Abstract
The wetlands on the Qinghai-Tibet Plateau are experiencing serious degradation, with more than 90,000 hectares of marshland converted to wet meadow or meadow after 40 years of drainage. However, little is known about the effects of wetland conversion on soil C stocks and the quality of soil organic carbon (SOC) (defined by the proportion of labile versus more resistant organic carbon compounds). SOC, microbial biomass carbon, light fraction organic carbon (LFOC), dissolved organic carbon, and the chemical composition of SOC in the soil surface layer (0–10 cm), were investigated along a wetland degradation gradient (marsh, wet meadow, and meadow). Wetland degradation caused a 16 % reduction in the carbon stocks from marsh (178.7 ± 15.2 kg C m−2) to wet meadow (150.6 ± 21.5 kg C m−2), and a 32 % reduction in C stocks of the 0–10 cm soil layer from marsh to meadow (122.2 ± 2.6 kg C m−2). Wetland degradation also led to a significant reduction in SOC quality, represented by the lability of the carbon pool as determined by a density fractionation method (L LFOC), and a significant increase in the stability of the carbon pool as reflected by the alkyl-C:O-alkyl-C ratio. 13C NMR spectroscopy showed that the labile form of C (O-alkyl-C) declined significantly after wetland degradation. These results assist in explaining the transformation of organic C in these plateau wetland soils and suggest that wetland degradation not only caused SOC loss, but also decreased the quality of the SOC of the surface soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil organic matter (SOM) is an important part of many terrestrial ecosystems, given its role in regulating the supply of nutrients to plants and microbes, and its influence on soil moisture and long-term carbon (C) storage (McLauchlan and Hobbie 2004). The various ecosystem functions associated with SOM, or soil organic carbon (SOC), depend on both its quantity and quality (Smith 2005), where the quality of SOC is the ease with which carbon in the SOM can be mineralized (Fissore et al. 2009).
Due to the impact of land use (Six et al. 2002a; Leifeld and Kögel 2005; Luan et al. 2010) and climate change (Kirschbaum 2006; Ågren 2000) on the vulnerability of SOC stocks, a number of SOC fractionation procedures have been proposed in order to distinguish the more easily decomposed SOC (i.e., relatively low stability, high-quality, labile) from that which is less easily decomposed (i.e., relatively high stability, low-quality, recalcitrant) (Six et al. 2002b; von Lützow et al. 2007). Labile carbon indicators, such as the light fraction organic carbon (LFOC), particulate organic carbon (POC) (Janzen et al. 1992; Six et al. 2002a), microbial biomass carbon (MBC), and dissolved organic carbon (DOC) (Neff and Asner 2001), were developed relying on physical or chemical fractionation methods. Although solid state 13C CP/MAS NMR has been developed to provide a semi-quantitative evaluation of soil chemical composition nondestructively (Huang et al. 2008; Dignac et al. 2002), no existing single biological, physical, or chemical fractionation technique adequately describes the continuum of SOM in nature (Paul et al. 2006). There is still no consensus as to which method or combination of methods is most suitable to evaluate the impacts of land use changes on the quality of SOC (Skjemstad et al. 2006). Therefore, in order to get a more accurate understanding of the effects of land use change on soil carbon, it is necessary to evaluate the quality of the SOC by employing multiple indices (Luan et al. 2010), particularly since the quality of the SOC is considered to be an important factor influencing the temperature sensitivity of SOC decomposition (Q10 values) both in field studies (Arevalo et al. 2010; Luan et al. 2013) and in laboratory studies (Arevalo et al. 2012; Xu et al. 2012; Fierer et al. 2005).
Wetlands store approximately one-third of the world’s soil C, despite only occupying approximately 2–6 % of the earth’s land surface (Whiting and Chanton 2001; Kayranli et al. 2010). Thus, wetlands play an important role in global C cycling, particularly in the context of climate change and other human disturbances that may affect the storage and decomposition of carbon (Wu 2012). As the largest wetland on the Eurasian continent, the Qinghai-Tibet Plateau accounts for 51.15 % (~48,075 km2) of China’s natural wetland (excluding lakes and floodplains)(Ding et al. 2004). However, wetlands in this region are experiencing a severe decline as a result of intensive human activities (e.g. drainage, grazing, peat harvest etc.) (Xiang et al. 2009; Wang et al. 2007a). Drainage through ditching has intensely changed the hydrologic condition and caused severe degradation of the Zoige wetland (Bai et al. 2009) in the northeast of Qinghai-Tibet Plateau, which is the largest high altitude wetland in the world. For example, around 1,000 km of channel (covering around 2,000 km2) was dug between 1965 and 1973, and another 50.5 km (covering 148 km2) was dug in the early 1990s, to meet the increasing demand for grazing land (Sun 1998a), which resulted in more than 905.1993 km2 of wetland loss through degrading into wet or drier meadows (Bai et al. 2009). There are three stages during wetland degradation in the Qinghai-Tibet plateau, and each stage is mainly determined by changes in hydrology: (1) permanently inundated wetland (marsh, representing intact wetland); (2) seasonally inundated wetland (wet meadow, representing intermediately degraded wetland); and (3) no inundated meadow (dry meadow, representing severely degraded wetland) (Huo et al. 2013). The impact of wetland degradation on the quantity and especially the quality of the SOC in these wetlands is unknown.
