sampled (36,331 on average). The RDP classifier was used to annotate the OTU representative sequence from domain to genus level (Wang et al. 2007). All samples were rarefied at 10,000 sequences to standardize sampling efforts. The relative abundance was plotted using Origin 9.0. VennDiagram and vegan packages in R 3.5.2 were used to draw Venn diagrams and calculate community diversity indices. Sample clustering of UPGMA (unweighted pair group method with arithmetic mean) was performed using Past 4.01, based on the Bray–Curtis distances. STAMP was used to perform principal component analysis (PCA) and pairwise comparisons of OTUs (Parks and Beiko 2010). Redundancy analysis (RDA) was performed using Canoco for Windows 4.5, and statistical testing was performed using SPSS 21.0.
Results and discussion
Results
Soil physicochemical properties and microbial diversity
The physicochemical properties of the Zoige wetland samples are shown in Table 1. All five soils were acidic with a pH of 5.41–6.49. When the soil moisture decreased, meadow soil acidity (LM and HM) was significantly (P < 0.05) lower than that of wetland with water (FW and UW) (Table 1 and Table S1). TOC was also significantly (P < 0.05) different, which was highest in FW and UW, middle in AW, and lowest in LM and HM. TN of the five soils presented approximately the same order and significance of TOC. DOC of HM and WSP of LM were significantly lower than that of other soils (P < 0.05). In addition, DON of FW was 3–5 times higher (P < 0.05) than other soils, with an average of 0.66 g kg−1.
Changes in the diversity of soil microbial community are illustrated in Table 2. The results showed that OTUs and total reads of UW were the lowest in the five soils. Chao1, ACE, and Shannon of FW were the highest, and Pielou of FW was in the middle. All diversity indices (chao1, ACE, Shannon, Simpson and Pielou) of UW were the lowest. Most of the diversity indices (except for Simpson) of HM were higher than those of LM. However, one-way analysis of variance (ANOVA) results showed that most diversity indices' differences did not reach the significant level (P > 0.05), except for chao1 of FW that was significantly higher than that of UW.
Shared and unique OTUs at the genus level of five soils are represented in Fig. 2. UW contains the least OTUs of 4547 (total of three replicates), followed by AW (5089), HM (5121), FW (5489) and LM (5693). OTUs shared by five soils (2129) accounted for 37.4–46.8% of each soil, with that of UW being the largest (46.8%). The specific OTUs in LM accounted for the largest proportion of its total (12.7%), followed by UW (11.1%), AW (10.4%), FW (8.6%) and HM (6.1%). When comparing the proportions of two-soil-shared OTUs to the total number of a certain soil (Table S2), the proportions of OTUs shared by UW and other soils (FW, AW, LM and HM) were always the smallest (54.3–60.8%), while the proportions of OTUs shared by FW and other soils (UW, AW, LM and HM) were relatively large (69.9–74.3%).
Composition of soil microbial communities
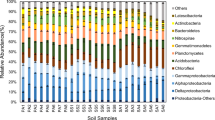
Sample clustering and relative abundance of soil microbes at the phylum level are shown in Fig. 3. There were eight dominant phyla (mean relative abundance > 1.0%) of soil microbes in the Zoige alpine wetland, and the sum of their mean relative abundance was 85.3% (Table S3). The mean relative abundance of Proteobacteria (36.5%), Acidobacteria (26.1%), Actinobacteria (9.4%) and Bacteroidetes (5.8%) was more than 5%, and mean relative abundance of Planctomycetes (2.6%), Gemmatimonadetes (2.0%), Chloroflexi (1.7%) and Firmicutes (1.1%) was 1–5%. One-way ANOVA showed that relative abundance of Proteobacteria (P = 0.001), Actinobacteria (P = 0.001), Gemmatimonadetes (P = 0.003) and Nitrospirae (P = 0.026) was significantly different (Table S4). Proteobacteria of UW (51.4%) was significantly higher than that of the other four soils, and UW’s relative abundance of Actinobacteria (1.6%), Gemmatimonadetes (0.9%) and Nitrospirae (0.03%) was significantly lower than those for other soils. Although one-way ANOVA showed no significant differences in the relative abundance of Firmicutes (P = 0.289), the value of LM (3.8%) was nearly 10 times the average of other soils (0.4%).
