Abstract
Day by day, the water sources are increasingly being adulterated due to various reasons including the uncontrolled discharge of pollutants from the point and nonpoint sources. Therefore, it is a timely need to develop suitable, inexpensive and efficient treatment techniques for water purification. This review aims at evaluating different water treatment technologies, their basic principles, cost and suitability for pollutants’ removal from wastewater. Among various water treatment technologies, adsorption technique appears to be techno-economically more attractive due to its inexpensiveness, universality and environment friendliness. Here, wide varieties of adsorbents (silica gel, activated alumina, clays, limestone, chitosan, activated carbon, zeolite, etc.) and their capacities for pollutant removal are described. The limitations of conventional adsorbent applications for water treatment are also discussed. Recently, nanotechnology has introduced nanoadsorbents, which have drawn additional attention due to their unique properties and are considered to be the viable alternative to conventional adsorbents. The potential applications, separation and regeneration of nanoadsorbents for wastewater treatment are also included in this review. Furthermore, prospects including commercial and health aspects of nanoadsorbents are also added.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The first United Nations World Water Development Report addressed water’s importance as “Water for People Water for Life.” Water is the prime need for sustaining all lives on the earth and plays a key role in maintaining ecosystems (Nemerrow 1978; Lehr et al. 1980; Franklin 1991; Helmer and Hespanhol 1997). But in the twenty-first-century reliable access to clean water remains a major global challenge (Qu et al. 2013a, b). Rapid population growth, enormous industrialization, enhancement of agricultural activities to meet food demand, other geological, environmental and global changes have contributed to both the increased variety and volume of pollutants continuously contaminating the water sources (Nemerow and Dasgupta 1991; Tchobanoglous and Franklin 1991; Yang 2011; Ali and Aboul-Enein 2004; da Silva and Gouveia 2020; Karimi-Maleh et al. 2019, 2020; Shamsadin-Azad et al. 2019). As a result, the quality of water sources for industrial, agricultural and human consumption is worsening globally. Therefore, water quality preservation and improvement become a serious concern for scientists, water regulatory authorities and governmental agencies (Ali et al. 2012). Generally, the water pollutants are classified as inorganic, organic and biological pollutants (Gupta et al. 2012; Ali et al. 2019; Elsayed et al. 2019; Karimi-Maleh et al. 2020).
In the last couple of decades, various technologies for water purification and recycling were reported in the literature. These technologies differ in their physical, chemical, biological, thermal and electrical principles. The most important water purification technologies include screening, coagulation–flocculation, filtration, biological treatment, oxidation, reverse osmosis, ion exchange process, distillation, electrochemical dialysis, adsorption, etc. (Barakat 2011; Wasewar 2010; Gupta et al. 2012; Hoque et al. 2018; Tlili and Alkanhal 2019; Blanco et al. 2019). Among these purification techniques, adsorption processes are the most popular treatment method due to its universality, low construction and maintenance costs and ease of operation (Gupta et al. 2012). The removal capacity of inorganic, organic and biological pollutants from wastewater via adsorption can be as high as 99.9% (Ali et al. 2012). The attributes which determine adsorption efficiency are porosity and pore size of adsorbents and their surface area (Singh and Kaushal 2013). Currently, common adsorbents used for wastewater purification process include clays (El-Guendi et al. 1995), activated alumina (Singh and Pant 2004), silica gel (Do 1998), limestone (Aziz et al. 2008), activated carbon (Aggarwal et al. 1999), zeolites (Ming and Dixon 1987), chitosan (Crini 2006), etc. Among all of them, activated carbon is the most extensively used adsorbent (Babel and Kurniawan 2003). Nevertheless, despite its versatility, activated carbon suffers from several shortcomings. Activated carbon shows limited porosity, surface area and pore volume and loses its adsorption capacity quickly. Recovery of adsorption capacity upon regeneration is also poor (Freeman 1998; Taiwo and Adesina 2005). Generally, surface functionalization of the conventional adsorbents is an attractive method to improve the adsorbent performance. However, these functionalized materials require complex, multistep synthesis process which is not amenable for their large-scale production and therefore are expensive (Bhatnagar et al. 2013). In addition, convenient separation of the adsorbent from treated water and removal of pollutants at ppb levels are major challenges for conventional adsorbent (Li et al. 2011; Mohan and Pittman 2006).