In this study, both the loss and change in quality of soil carbon were quantified along a degradation gradient from marsh to wet meadow, and then meadow. Our objectives were to: (1) investigate the effect of wetland degradation on the labile organic carbon, chemical composition and the quality of SOC; and (2) identify the similarities or differences among carbon fractionation methods for describing the continuum of SOM.
Materials and methods
Site description and soil sampling
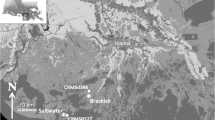
The study sites were located at the Wetland Ecological Research Station in the Zoige National Wetland Reserve (33°56′N, 102°52′E, 3,430 m.a.s.l.), Sichuan Province, China. The area is characterized by a mild, cold, temperate continental monsoon climate. The annual mean temperature is about 0.7–1.1 °C, with the highest monthly mean being 10.8 °C in July and the lowest being −10.6 °C in January. The annual mean precipitation is 656.8 mm, with 86 % of this occurring between April and October (Xiang et al. 2009). Based on the three wetland degradation stages (i.e., intact wetland, early degrading, and degraded), we chose five marshes (Gangna-a, Fenqu-a, Shebao, Fenqu-b, Hua lake-b; marsh), three wet meadows (Chuke, Hua lake-a, Gangna-b; wet meadow), and four meadows (Angda, A’Xi-a, A’Xi-b, A’Xi-c; meadow) for the investigations. These wet meadows and meadows were converted from marshes after 20–40 years of drainage. Alpine peat (Histosols, FAO; Fibrists, USDA), and alpine meadow soils (mat cryic cambisols, FAO) (Wang et al. 2007b) are common in this area, with perennial or seasonal and temporary water logging as a result of the flat topography. The soil parent material is mainly homogeneous silt and clay (Sun 1998b). All sample sites were exposed to grazing by yak and sheep. The location of the sampling sites is shown in Fig. 1, and a common set of site attributes is summarized in Table 1.
At each site, three 5 × 5 m2 plots over 10 m apart from each other were established. Three soil cores from each plot were extracted (7 cm in diameter, 10 cm in depth) and thoroughly mixed, and then each sample was divided into two parts. One part of the sample was air dried for pH, mass-based SOC, TN, LFOC, and solid-state 13C cross polarization with magic angle spinning nuclear magnetic resonance (13C CP/MAS NMR) measurement (three replicates from each site were mixed into one sample for 13C CP/MAS NMR analysis). The other part was kept at field moisture content at 4 °C for MBC, microbial biomass nitrogen (MBN), extractable DOC, and K2SO4 extractable nitrogen (DON) measurements after 2 weeks; 100 mL (50.46 mm diameter, 50 mm height) sampling cylinders were used for analyses of bulk density (BD).
Soil analysis
Physical fractionation
Light fraction SOM was obtained by density fractionation based on a study by Six et al. (1998), but with a modification using a CaCl2 solution (density of 1.5 g mL−1) as Luan et al. (2011) described. The soil structure of a suspension of 10 g of soil and 25 mL CaCl2 solution were disrupted by ultrasonication with an energy of 225 J mL−1 applied over 12 min. The supernatant solution containing the light fraction was vacuum filtered through a 0.45 μm fiberglass filter (previously dried at 50 °C and weighed) after centrifuging. The filter and the retained light fraction were rinsed with distilled water, dried at 65 °C, ground and analyzed for organic C and N. Bulk SOC and LFOC contents were determined using a wet oxidation method with 133 mmol L−1 K2Cr2O7 at 170–180 °C (Lu 2000). Soil nitrogen concentration in bulk soil (TN) and light fractions (LFN) were determined using the micro-Kjeldahl method. Soil pH was measured from soil–water suspensions (1:5, v/v).
Chemical fractionation
MBC and MBN of each sample was determined using the chloroform fumigation-extraction method (modified from Vance et al. 1987), while the waterlogged soil was fumigated according to Inubushi et al. (1991) method. Both fumigated and nonfumigated soils were extracted with 50 mL 0.5 M K2SO4 and then filtered after removal of the CHCl3 from the soil by repeated evacuations. The extracts were kept frozen at −30 °C before analysis using a MULTI N/C 3100 (Analytik Jena AG). The total amount of MBC and MBN were determined by the difference between K2SO4-extractable C and N in fumigated and non-fumigated soil, with correction factors of KEC = 0.45 (Sparling et al. 1990) and KEN = 0.54 (Brookes et al. 1985) for MBC and MBN, respectively. Extractable DOC was determined as total organic C in extracts from non-fumigated soil.