The numbers of OTUs with significant difference (P < 0.05) at the genus level between different soils were counted according to the phylum to they belonged (Table S5). There were more OTUs with significant differences between FW, UW and AW (433 with 67.4% were FW > UW, 334 with 54.5% were FW > AW, and 396 with 39.9% were UW > AW), less between AW and LM (224 with 79.8% were AM > LM), and the least between LM and HM (108 with 15.7% were LM > HM) (Tables S6–S10). In general, dominant phyla (Proteobacteria, Acidobacteria, Actinobacteria, Bacteroidetes, Planctomycetes, Gemmatimonadetes, Chloroflexi and Firmicutes) had more significant differences, except for Bacteroidetes between LM and HM (only one). Among other phyla with low relative abundance (< 1.0%), there were also significant differences in Armatimonadetes, candidate division WPS-1 and candidate division WPS-2 between FW and UW (with number of 5–7), Nitrospirae between UW and AW (with number of 6), and Armatimonadetes between LM and HM (with number of 7).
In order to find the most significant differences in the composition of microbial communities, OTUs with high (top 30) DPs (difference in mean proportions) in pairwise comparison were selected. Many significantly different-OTUs between FW and UW (Fig. S1), UW and AW (Fig. S2) belong to Proteobacteria, including Rhizobiales, Burkholderiales, Myxococcales1 and Xanthomonadales at order level, and OTUs with three largest DPs all belong to Rhizobiales and are higher in UW. In the result between FW and AW (Fig. S3), there were many OTUs belongs to Acidobacteria (including GP1, GP3, GP4, GP6, GP7 and GP17 at class level) and Proteobacteria (including Burkholderiales, Xanthomonadales, Rhodospirillales and Rhizobiales at order level), with the number of 11 and 10, respectively. When comparing LM with AW (Fig. S4) and HM (Fig. S5), most significantly different-OTUs were lower in LM, and they belonged to Acidobacteria, Proteobacteria, Actinobacteria, Planctomycetes, Verrucomicrobia, Gemmatimonadetes, Bacteroidetes and Armatimonadetes at the phylum level.
Soil microbial communities and environmental factors
The result of PCA is shown in Fig. 4. The PC1, PC2 and PC3 axes explained 30.9%, 25.3% and 11.7% of the changes, respectively. UW was in the positive direction of PC1 and PC2 axes, while FW, AW, LM, and HM were all distributed in the negative direction of PC1 axis, and both positive and negative directions of PC2 axis. FW and AW were both distributed in the negative direction of PC3 axis; LM and HM were both distributed in the positive direction of PC3 axis, while UW was distributed in both the positive and negative direction of PC3 axis. In general, the distribution of samples in UW was the most different from those in the other four soils, indicating that the structure of the soil microbial communities of UW was the most specific among the five soils in the Zoige wetland.
Constrained ranking can be used to study the impact of environmental factors on soil microbial species. Common methods include redundant analysis (RDA) (based on a linear model) and canonical correspondence analysis (CCA) (based on a single peak model). The length of the first axis in detrended correspondence analysis (DCA) was 0.65 (Table S11), so RDA was used in this study. The first two axes of RDA explain 48.7% and 7.1% of total variance (Fig. 5). The results showed that there were strong positive correlations between TOC and TN, DOC and DON; a strong negative correlation between pH and WSP in this study.
The impacts of environmental factors on microbes varied with microbial taxa. In this study (Fig. 5), Proteobacteria and Verrucomicrobia had positive correlations with WSP; Acidobacteria had strong positive correlations with DON and DOC; Actinobacteria and Bacteroidetes had strong negative correlations with TN and TOC; Gemmatimonadetes was negatively correlated with WSP and positively correlated with pH. Moreover, UW was mainly affected by WSP, TN and TOC; FW and LM were mainly affected by pH, DOC and DON; HM was affected by pH, while AW was moderately affected by all environmental factors. In addition, the distance between UW and other soils (FW, AW, LM and HM) was the largest, which was consistent with PCA.