Recent advances in nanotechnology showed the potential routes to synthesize nanomaterials are mechanical, solgel, combustion, sonochemical, chemical, microwave and so on from numerous sources such as biomass residues, agriwastes and residues (Biswas et al. 2017, 2019; Rangari et a. 2017). To overcome the aforementioned limitations of the conventional adsorbents, researchers introduced next-generation adsorbents (nanoadsorbents) for the water treatment system (Förstner and Wittmann 1985; Ali et al. 2012). Nanoadsorbent possesses an array of exceptional physical and chemical properties like high surface area, high chemical reactivity, conductivity, catalytic, selectivity, magnetic and optical properties. The high surface area offers a higher number of active sites for various pollutants to react with nanoadsorbent. These properties make them better adsorbent than their corresponding bulk materials (Kalfa et al. 2009; Liu et al. 2005; Hurt et al. 2006; Ilisz et al. 2004). Nanoadsorbents open a potential market in environmental industries at both the domestic and international levels. It is expected that the market value of the nanoadsorbents will reach €1.6 trillion by 2020 and create over a million jobs (Donlan et al. 2009).
Nature of water pollutants
Before addressing water treatment technologies, it is important to have a good idea of the nature of the major water pollutants both qualitatively and quantitatively. The nature and amount of the pollutants found in wastewater vary based on sources such as agricultural, industrial and municipal discharge effluents. The toxicity of these pollutants is only observed when they exceed the permissible limit. Generally, wastewater pollutants are classified into three categories such as organic, inorganic and biological pollutants. These water pollutants may be present in water either in colloidal, solvated or in the suspended form (Gupta et al. 2012).
Inorganic pollutants
Inorganic pollutants include heavy metals found in various waste streams that include industrial wastewater, river sediments, mine drainage, ashes and electronic scraps wastewater (Hoque and Philip 2011). Heavy metal comprises cations of lead, zinc, chromium, cadmium, iron, copper, arsenic and mercury, and anions of nitrates, sulfates, phosphates, fluorides, chlorides and oxalates are common inorganic water pollutants (Gaston 1979; Hutson and Roberts 1990; John 1990). The maximum contaminant level (MCL) established by USEPA (US Environmental Protection Agency) for some heavy metals is mentioned in Table 1 (Babel and Kurniawan 2003).
Organic pollutants
The organic pollutants are very stable in the environment due to one or more cyclic rings in chemical structure, absence of polar functional group and a few halogen substitutions (Ali et al. 2012). The toxic organic pollutants are comprised of pesticides (insecticides, fungicides, herbicides), phenols, fertilizers, plasticizers, biphenyls, polybrominated biphenyls, formaldehyde, greases, detergents, hydrocarbons, oils, pharmaceuticals, etc. (Gaston 1979; Hutson and Roberts 1990; John 1990). Agricultural and chemical industries are the most important sources of organic pollutants.
Biological pollutants
Various biological agents like viruses, bacteria, algae, fungi, amoeba and other worms also pollute water and induce different types of diseases (Gaston 1979; Hutson and Roberts 1990; John 1990). The water pollutants cause serious health problems like kidney damage, nausea, rheumatoid arthritis, chronic asthma, diseases of the nervous system, circulatory system and, in extreme cases, death (Barakat 2011).
Wastewater treatment technology
The wastewater treatment technologies have been classified into three stages: (1) primary, (2) secondary methods and (3) tertiary (Fig. 1). The primary stage includes screening, filtration, coagulation–flocculation, centrifugation, gravity and sedimentation methods. Generally, screening is the first operation in wastewater treatment. These techniques can protect the equipment and/or water plant from potential damage or blockages by removing large non-biodegradable floating solids or sediments present in the wastewater, such as cloth debris, paper, fiber, fecal solids, wood, hair and kitchen refuse. Sometimes, activated silica, alum or iron materials are used to remove non-settable solids by coagulation process (Franklin 1991; Nemerow and Dasgupta 1991; Latifossglu et al. 1997).