Carbon pool ‘lability’ (or SOC quality) defined by different fractionation methods was calculated using the method described by Luan et al. (2010):
where LFOC is the light fraction organic carbon; SOC is the soil organic carbon; DOC is the extractable dissolved organic carbon; and MBC is the microbial biomass carbon.
Solid-state 13CP/MAS NMR
To remove paramagnetic compounds and to increase the C content of the mineral soil fractions, soil samples were treated with hydrofluoric acid (10 %, v/v) prior to CPMAS 13C NMR spectroscopy, as previously demonstrated by Schmidt et al. (1997). Soil samples were packed in a ZrO2 rotor (7 mm outside diameter). Solid-state 13CP/MAS NMR measurements were performed at magic angle spinning (MAS) rates of 5 kHz using a Bruker AVANCE III 400 spectrometer, operating at 100.64 MHz. Single contact time of 1 ms was applied with an acquisition time of 42 ms, and a recycle delay of 1 s. Twenty thousand transients were collected for all samples, and a Lorentzian line broadening function of 50 Hz was applied to all spectra. Chemical shift values were referenced externally to glycine at 176.03 ppm, which is equivalent to tetramethylsilane at 0 ppm (Wang et al. 2010b).
The 13C NMR spectra was divided into four chemical shift regions: (i) aliphatic or alkyl-C (0–50 ppm) of lipids, fatty acids, and plant aliphatic polymers (recalcitrant form of C); (ii) O-alkyl-C (50–112 ppm) derived primarily from polysaccharides (cellulose and hemicelluloses), but also from proteins and side chains of lignin (labile form of C); (iii) aromatic or aryl-C (112–163 ppm) derived from lignin and/or protein; and finally, (iv) carbonyl-C (163–190 ppm) derived from aliphatic esters, carboxyl groups and amide carbonyls (Faz Cano et al. 2002). Areas of the chemical-shift regions were measured by integration and were expressed as a percentage of the total area (relative intensity). As recommended by Baldock et al. (1997), this study considers the ratio of alkyl-C to O-alkyl-C as an index of the extent of decomposition or substrate quality for microbes. An aromaticity index has been used to characterize the extent of humification of SOM (Dai et al. 2001), with the index defined as:
Statistical analysis
One way analysis of variance (ANOVA) with the least significant difference (LSD) was performed to assess the effect of wetland degradation on labile carbon, nitrogen concentration, C:N ratio, carbon pool lability, and the relative intensity of C in the region, alkyl-C:O-alkyl-C ratio, and aromaticity. Regression analysis was used to examine the relationships among labile carbon indicators, carbon pool lability, and the extent of decomposition. Statistical analyses were performed using the SPSS 13.0 software package for Windows.
Results
Soil C stocks
Soil BD was significantly increased after wetland degradation, from 0.31 ± 0.01(SD) g cm−3 in the marsh to 0.58 ± 0.08 g cm−3 in the wet meadow, and 1.06 ± 0.06 g cm−3 in the meadow at the 0–10 cm soil layer (F = 70.4, P < 0.001). However, the SOC stock in the 0–10 cm soil layer was reduced, from 178.7 ± 15.2 kg C m−2 in the marsh to 150.6 ± 21.5 kg C m−2 in the wet meadow, and 122.2 ± 2.6 kg C m−2 in the meadow (F = 4.29, P < 0.05). No significant change in soil pH was observed (F = 2.22, P = 1.64) (Table 1), and a negative exponential model described the relationship between BD and SOC (SOC = 1,021.9e −2.09×BD, R2 = 0.94, P < 0.001).
Labile organic carbons and carbon pool lability
Soil MBC, LFOC, MBN, extractable DON, and LFN were reduced, while extractable DOC increased in the 0–10 cm layer after degradation from marsh to wet meadow (Fig. 2). Wetland degradation also led to a significant decrease in LF C:N ratio and a significant increase of DO C:N (Fig. 2). Additionally, an increase of carbon pool lability, as shown by density fractionation method (L LFOC), was found after wetland degradation (Table 2). Both MBC and LFOC strongly correlated with SOC (R2 = 0.59 for MBC and 0.94 for LFOC, P < 0.001) (Fig. 3a, c). Significant correlation between DOC and SOC (R2 = 0.66, P < 0.001) was only found after data from the marsh was excluded (Fig. 3b).