Discussion
With current level of global warming, emission of stored carbon in wetlands may strengthen the greenhouse effects (Bridgham et al. 1995). Based on incubation experiments, Gao et al. (2011a) found that CO2 and CH4 emission rates in the Zoige alpine wetland increased with the increase in temperature, and increasing water table reduced CO2 emissions and increased CH4 emissions. The results also showed that the carbon mineralization process in the Zoige wetland was more sensitive at low temperature (Gao et al. 2011a). Our research found that with the decrease in soil moisture (from FW to HM), the acidity of soil was weakened, and the soil nutrients tended to decrease, which is consistent with previous studies (Gao et al. 2014a; Gu et al. 2018; Ma et al. 2016), possibly because the reduced soil moisture in the wetland increased soil permeability and thus promoted the decomposition of soil organic carbon and other nutrients. In addition, Fenner and Freeman (2011) found that aridification reduced the content of phenolic compounds in peatlands and promoted microbial growth, which in turn exacerbated soil carbon mineralization. Microbial activity also affects soil properties and nutrient conversion. Previous research showed that the mineralization rates of carbon, nitrogen and phosphorus are positively correlated with soil microbial biomass (McLatchey and Reddy 1998).
Soil organisms (especially microbes) are closely related to soil and water conservation, decomposition of organic matter, nutrient cycling, detoxification of poisons and inhibition of pathogenic bacteria in soil ecosystems (Doran and Zeiss 2000). Generally speaking, soil microbial diversity of natural wetland is higher than that of constructed wetland, and its microbial community difference is also smaller than that of constructed wetland (Ansola et al. 2014). In addition, previous studies found that drought has a long-term effect on microbial communities by affecting vegetation and soil moisture, with bacteria being more resilient but less resistant than fungi (Bapiri et al. 2010; Barnard et al. 2013; De Vries et al. 2018). In our study, there was no significant difference of richness and diversity indices of microbial communities between different soils, except for Chao1′s difference between FW and UW. Zhong et al. (2017) also found that statistical significance of diversity reduction only appeared in the middle depth of the soil, but not in the top soil or deep soil. In our study, samples of similar soil types were clustered together in PCA, with the distance between UW and other soils being the largest, indicating that soil types largely shaped microbial communities in the Zoige alpine wetland (Tang et al. 2012). In addition, diversity indices of HM were higher than those of LM, probably due to the higher metabolic activity of the soil microorganisms in HM and their stronger ability to utilize substrates.
The most dominant phyla in our study accounted for more than half of the total microbes, including Proteobacteria (36.5%), Acidobacteria (26.1%), Actinobacteria (9.4%), Bacteroidetes (5.8%), Planctomycetes (2.6%), Gemmatimonadetes (2.0%), Chloroflexi (1.7%) and Firmicutes (1.1%). Notwithstanding the heterogeneity in soil types and soil properties, microbial communities in the Zoige wetland shared similar dominant taxa. Based on DGGE, Tang et al. (2012) found bacterial phyla of Proteobacteria and Bacteroidetes in swamp soil, peat soil, meadow soil and sand soil. Gu et al. (2018) found that swamp soil, meadow soil and sandy soil were dominated by Proteobacteria (24.6–31.6%), Acidobacteria (12.3–19.7%) and Chloroflexi (10.8–19.7%). Cui et al. (2015b) observed similar pattern in different incubation groups, with the 10 top abundant phyla being Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes, Acidobacteria, Planctomycetes, Verrucomicrobia, Chlorobi, Gemmatimonadetes and OP8. Cui et al. (2015a) also found that methanogenic bacterial communities in wetlands constituted by different plant species remained relatively constant and were not significantly affected by the temperature increases. However, there were still specificities in the microbial communities of the Zoige wetland. We found that Proteobacteria (especially Rhizobiales at the order level) of UW was significantly higher than that of other soils, Actinobacteria and Gemmatimonadetes of UW were significantly lower than those of others, and Firmicutes of LM were higher than that of other soils. These differences might be caused by the physiological preferences (e.g., oxygen content, hydrothermal condition and nutrient content) of different phyla.
The microbial community in the Zoige wetland was complicatedly affected by environmental factors, and the complex mechanism of microbial metabolism can be explained by the relationship between specific microbial taxa and environmental factors. Our results suggested the strong negative correlations between TOC(TN) and Actinobacteria (Bacteroidetes), indicating that TOC and TN may be the key factors that inhibit the growth of Actinobacteria and Bacteroidetes. The strong positive correlations between Acidobacteria and dissolved nutrients (DOC and DON) also indicated the effect of nutrient conditions on Acidobacteria. Tang et al. (2012) found that soil organic carbon and TN were more strongly correlated with microbial biomass than soil water content. Zhong et al. (2017) found that soil pH, TOC and TN were the most important parameters influencing the structure of soil microbial communities, which confirmed our results of RDA. What is more, researchers also found that drought indirectly increased the abundance of denitrification and ammonia-oxidizing genes to increase soil nitrogen availability (De Vries et al. 2018), and altitude change significantly affected ammonia-oxidizing microorganisms (Zhang et al. 2009). In addition, Gu et al. (2018) found that soil total potassium was the strongest driver that affected the shaping of microbial communities in the Zoige wetland.