In secondary water treatment systems, different biological microbes (usually bacterial and fungal strains) are employed for the removal of different pollutants (Franklin 1991; Kato et al. 1997; Zinkus et al. 1998). These microbes produce different by-products like water, carbon dioxide and ammonia gas after reacting with organic pollutants (Pendashteh et al. 2010; Joss et al. 2006). Tertiary water treatment technologies are needed to achieve a water quality that meets the existing standards for human consumption. This is the final treatment strategy for water purification. The technique includes oxidation, precipitation, ion exchange, adsorption, reverse osmosis, etc. (Gupta et al. 2012).
These water treatment technologies are applied for groundwater, surface water and wastewater treatment purposes. Due to uncontrolled industrial discharge, groundwater is adulterated by toxic metal cations and anions continuously. Tertiary treatment is necessary to remove these pollutants. On the other hand, both secondary and tertiary treatment methods are required for surface water, where contamination is initiated by biological, organic and inorganic pollutants. Generally, utilization of tertiary water treatment methods varies depending on wastewater nature, economic feasibility and efficiency of pollutant removal. For better water treatment a good hybridization of these three treatment technologies is always required. (Gupta et al. 2012).
Current state of the art for wastewater treatment
Generally, water treatment processes are employed for three purposes, i.e., wastewater treatment, water source reduction and recycling. Researchers all over the world are looking for low-cost and effective methods for wastewater treatment and reuse (Gupta et al. 2012). Over the last few decades, numerous processes have been applied to remove metal ions from industrial wastewater. The commonly used techniques for removing water pollutants are filtration, biological treatment, ion exchange process, chemical precipitation, oxidation, electrochemical dialysis and adsorption (Barakat 2011). Here, these water treatment techniques are shortly discussed mentioning their mechanism of action, advantages and shortcomings.
Chemical precipitation
In most of the countries, chemical precipitation is a commonly applied method for the elimination of heavy metal ions from wastewater due to availability of cheap precipitant like lime and limestone (Mirbagherp and Hosseini 2004; Aziz et al. 2008). Besides these, alum, sodium bicarbonates, ferric chloride and ferrous sulfate are also commonly applied precipitants for the chemical precipitation purposes (Gupta et al. 2012). Inorganic effluent with a metal concentration of higher than 1000 mg/L can be treated by lime precipitation as reported in the literature (Aziz et al. 2008). Limestone plays the prime role to remove heavy metals via chemisorption reaction because of its rough surface which provides higher contact areas to react even at very low concentrations. Besides, the pH of the resultant solution goes up above the solubility value due to calcium carbonate of limestones, thus allowing metal impurities to precipitate via forming metal oxides and carbonates (Sturchio et al. 2003; Xu et al. 1996; Stipp et al. 1992). The theoretical mechanism of heavy metal elimination via chemical precipitation technique is shown by the following reaction (Wang et al. 2004):
where M2+, OH− and M(OH)2 imply dissolved metal ions, precipitant and insoluble metal hydroxide, respectively. Here, the removal efficiency can be improved by adjusting the temperature and alkalinity (pH 9–11) (Wang et al. 2004). Aziz et al. (2008) reported that limestone significantly removed (more than 90%) Cd2+ and Cu2+ from aqueous solution at pH 8.5 (Aziz et al. 2008). Though it can remove heavy metals from industrial effluent, the requirement of a large amount of chemicals, excessive sludge formation, poor metal precipitation, slow settling, presence of oil and grease as well as the aggregation of metal precipitates during treatment make its application limited for a broad range of applications (Aziz et al. 2008).
Biological treatment
In this system, organic compounds are degraded in modified sludge tanks through the application of aerobic or anaerobic conditions, under the strict control of temperature and the system’s chemical oxygen demand (COD) (Britto and Rangel 2008).