Solid-state 13CP/MAS NMR spectroscopy
Soil samples in the 0–10 cm layer showed similar distribution of functional groups across the three sites, in the order of O-alkyl > alkyl > aromatic > carboxyl (Table 2). However, wetland degradation significantly increased the alkyl-C (%), while it reduced the O-alkyl-C (%) (Table 2). Alkyl-C:O-alkyl-C ratio and the aromaticity of 12 sites varied from 0.37 to 0.69 and 17.35 to 5.71 %, respectively. The alkyl-C:O-alkyl-C ratio increased while the aromaticity reduced significantly after degradation (Table 2).
Relationships among soil labile carbon indicators
A positive correlation between MBC and LFOC was found (R2 = 0.62, P < 0.001, Fig. 3e), while a positive correlation between MBC with extractable DOC was only found when data from the marsh were excluded (Fig. 3d). The variance of O-alkyl-C (%) can be explained by the MBC and LFOC (R2 = 0.55, and 0.80, respectively) (Table 3), and the variation of alkyl-C:O-alkyl-C ratio can be explained by the L LFOC, and LF C:N ratio (R2 = 0.43–0.92) (Table 3). We found a positive linear relationship between the L LFOC and LF C:N ratio (Fig. 3f). The L LFOC explains the variation of aromaticity among sites (R2 > 0.35) (Fig. 4).
Discussion
Soil organic carbon and carbon pool lability
Wetland degradation results in a significant reduction in the 0–10 cm soil layer C stocks (e.g., 15.8 and 31.6 % of reduction in the wet meadow and meadow respectively as compared with the marsh). This reduction is mainly attributed to the reduction in the SOC concentration (Table 1) because the BD was significantly increased. Both the increase in decomposition and the reduction in biomass input after degradation (e.g., a higher grazing intensity in the meadow and wet meadow because of easier access) can be a consequence of the decrease in the surface soil C stocks.
The MBC concentrations in the meadow (0.20 ± 0.05 mg g−1) and wet meadow (0.54 ± 0.19 mg g−1) are comparable to those reported in the Kobresia meadow in Haibei, where they ranged 0.47–0.80 mg g−1 (Wang et al. 2010a). In our study, however, the MBC only contributed 0.17, 0.19, and 0.20 % to the SOC in the meadow, wet meadow, and marsh, respectively. These contributions are lower than those reported in other studies, which showed a contribution of 1–5 % (e.g., Haynes 2005). A lower prevalence of the MBC in the SOC at our sites may be attributed to the lower mean annual temperature at this high elevation, hence the lower microbial community and activity (Plante et al. 2011). A significant reduction in the MBC after wetland degradation (Fig. 2) could be partly attributed to the reduction in the substrate supply, such as LFOC (Fig. 4b).
The marsh was found to have a lower extractable DOC than the wet meadow (a moderately degraded wetland). Similarly, Song et al. (2011) observed a greater loss of the DOC in the river from the degraded wetland compared to the pristine marshy rivers. The lower concentrations of the soil extractable DOC in the marsh may be caused by its waterlogged conditions, because the DOC efflux may be increased due to a higher leaching frequency (Christ and David 1996; Godde et al. 1996). However, no significant difference in the extractable DON between the marsh and wet meadow was detected. This finding suggests that the dissolved OM with a high C:N ratio is lost from the marsh, leading to a significantly lower C:N ratio of the extractable OM in the top soil (Fig. 2b). Further, the urine and feces of yaks and sheep, with a high N concentration, also contributes to the lower DO C:N in these grazing sites. Different pathways for the C and N losses may also explain the differences (i.e., the soluble C was respired and the soluble N was immobilized or simply not leached). The wet and dry cycles increase the solubility of OM in the soil (Miller et al. 2005), which results in a higher extractable DOC in the wet meadow than in the meadow.
The LFOC concentrations (i.e., 212.2, 56.9, and 11.5 g C kg−1 in the marsh, wet meadow, and meadow, respectively) and proportions (i.e., 36.5, 17.6, and 9.8 % in the marsh, wet meadow, and meadow, respectively) are higher than those reported in the forests (i.e., 6.62–23.09 g C kg−1, 9.5–20.4 % (Luan et al. 2010)), which are also located in the east edge of the Qinghai-Tibet plateau. However, the carbon content of the LFN (e.g., 267.42, 308.09, and 387.82 g C kg−1 light fraction in the meadow, wet meadow, and marsh, respectively) are similar to the previous studies in the forests (e.g., 270.6–314.4 g C kg−1 light fraction (Luan et al. 2010)), indicating a similar composition of partly decomposed OM (i.e., light fraction OM). Interestingly, a significantly higher C:N ratio of LF was found in the marsh than in the meadows (Fig. 2c), suggesting that wetland degradation results in a higher decomposition extent of light fraction. The significantly higher L LFOC and aromaticity in the marsh than in the meadows further confirm our speculation (Table 2, 3). Nonetheless, the litter input from different species among our sites may also account for the differences in the LFOC, because the plant trait is a major control factor on the soil C sequestration (Russell et al. 2004).