In summary, changes in nutrient content and availability caused by aridification in the Zoige wetland would affect the metabolism of soil microorganisms and thus affect the function and stability of the entire wetland ecosystem. Some studies found that climate warming would promote the development of vascular plants in peat wetlands, while the rhizosphere initiation promotes the heterotrophic decomposition of peat microbes, thereby increasing the degree of humification of dissolved organic matter (Gavazov et al. 2018). In addition, seasonal freezing and thawing, extreme weather such as summer rainstorm in alpine regions, also cause soil moisture changes (Edwards et al. 2007), which will further affect soil physical properties, nutrient dynamics and microbial activity. Moreover, research showed that recovery of water may further accelerate carbon loss in peatlands (Fenner and Freeman 2011), which has an important reference value for the restoration of the Zoige alpine wetland, and also illustrates the need for further understanding the ecological role of microbial communities in the degradation of wetland ecosystem.
Conclusion
We studied the microbial community composition and the diversity of five soils with different moisture contents in the Zoige alpine wetland and found that (1) soil acidity and nutrients tended to decrease when the water content decreased; (2) the flooded wetland had the highest microbial diversity, while the unflooded mound near the flooded wetland had the lowest microbial diversity, and its microbial composition was significantly different from the other four soils; (3) dominant phyla in five soils were Proteobacteria, Acidobacteria, Actinobacteria and Bacteroidetes, and they responded differently to environmental factors, while the specific mechanism and quantitative relationship are still unclear. Alpine soil microbial composition and its variation with aridification caused by climate change in a natural environment are of great ecological significance. In the future, we can combine other advanced methods such as metagenomics and metabolomics to further study the spatial and temporal response of soil microbes to environmental changes such as warming, the mechanisms of carbon, nitrogen and phosphorus transformation, and the quantitative relationships between certain functional bacteria and environmental processes in alpine wetlands.
References
Andersen R, Chapman SJ, Artz RRE (2013) Microbial communities in natural and disturbed peatlands: a review. Soil Biol Biochem 57:979–994. https://doi.org/10.1016/j.soilbio.2012.10.003
Ansola G, Arroyo P, de Miera LES (2014) Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci Total Environ 473:63–71. https://doi.org/10.1016/j.scitotenv.2013.11.125
Bai JH, Lu QQ, Zhao QQ, Wang JJ, Ouyang H (2013) Effects of Alpine Wetland landscapes on regional climate on the Zoige Plateau of China. Adv Meteorol. https://doi.org/10.1155/2013/972430
Bapiri A, Baath E, Rousk J (2010) Drying-rewetting cycles affect fungal and bacterial growth differently in an arable soil. Microb Ecol 60:419–428. https://doi.org/10.1007/s00248-010-9723-5
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J 7:2229–2241. https://doi.org/10.1038/ismej.2013.104
Bokulich NA et al (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. https://doi.org/10.1038/nmeth.2276
Bridgham SD, Johnston CA, Pastor J, Updegraff K (1995) Potential feedbacks of northern wetlands on climate-change - an outline of an approach to predict climate-change impact. Bioscience 45:262–274. https://doi.org/10.2307/1312419
Cui M, Ma A, Qi H, Zhuang X, Zhuang G, Zhao G (2015a) Warmer temperature accelerates methane emissions from the Zoige wetland on the Tibetan Plateau without changing methanogenic community composition. Sci Rep. https://doi.org/10.1038/srep11616
Cui MM, Ma AZ, Qi HY, Zhuang XL, Zhuang GQ, Zhao GH (2015b) Warmer temperature accelerates methane emissions from the Zoige wetland on the Tibetan Plateau without changing methanogenic community composition. Sci Rep 5:12. https://doi.org/10.1038/srep11616