Aerobic treatment
In aerobic treatment, bacteria and fungi are the most widely used because of their ability to destroy pollutants in the presence of oxygen in biological treatment processes. The prime benefit of the aerobic approach is the production of the non-toxic end product as well as significant influence to remove nitrates, phosphates, BOD, COD, dissolved and suspended organics, etc. (Gupta et al. 2012). Different enzyme-secreting bacteria in the wastewater are selected for their pollutants breakdown capacity (Rai et al. 2005). For example, Kurthia sp strain can efficiently decolorize (92–100%) a wide variety of dyes (organic pollutants) like magenta, pararosaniline, brilliant green, ethyl violet, crystal violet and malachite green (Sani and Banerjee 1999). The successful decolorization of pollutants depends on several factors like temperature and initial pH of the effluent, the concentration of pollutants and the concentrations of dyes (Christie 2007). The aerobic treatment has some shortcomings (Husain 2006; Kulla 1981; Gupta et al. 2012):
-
(i)
development of aerobic bacterial strains is costly and challenging
-
(ii)
no uniform decomposition of synthetic dyes and
-
(iii)
costly due to high production of biosolids and its management
Anaerobic treatment
The anaerobic decomposition or putrefaction occurs when dissolved oxygen is not available in the wastewater (Venkata Mohan et al. 2007; Van Der zee and Villaverde 2005). Delee et al. (1998) demonstrated the potential applications of anaerobic approach for a wide range of artificial dyes. The anaerobic treatment process offers the following advantages over an aerobic treatment process (Delee et al. 1998).
-
cheap and alternative of aerobic process where costly aeration and bulk sludge are a major concern,
-
dye decolorization can be done via the reduction process with low cost but efficient removal of BOD levels,
-
heavy metals can be removed via sulfate reduction,
-
high effluent temperatures are favorable and
-
high pH of effluent can be reduced and thus initiates neutralization of organics.
Zee van der et al. (2001) showed the treatment and decolorization of 20 azo dyes using anaerobic granular sludge and investigated their viability of the process (Van der Zee et al. 2001). Despite these advantages, still, the anaerobic process faces some challenges like removal of BOD is not sufficient, dyes and other organics are not neutralized, nutrients (N, P) are not eliminated and sulfates form sulfide (Delee et al. 1998).
This treatment process requires 20 to 200 US$ per million liters depending on the material used for treatment purposes (Franklin 1991; Zinkus et al. 1998). A simplified representation of the biological treatment is shown in Fig. 2.
Filtration technology
This technology is considered to be the primary technique that received huge attention for drinking water and wastewater treatment applications. This approach showed excellent performance not only for removing suspended solids and organic matters but also for heavy metals and other inorganics (Barakat 2011). Numerous membrane filtration techniques are available that include microfiltration, nanofiltration, ultrafiltration, reverse osmosis and so on which are applicable depending on particle size to be removed (Fig. 3) (Barakat 2011; Gupta and Suhas 2009). To evaluate the potential of ultrafiltration technique, Saffaj et al. (2004) used cheap ZnAl2O4–TiO2 ultrafiltration membranes and achieved 93% Cd (II) and 86% Cr(III) rejection, respectively. This high removal efficiency might be because of higher interactions between the divalent cations (pollutant) and the filter membrane (Saffaj et al. 2004). Based on membrane properties, this ultrafiltration method can attain more than 90% removal capacity at a wide pH range and 2–5 bar of pressure for metal concentration with a range of 10 to 112 mg/L (Kurniawan et al. 2006a, b). Recently, a polymer-supported ultrafiltration method has been introduced for the removal of heavy metal ions from industrial effluent. This method uses soluble polymeric ligand that binds to metal or ions of interest and form macromolecules complexes. These complexes are rejected by the membrane and produce an effluent without targeted metal ions (Rether and Schuster 2003).