In addition, the variation in the SOC among our sites strongly correlated with both the MBC and LFOC (Fig. 3), which confirms that these labile indicators are effective indicators of soil carbon due to land use change. However, the carbon pool lability represented by the different fractionation methods has different sensitivities to degradation effects (Tables 2, 3). For example, significant reductions in the carbon pool lability after degradation can only be detected by the density fractionation method and 13C NMR technique, indicating that different techniques fractionate the soil labile C pool differently (McLauchlan and Hobbie 2004).
Solid-state 13CP/MAS NMR spectroscopy
Our study found that wetland degradation significantly affects the chemical composition of the SOC (Table 2). The spectra of the top soil in the three sites showed similar structures with various proportions. Among the four C functional groups, O-alkyl-C showed the highest relative intensity, which is consistent with the previous reports in the grasslands (Helfrich et al. 2006) and marshes (Wang et al. 2011). High O-alkyl-C signals in the 13C NMR spectra, representing mainly the plant polysaccharides, suggests a high potential of decomposition in the soil C (Almendros et al. 2000). Therefore, the highest relative intensity of the O-alkyl-C signal further confirms that a low annual temperature results in an accumulation of the labile organic carbon. Nevertheless, wetland degradation leads to a significant reduction in the relative intensity of O-alkyl-C and a significant increase in the relative intensity of alkyl-C, indicating an increase in the proportion of the recalcitrant SOC (Lorenz et al. 2007). Therefore, the decline in the SOC quality caused by wetland degradation results in a higher risk of C loss in the degraded wetlands under the projected future warming because the fractions of the more recalcitrant (lower quality) soil carbon are more sensitive to changes in temperature (Xu et al. 2012; Hartley and Ineson 2008; Craine et al. 2010).
The aryl-C:O-alkyl-C ratio, a useful indicator of the degree of SOM decomposition (Baldock et al. 1997) or substrate quality for microbes, is also significantly affected by the degradation, with the highest value occurring in the meadow and the lowest occurring in the marsh (Table 2). The aryl-C:O-alkyl-C ratio trend among the wetland degradation gradient confirms the effect of wetland degradation on the SOC quality. Similarly, aromaticity (%) has been used to characterize the extent of humification of the SOM (Dai et al. 2001). The lowest aromaticity was found in the meadow (Table 2), and this observation reflects the lowest extent of humification of the SOM in the meadow compared with the marsh and wet meadow. A lower redox potential in the marsh (−157 to −130 mV) than in the wet meadow (−114 to −63 mV) was reported in the same area (Wang et al. 2003; Chen et al. 2008), which can potentially help explain the lower humification extent in the marshes and account for why there are significant relationships among some site variables only when the marshes are omitted from our analyses (e.g., Fig. 3b, d).
Carbon pool lability indicators
There are significant correlations among the labile carbon indicators (i.e., the extractable DOC, MBC and LFOC; Fig. 3). This finding is consistent with previous reports (Laik et al. 2009; McLauchlan and Hobbie 2004; Sparling et al. 1998). A higher proportion of the labile C in the SOM, represented by the labile carbon indicators (e.g., MBC and LFOC), can support a higher microbial biomass, and thus an enhanced microbial activity, which favors the production and/or accumulation of O-alkyl-C (Chen et al. 2004). Therefore, positive linear relationships between the O-alkyl-C intensity and the labile carbon indicators (e.g., MBC and LFOC) were found (Table 3).
Recently, the simpler and more rapid indices of SOC stability or quality are increasingly applied (Plante et al. 2011; Luan et al. 2010; Baldock et al. 1997). Several studies have compared these methods for measuring the labile SOC with significantly different sizes, and found positive correlations among various SOC quality indicators (McLauchlan and Hobbie 2004; Luan et al. 2010; Chen et al. 2004; Plante et al. 2011). In this study, the L LFOC, L MBC, L extractable DOC, aryl-C:O-alkyl-C ratio, and aromaticity (%) were also employed to evaluate the SOC quality based on different fractionation methods. Most of them can reasonably reflect the decomposition or the SOC quality. These indicators were also found to have tight connections to one another, especially the significantly linear relationship between the aryl-C:O-alkyl-C ratio and L LFOC, indicating that the density fractionation method and solid state 13C NMR spectroscopy can detect the soil decomposition extent or quality. Mathers et al. (2003) reported that the aromaticity index is probably a better estimate for the degree of decomposition compared with the aryl-C:O-alkyl-C ratio. However, we did not find a higher sensitivity of aromaticity to wetland degradation than that of the aryl-C:O-alkyl-C ratio (Table 2). Further, the aryl-C:O-alkyl-C ratio better explained the variation of L LFOC among study sites (R2 = 0.92, Table 3) than the aromaticity (R2 = 0.36, Fig. 4). This result suggests that the aryl-C:O-alkyl-C ratio is a better indicator for the SOC quality and decomposition extent.