De Vries FT et al (2018) Soil bacterial networks are less stable under drought than fungal networks. Nat Commun. https://doi.org/10.1038/s41467-018-05516-7
Dequiedt S et al (2011) Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Glob Ecol Biogeogr 20:641–652. https://doi.org/10.1111/j.1466-8238.2010.00628.x
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol 15:3–11. https://doi.org/10.1016/s0929-1393(00)00067-6
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. https://doi.org/10.1038/nmeth.2604
Edwards AC, Scalenghe R, Freppaz M (2007) Changes in the seasonal snow cover of alpine regions and its effect on soil processes: a review. Quatern Int 162:172–181. https://doi.org/10.1016/j.quaint.2006.10.027
Fenner N, Freeman C (2011) Drought-induced carbon loss in peatlands. Nat Geosci 4:895–900. https://doi.org/10.1038/ngeo1323
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590. https://doi.org/10.1038/nrmicro.2017.87
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631. https://doi.org/10.1073/pnas.0507535103
Fu L, Song T, Lu Y (2015) Snapshot of methanogen sensitivity to temperature in Zoige wetland from Tibetan plateau. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00131
Gao J, Ouyang H, Lei G, Xu X, Zhang M (2011a) Effects of temperature, soil moisture, soil type and their interactions on soil carbon mineralization in Zoig alpine wetland Qinghai-Tibet Plateau. Chin Geogr Sci 21:27–35. https://doi.org/10.1007/s11769-011-0439-3
Gao J, Lei G, Zhang X, Wang G (2014a) Can delta C-13 abundance, water-soluble carbon, and light fraction carbon be potential indicators of soil organic carbon dynamics in Zoige wetland? CATENA 119:21–27. https://doi.org/10.1016/j.catena.2014.03.005
Gao JQ, Zhang XW, Lei GC, Wang GX (2014b) Soil organic carbon and its fractions in relation to degradation and restoration of wetlands on the Zoige Plateau. China Wetlands 34:235–241. https://doi.org/10.1007/s13157-013-0487-9
Gavazov K et al (2018) Vascular plant-mediated controls on atmospheric carbon assimilation and peat carbon decomposition under climate change. Glob Change Biol 24:3911–3921. https://doi.org/10.1111/gcb.14140
Gu Y et al (2018) Degradation shaped bacterial and archaeal communities with predictable taxa and their association patterns in Zoige wetland at Tibet plateau. Sci Rep. https://doi.org/10.1038/s41598-018-21874-0
Gu YF, Liu T, Bai Y, Xiang QJ, Zhang XP, Chen Q (2019) Pyrosequencing of nirS gene revealed spatial variation of denitrifying bacterial assemblages in response to wetland desertification at Tibet plateau. J Mt Sci 16:1121–1132. https://doi.org/10.1007/s11629-018-5147-3
Hao YB et al (2011) Predominance of precipitation and temperature controls on ecosystem CO2 exchange in Zoige Alpine Wetlands of Southwest China. Wetlands 31:413–422. https://doi.org/10.1007/s13157-011-0151-1
Huo L, Chen Z, Zou Y, Lu X, Guo J, Tang X (2013) Effect of Zoige alpine wetland degradation on the density and fractions of soil organic carbon. Ecol Eng 51:287–295. https://doi.org/10.1016/j.ecoleng.2012.12.020
Iqbal A et al (2019) Pattern of microbial community composition and functional gene repertoire associated with methane emission from Zoige wetlands China-A review. Sci Total Environ 694:12. https://doi.org/10.1016/j.scitotenv.2019.133675
Jiang W, Lv J, Wang C, Chen Z, Liu Y (2017) Marsh wetland degradation risk assessment and change analysis: a case study in the Zoige Plateau, China. Ecol Indic 82:316–326. https://doi.org/10.1016/j.ecolind.2017.06.059
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999. https://doi.org/10.1016/j.soilbio.2005.08.012
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Li BQ et al (2014) Effects of climate variations and human activities on runoff in the Zoige Alpine Wetland in the eastern edge of the Tibetan Plateau. J Hydrol Eng 19:1026–1035. https://doi.org/10.1061/(asce)he.1943-5584.0000868
Liang Y et al (2015) Long-term soil transplant simulating climate change with latitude significantly alters microbial temporal turnover. ISME J 9:2561–2572. https://doi.org/10.1038/ismej.2015.78
Luan JW, Cui LJ, Xiang CH, Wu JH, Song HT, Ma QF (2014) Soil carbon stocks and quality across intact and degraded alpine wetlands in Zoige, east Qinghai-Tibet Plateau. Wetl Ecol Manag 22:427–438. https://doi.org/10.1007/s11273-014-9344-8