In addition to the ultrafiltration method, sometimes polymers have been used as binders to remove metal ions reported in the literature. Those polymers include carboxyl methylcellulose (Barakat 2008), polyethyleneimine (Aroua et al. 2007), diethylaminoethyl cellulose (Trivunac and Stevanovic 2006), etc. The selection of appropriate membranes for filtration technique depends on several factors including characteristics of the wastewater, heavy metals concentrations, pH, temperature, compatibility with the polluted water and cleaning agents to abate surface fouling (Madaeni and Mansourpanah 2003). However, surface fouling exhibits prime difficulty in the filtration approach, which reduces productivity and wide application (Zularisama et al. 2006). Researchers use both physical and chemical procedures for recovering membrane permeability. The membranes have been reported to regain the initial membrane efficiency by the treatment with different chemical agents like sodium hypochlorite (NaOCl), sodium hydroxide (NaOH), hydrochloric acid (HCl) and nitric acid (HNO3). But these regeneration processes are very expensive and may cause unavoidable membrane damage and generate toxic by-products (Park et al. 2002). The cost of this water treatment process ranges from 25 to 450 US$ per million liters of treated water (Franklin 1991; Nemerow and Dasgupta 1991).
Oxidation
Oxidation is one of the most widely used approaches for decolorization of organic matters like dyes. Generally, two forms of oxidation processes are used for industrial wastewater treatment such as chemical oxidation and UV-assisted oxidation. Various kinds of oxidizing agents such as chlorine (Namboodri et al. 1994), hydrogen peroxide (Hage and Lienke 2006), Fenton’s reagent (H2O2 + Fe catalyst) (Wang 2008) and ozone (Wu et al. 2008) are applied for the effluent treatment after the primary treatment (sedimentation) process. Oxidation processes completely degrade the pollutants into low molar mass substances such as nitrogen, sulfates, aldehydes and carboxylates (Gupta and Suhas 2009). It is worth mentioning that the pH of the solution and applied catalysts greatly influence the efficiency of the oxidation process. For example, in the decomposition of the metal–dye complex like chromium, copper, iron and nickel are generated and exhibit a catalytic effect that enhances decolorization. Chlorine is used as a strong oxidizing agent in low-cost approach for decolorizing dye water, but unfortunately, it shows unavoidable side reactions, generates toxic compounds such as trihalomethane, increasing adsorbable organic halogen content in the treated water, and liberates metals in metal–dye complex degradation which encounters corrosion in metallic containers (Gupta and Suhas 2009). The cost of wastewater treatment by oxidation ranges from 100 to 2000 US$ per million liters of clean water (Gupta et al. 2012).
Advanced oxidation processes (AOPs)
Sometimes, a single oxidation process fails to degrade the organic pollutants completely. The process where more than one oxidation processes are applied simultaneously to produce powerful, non-selective hydroxyl radicals in an aqueous medium for pollutant degradation is termed as advanced oxidation processes (Garciamontano et al. 2006). These hydroxyl radicals are highly reactive but unstable due to their high oxidation potential (Mahamuni and Adewuyi 2010). In 2009, Klavarioti and his co-worker discussed and compared different types of advanced oxidation processes, such as ozone-assisted chemical oxidation processes, combined ozone and peroxide, ultraviolet-enhanced oxidation such as UV/H2O2, UV/ozone, UV/air, wet air oxidation, electrochemical oxidation and catalytic wet air oxidation (Klavarioti et al. 2009).
The reaction between hydroxyl (.OH) radical and natural organic matter proceeds in three steps: (i) addition of .OH to double bonds, (ii) abstraction of H-atom resulting carbon-centered radicals and (iii) organic substituents donate an electron to .OH, radical resulting carbon-contained radicals, which react very quickly with oxygen and form peroxyl radicals. At the end of the reaction, ketones or aldehydes and/or carbon dioxide are formed (Kleiser and Frimmel 2000). The rate of the reaction depends on several factors like oxygen, the presence of free radicals and also pollutant concentrations (Parsons 2004). Techniques included in the advanced oxidation processes are photo-Fenton’s oxidation process (Moncayo-Lasso et al. 2009), ultraviolet (UV) photolysis (Gjessing and Kallovist 1991) and ultrasonic irradiation (Nagata et al. 1996). At ambient temperature and pressure, advanced oxidation processes are capable of complete degradation of dyes and also show benefit over biological approach for wastewater treatment comprising toxic or bio-contaminants (Matilainen and Sillanpaa 2010).