Conclusions
Our results indicate that the marsh and wet meadow have a larger capacity for storing the SOC in the top 10 cm soil layer, especially those of the labile fractions, compared to the meadow. Also, wetland degradation will lead to a decrease in the carbon pool lability or SOC quality (an increase in the proportion of the recalcitrant organic C). Therefore, there is a high risk of soil carbon loss because both environment change and human activity disturbance can result in an intensive degradation of wetland in the Qinghai-Tibet plateau. In addition, the density fractionation method obtains similar results to the 13C NMR technique in evaluating the SOC quality.
Abbreviations
- SOM:
-
Soil organic matter
- SOC:
-
Soil organic carbon
- TN:
-
Total nitrogen
- C:N:
-
Carbon to nitrogen ratio
- BD:
-
Soil bulk density
- LFOC:
-
Light fraction organic carbon
- LFN:
-
Light fraction nitrogen
- MBC:
-
Microbial biomass carbon
- MBN:
-
Microbial biomass nitrogen
- DOC:
-
Dissolved organic carbon
- DON:
-
Dissolved organic nitrogen
- 13C CP/MAS NMR:
-
Solid-state 13C cross polarization with magic angle spinning nuclear magnetic resonance
- DO C:N:
-
DOC-to-DON ratio
- LF C:N:
-
LFOC-to-LFN ratio
References
Ågren GI (2000) Temperature dependence of old soil organic matter. J Human Environ 29(1):55
Almendros G, Dorado J, González-Vila FJ, Blanco MJ, Lankes U (2000) 13C NMR assessment of decomposition patterns during composting of forest and shrub biomass. Soil Biol Biochem 32(6):793–804
Arevalo CBM, Bhatti JS, Chang SX, Jassal RS, Sidders D (2010) Soil respiration in four different land use systems in north central Alberta, Canada. J Geophys Res 115(G1):G01003. doi:10.1029/2009jg001006
Arevalo CBM, Chang SX, Bhatti JS, Sidders D (2012) Mineralization potential and temperature sensitivity of soil organic carbon under different land uses in the parkland region of Alberta, Canada. Soil Sci Soc Am J 76(1):241–251. doi:10.2136/sssaj2011.0126
Bai J, Ouyang H, Wang Q, Gao H, Ding Q (2009) Changes in landscape patterns of alpine wetlands in Roige plateau before and after drainage. Trans CSAE 25(Supp 1):64–68 (in Chinese with english abstract)
Baldock JA, Oades JM, Nelson PN, Skene TM, Golchin A, Clarke P (1997) Assessing the extent of decomposition of natural organic materials using solid-state 13C NMR spectroscopy. Aust J Soil Res 35(5):1061–1084. doi:10.1071/S97004
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Chen CR, Xu ZH, Mathers NJ (2004) Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Sci Soc Am J 68(1):282–291. doi:10.2136/sssaj2004.2820
Chen H, Gao Y, Yao S, Ning W, Wang Y, Luo P, Tian J (2008) Spatiotemporal variation of methane emissions from alpine wetlands in Zoige Plateau. Acta Ecol Sin 28(7):3425–3437
Christ MJ, David MB (1996) Temperature and moisture effects on the production of dissolved organic carbon in a spodosol. Soil Biol Biochem 28(9):1191–1199
Craine JM, Fierer N, McLauchlan KK (2010) Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat Geosci 3 (12):854–857. doi:10.1038/ngeo1009
Dai KO, Johnson C, Driscoll C (2001) Organic matter chemistry and dynamics in clear-cut and unmanaged hardwood forest ecosystems. Biogeochemistry 54(1):51–83. doi:10.1023/a:1010697518227
Dignac M-F, Knicker H, Kögel-Knabner I (2002) Effect of N content and soil texture on the decomposition of organic matter in forest soils as revealed by solid-state 13CPMAS NMR spectroscopy. Org Geochem 33(12):1715–1726
Ding W, Cai Z, Wang D (2004) Preliminary budget of methane emissions from natural wetlands in China. Atmos Environ 38(5):751–759
Faz Cano A, Mermut AR, Ortiz R, Benke MB, Chatson B (2002) 13C CP/MAS NMR spectra of organic matter as influenced by vegetation, climate, and soil characteristics in soils from Murcia, Spain. Can J Soil Sci 82(4):403–411. doi:10.4141/S02-031
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86(2):320–326
Fissore C, Giardina CP, Kolka RK, Trettin CC (2009) Soil organic carbon quality in forested mineral wetlands at different mean annual temperature. Soil Biol Biochem 41(3):458–466. doi:10.1016/j.soilbio.2008.11.004
Godde M, David MB, Christ MJ, Kaupenjohann M, Vance GF (1996) Carbon mobilization from the forest floor under red spruce in the northeastern USA. Soil Biol Biochem 28:1181–1189
Hartley IP, Ineson P (2008) Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biol Biochem 40:1567–1574