Ma K, Zhang Y, Tang SX, Liu JG (2016) Spatial distribution of soil organic carbon in the Zoige alpine wetland, northeastern Qinghai-Tibet Plateau. CATENA 144:102–108. https://doi.org/10.1016/j.catena.2016.05.014
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
McLatchey GP, Reddy KR (1998) Regulation of organic matter decomposition and nutrient release in a wetland soil. J Environ Qual 27:1268–1274. https://doi.org/10.2134/jeq1998.00472425002700050036x
Pang A, Li C, Wang X, Hu J (2010) Land use/cover change in response to driving forces of Zoige County, China. In: Yang Z, Chen B (eds) International conference on ecological informatics and ecosystem conservation, vol 2, Procedia Environmental Sciences, pp 1074–1082. https://doi.org/10.1016/j.proenv.2010.10.119
Parks DH, Beiko RG (2010) Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26:715–721. https://doi.org/10.1093/bioinformatics/btq041
Qiu J (2008) The third pole. Nature 454:393–396. https://doi.org/10.1038/454393a
Serna-Chavez HM, Fierer N, van Bodegom PM (2013) Global drivers and patterns of microbial abundance in soil. Glob Ecol Biogeogr 22:1162–1172. https://doi.org/10.1111/geb.12070
Tang J et al (2012) Effects of wetland degradation on bacterial community in the Zoige Wetland of Qinghai-Tibetan Plateau (China). World J Microbiol Biotechnol 28:649–657. https://doi.org/10.1007/s11274-011-0858-4
Tian J et al (2015) Response of archaeal communities to water regimes under simulated warming and drought conditions in Tibetan Plateau wetlands. J Soils Sedim 15:179–188. https://doi.org/10.1007/s11368-014-0978-1
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/aem.00062-07
Wu L-s, Feng S, Nie Y-y, Zhou J-h, Yang Z-r, Zhang J (2015) Soil cellulase activity and fungal community responses to wetland degradation in the Zoige Plateau China. J Mt Sci 12:471–482. https://doi.org/10.1007/s11629-014-3183-1
Wu L-s, Nie Y-y, Yang Z-r, Zhang J (2016) Responses of soil inhabiting nitrogen-cycling microbial communities to wetland degradation on the Zoige Plateau China. J Mt Sci 13:2192–2204. https://doi.org/10.1007/s11629-016-4004-5
Xiang SA, Guo RQ, Wu N, Sun SC (2009) Current status and future prospects of Zoige Marsh in Eastern Qinghai-Tibet Plateau. Ecol Eng 35:553–562. https://doi.org/10.1016/j.ecoleng.2008.02.016
Zhang GS, Tian JQ, Jiang N, Guo XP, Wang YF, Dong XZ (2008) Methanogen community in Zoige wetland of Tibetan plateau and phenotypic characterization of a dominant uncultured methanogen cluster ZC-I. Environ Microbiol 10:1850–1860. https://doi.org/10.1111/j.1462-2920.2008.01606.x
Zhang L-M, Wang M, Prosser JI, Zheng Y-M, He J-Z (2009) Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest Fems. Microbiol Ecol 70:208–217. https://doi.org/10.1111/j.1574-6941.2009.00775.x
Zhang K et al (2016) Effects of short-term warming and altered precipitation on soil microbial communities in Alpine Grassland of the Tibetan Plateau. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01032
Zhong Q et al (2017) Water table drawdown shapes the depth-dependent variations in prokaryotic diversity and structure in Zoige peatlands Fems. Microbiol Ecol. https://doi.org/10.1093/femsec/fix049
Acknowledgements
Thanks are due to Nanjing Institute of Soil Science of the Chinese Academy of Sciences for their support for high-throughput sequencing.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 41271094).
Author information
Authors and Affiliations
Contributions
SF and HS conceived the idea and designed the study. SF, YC and ZJ collected samples and performed the experiment. SF and JQ interpreted the data and wrote this paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Rupali Datta.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, S., Qin, J., Sun, H. et al. Alpine soil microbial community structure and diversity are largely influenced by moisture content in the Zoige wetland. Int. J. Environ. Sci. Technol. 19, 4369–4378 (2022). https://doi.org/10.1007/s13762-021-03287-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03287-1