Namboodri and Walsh (1996) performed a comparative study between oxidation and advanced oxidation for color removal. They reported that the oxidation alone can only remove 10–20% color, but after the addition of peroxide the removal efficiency reached 90% (Namboodri and Walsh 1996). De Witte et al. (2009) investigated the advanced oxidation process with a 120 mL/min O3 flow rate for removal of ciprofloxacin (pharmaceutical activated product). The highest degradation was found with the highest concentration of ozone (660–3680 ppm) and the lowest concentration of ciprofloxacin (22.64–135.81 µM). The degradation was pH-dependent. At pH 10, the highest concentration (99%) of the degraded product (diethylene ciprofloxacin) was identified using HPLC–MS technique (De Witte et al. 2009). Common pharmaceuticals (diclofenac) were completely removed within 100 min from water by solar-driven photocatalysis. The stock solution concentration was 50 mg/L (Pérez-Estrada et al. 2005). Though advanced oxidation processes have proven potential for dye removal, it has some shortcomings like the formation of undesired by-products, incomplete mineralization, dependent on pH, a high degree of pretreatment obligation and operational challenges. Moreover, the processes are found to be pretty expensive in the small-scale sector, especially in developing countries (Gupta and Suhas 2009; Comninellis et al. 2008).
Ion exchange treatment
Generally, ion exchange involves adsorption of dissolved and colloidal matters from industrial effluent (ionic matter) (Kurniawan et al. 2006a, b). A solid sorbent (ion exchanger) can accomplish exchanging cations or anions with the surrounding substances (Choi et al. 2007). This treatment can remove pollutants at lower concentrations (up to 250 mg L−1) (Gupta et al. 2012). There are two types of ion exchange membranes like anion or cation exchangers. In anion exchanger, the membrane infused with the positively charged ions (NH3+, NH2+, NR3+) which permit anions to pass through, but discard cations. On the other hand, the cationic membranes infused with negatively charged ions (SO32−, RCOO−, PO33−) permit cations to pass through rejecting anions. Based on the preparation method, the membranes are categorized into heterogeneous and homogeneous (Xu 2005). Polymeric membrane (styrene and acrylic resins) is mostly used because of its structural integrity, chemical inertness and great selectivity (Dickert 2007). The basic principle of the ion exchange process is described in Fig. 4.
Synthetic organic ion exchange resins (Barakat 2011), zeolites (Vassilis 2010) are found to remove pollutants such as Cr3+, Fe3+, Cu2+, Pb2+ and NH3. However, this kind of technique has some disadvantages like expensive membranes, poor electrochemical properties and high porosity (Xu 2005; Nagarale et al. 2006). Moreover, it needs backwashing and regeneration after long use. Sometimes, the organic materials fix the resin and cause the appearance of fouling in the membrane (Üstün et al. 2007). Ion exchange is a low-cost reversible process because the adsorbent resin can be used for a long period before replacement is mandatory (Homem and Santos 2011). The cost varies from 50 to 200 US$ for the treatment of one million liters of wastewater (Gupta et al. 2012).
Electrodialysis
Electrodialysis technique for water pollution management was developed based on electrochemistry principles, where ionized species are separated by applying a high voltage on both sides of an ion exchange membrane (Barakat 2011) (Fig. 5). The basic difference between electrodialysis and ion exchange membrane treatment process is that in the ion exchange process the concentration difference (diffusion and dialysis) is the driving forces, whereas, in electrodialysis, the externally applied electric potential is the driving force (Koter and Warszawski 2000). Generally, electrodialysis cell is made of thin films of polymeric products having either anionic or cationic in nature (anion exchange membrane and cation exchange membrane) (Chen 2004). The ionic functional groups of the membrane originate from the chemical nature of the polymer (Blackburn 1999). Such anionic exchange membranes have positive fixed charges due to the presence of quaternary amine groups in the polymer; in contrary, cation exchange membranes have negative charges resulted due to the presence of sulfonic acid groups in the polymer. A solution containing ionic species is added to an electrodialysis cell. Then, potential is applied by the electrodes on both sides of the dialysis cell. The anionic exchange membranes with fixed positive charges attract anions and allow them to pass through and reject cations. The reverse scenario works in a cationic exchange membrane (Blackburn 1999).