Haynes RJ (2005) Labile organic matter fractions as central components of the quality of agricultural soils: an overview. Adv Agron 85:221–268
Helfrich M, Ludwig B, Buurman P, Flessa H (2006) Effect of land use on the composition of soil organic matter in density and aggregate fractions as revealed by solid-state 13C NMR spectroscopy. Geoderma 136(1–2):331–341
Huang Z, Xu Z, Chen C, Boyd S (2008) Changes in soil carbon during the establishment of a hardwood plantation in subtropical Australia. For Ecol Manag 254(1):46–55
Huo L, Chen Z, Zou Y, Lu X, Guo J, Tang X (2013) Effect of Zoige alpine wetland degradation on the density and fractions of soil organic carbon. Ecol Eng 51:287–295. doi:10.1016/j.ecoleng.2012.12.020
Inubushi K, Brookes PC, Jenkinson DS (1991) Soil microbial biomass C, N and ninhydrin-N in aerobic and anaerobic soils measured by the fumigation-extraction method. Soil Biol Biochem 23(8):737–741
Janzen HH, Campbell CA, Brandt SA, Lafond GP, Townley-Smith L (1992) Light fraction organic matter in soils from long term crop rotations. Soil Sci Soc Am J 56:1799–1806
Kayranli B, Scholz M, Mustafa A, Hedmark Å (2010) Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands 30(1):111–124
Kirschbaum MUF (2006) The temperature dependence of organic-matter decomposition—still a topic of debate. Soil Biol Biochem 38(9):2510–2518
Laik R, Kumar K, Das DK, Chaturvedi OP (2009) Labile soil organic matter pools in a calciorthent after 18 years of afforestation by different plantations. Appl Soil Ecol 42(2):71–78
Leifeld J, Kögel K (2005) Soil organic matter fractions as early indicators for carbon stock changes under different land-use? Geoderma 124:143–155
Lorenz K, Lal R, Preston CM, Nierop KGJ (2007) Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio(macro)molecules. Geoderma 142(1–2):1–10
Lu R (2000) Soil and agricultural chemistry analysis methods. Chinese Agricultural Scientific and Technology Press, Beijing (in Chinese)
Luan J, Xiang C, Liu S, Luo Z, Gong Y, Zhu X (2010) Assessments of the impacts of Chinese fir plantation and natural regenerated forest on soil organic matter quality at Longmen mountain, Sichuan, China. Geoderma 156(3–4):228–236
Luan J, Liu S, Wang J, Zhu X, Shi Z (2011) Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol Biochem 43(3):503–512
Luan J, Liu S, Wang J, Zhu X (2013) Factors affecting spatial variation of annual apparent Q10 of soil respiration in two warm temperate forests. PLoS One 8(5):e64167. doi:10.1371/journal.pone.0064167
Mathers NJ, Mendham DS, O’connell AM, Grove TS, Xu Z, Saffigna PG (2003) How does residue management impact soil organic matter composition and quality under Eucalyptus globulus plantations in southwestern Australia? For Ecol Manag 179(1–3):253–267
McLauchlan K, Hobbie S (2004) Comparison of labile soil organic matter fractionation techniques. Soil Sci Soc Am J 68(2):1616–1625
Miller AE, Schimel JP, Meixner T, Sickman JO, Melack JM (2005) Episodic rewetting enhances carbon and nitrogen release from chaparral soils. Soil Biol Biochem 37(12):2195–2204
Neff JC, Asner GP (2001) Dissolved organic carbon in terrestrial ecosystems: synthesis and a model. Ecosystems 4(1):29–48
Paul EA, Morris SJ, Conant RT, Plante AF (2006) Does the acid hydrolysis-incubation method measure meaningful soil organic carbon pools? Soil Sci Soc Am J 70(3):1023–1035. doi:10.2136/sssaj2005.0103
Plante AF, Fernández JM, Haddix ML, Steinweg JM, Conant RT (2011) Biological, chemical and thermal indices of soil organic matter stability in four grassland soils. Soil Biol Biochem 43(5):1051–1058
Russell AE, Cambardella CA, Ewel JJ, Parkin TB (2004) Species, rotation, and life-form diversity effects on soil carbon in experimental tropical ecosystems. Ecol Appl 14(1):47–60. doi:10.1890/02-5299
Schmidt MWI, Knicker H, Hatcher PG, Kogel-Knabner I (1997) Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10% hydrofluoric acid. Eur J Soil Sci 48(2):319–328
Six J, Elliott ET, Paustian K, Doran JW (1998) Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci Soc Am J 62:1367–1377
Six J, Callewaert P, Lenders S (2002a) Measuring and understanding carbon storage in afforested soils by physical fractionation. Soil Sci Soc Am J 66(6):1981–1987
Six J, Contant RT, Paul EA, Paustian K (2002b) Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–176
Skjemstad JO, Swift RS, McGowan JA (2006) Comparison of the particulate organic carbon and permanganate oxidation methods for estimating labile soil organic carbon. Soil Res 44(3):255–263. doi:10.1071/SR05124
Smith P (2005) An overview of the permanence of soil organic carbon stocks: influence of direct human-induced, indirect and natural effects. Eur J Soil Sci 56(5):673–680
Song CC, Wang LL, Guo YD, Song YY, Yang GS, Li YC (2011) Impacts of natural wetland degradation on dissolved carbon dynamics in the Sanjiang Plain, Northeastern China. J Hydrol 398(1–2):26–32
Sparling GP, Feltham CW, Reynolds J, West AW, Singleton P (1990) Estimation of soil microbial c by a fumigation-extraction method: use on soils of high organic matter content, and a reassessment of the kec-factor. Soil Biol Biochem 22(3):301–307
Sparling G, Vojvodic-Vukovic M, Schipper LA (1998) Hot-water-soluble C as a simple measure of labile soil organic matter: the relationship with microbial biomass C. Soil Biol Biochem 30(10/11):1469–1472
Sun G (1998a) Formation and evolvement of marsh. Territ Nat Res 4:33–35 (in Chinese with english abstract)
Sun GY (1998b) Swamps and peat in Hengduan mountains. Comprehensive scientific expedition for Tibetan of Chinese Academy Sciences. Science Press, Beijing
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707
von Lützow M, Kögel-Knabner I, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, Marschner B (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39(9):2183–2207
Wang D, Ding W, Wang Y (2003) Influence of major environmental factors on difference of methane emission from Zoige Plateau and Sanjiang Plain wetland. Wetl Sci 1(1):63–67 (in Chinese)
Wang G, Li Y, Wang Y, Chen L (2007a) Typical alpine wetland system changes on the Qinghai-Tibet Plateau in recent 40 years. Acta Geogr Sin 62(5):481–491 (in Chinese with english abstract)
Wang G, Wang Y, Li Y, Cheng H (2007b) Influences of alpine ecosystem responses to climatic change on soil properties on the Qinghai-Tibet Plateau, China. Catena 70(3):506–514. doi:10.1016/j.catena.2007.01.001
Wang C, Long R, Wang Q, Liu W, Jing Z, Zhang L (2010a) Fertilization and litter effects on the functional group biomass, species diversity of plants, microbial biomass, and enzyme activity of two alpine meadow communities. Plant Soil 331(1):377–389
Wang H, Liu S-R, Mo J-M, Wang J-X, Makeschin F, Wolff M (2010b) Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China. Ecol Res 25(6):1071–1079
Wang J, Dodla S, DeLaune R, Hudnall W, Cook R (2011) Soil carbon characteristics in two Mississippi river deltaic marshland profiles. Wetlands 31(1):157–166
Whiting GJ, Chanton JP (2001) Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration. Tellus B 53(5):521–528
Wu J (2012) Response of peatland development and carbon cycling to climate change: a dynamic system modeling approach. Environ Earth Sci 65(1):141–151. doi:10.1007/s12665-011-1073-1
Xiang S, Guo R, Wu N, Sun S (2009) Current status and future prospects of Zoige Marsh in eastern Qinghai-Tibet Plateau. Ecol Eng 35(4):553–562. doi:10.1016/j.ecoleng.2008.02.016
Xu X, Luo Y, Zhou J (2012) Carbon quality and the temperature sensitivity of soil organic carbon decomposition in a tallgrass prairie. Soil Biol Biochem 50:142–148. doi:10.1016/j.soilbio.2012.03.007
Acknowledgments
We gratefully acknowledge the support of Dangzhou Hamo, Ta Baima, as well as the Roige National Wetland Reserve for their assistance in field sampling. We would also like to thank Professor Max Finlayson and three anonymous reviewers for their valuable comments on this manuscript. Thanks to Beverly Young and Sarah Furlotte for their improvements on the language.
Funding
This study was funded by the Research Institute of Forestry New Technology (CAFINT2011C09), the Chinese Ministry of Finance (No. 201204201), and China’s National Natural Science Foundation (No. 31200370). J Wu would like to thank Humber River Basin research initiative, Agricultural Research Initiative of NL, and Grenfell Campus’s Start-up research fund for research funding.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luan, J., Cui, L., Xiang, C. et al. Soil carbon stocks and quality across intact and degraded alpine wetlands in Zoige, east Qinghai-Tibet Plateau. Wetlands Ecol Manage 22, 427–438 (2014). https://doi.org/10.1007/s11273-014-9344-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-014-9344-8