Electrodialysis can provide high selectivity for successful separation and recovery of the desired compounds and metals (Scott 1995). The electrodialysis cell can be used as a reactor for obtaining desirable products from both electrolytes and chemicals. The successful operation of the cell depends on selecting the cell structure and the right operating conditions. Electrodialysis ensures high water recovery after treatment. It also reduces water hardness. The flexibility to operate when needed and no requirement of any toxic chemical make this treatment process attractive compared to other processes (Koter and Warszawski 2000).
Moreover, electrodialysis not only removes undesirable impurities from water but also produces highly concentrated liquor for recovery for reuse, such as valuable metals Cr and Cu (Blackburn 1999). The success of the electrodialysis depends on membrane characteristics, feedstock nature, composition and process parameters such as initial flow rate, operation conditions and cell geometry (Mohammadi et al. 2004). Due to long use and higher current, membrane fouling occurs and consequently decreases the efficiency of the membrane. The presence of other ions also decreases the efficacy of the membrane separation process (Korngold et al. 1998; Messalem et al. 1998; Hansen et al. 1997). Pretreatment is necessary to avoid membrane fouling. For better performance, electrodialysis process needs a continuous supply of new feedstock, continuous monitoring on the operation and regular maintenance to avoid damages. The treatment cost ranges from 15 to 400 USD (Gupta et al. 2012) (Table 2).
Sometimes, combined techniques are applied for water pollutants removal; for example, in India, Kenya, Senegal and Tanzania, the most commonly used technique for defluoridation is the Nalgonda technique. In this method, the required amounts of alum, bleaching powder and lime are applied to treat water. After that, the water proceeded with disinfection, flocculation, filtration and sedimentation. The whole process takes 2–3 h in a batch experiment. But this treated water contains 2–7 mg/L residual aluminum concentration which is higher than the WHO standard (0.2 mg/L) (Ayoob et al. 2008; WHO 2004; Meenakshi and Maheshwari 2006).
Adsorption
Reverse osmosis, ion exchange and oxidation are commonly used technologies for wastewater treatment. These methods introduce toxic secondary pollutants into the ecosystem which make their use limited in wastewater treatment at potable and industrial levels (Crini 2005; Gaya and Abdullah 2008). Therefore, there is a need to explore other alternative techniques, which are effective in removing pollutants and are economical at the same time. The increasing environmental awareness and concern among scientists led to developing the adsorption technique, which is effective for purification and separation of water and wastewater pollutants. Among all the water purification treatment methods, adsorption is one of the popular techniques which is meant to be an efficient, economical and eco-friendly technique for wastewater treatment (Crini 2006). Adsorption is the mass transfer process where pollutants are concentrated or adsorbed on a solid substrate from the liquid phase or gaseous surroundings in contact with the substrate. Here, pollutants are referred to as adsorbate and substrate as an adsorbent (Singh and Kaushal 2013).
Kayser in 1881 first introduced the term adsorption in the literature and postulated the adsorption process as a surface accumulation of materials (Dabrowski 2001). It is a surface phenomenon if the adsorbate or the species are attached to the adsorbent surface physically without any chemical bonding; the process is denoted as physisorption. In physisorption, the bonding forces are van der Waal forces, weak H-bonds, hydrophobicity, interaction due to polarity and steric effect, dipole–dipole interaction, π–π interaction, etc. (Ali et al. 2012). On the other hand, a chemical bonding also participates in the adsorption process, referred to as chemisorption (Ali et al. 2012). It is hard to remove chemisorbed compounds from the substrate (Yadla et al. 2012). The pollutants are adsorbed onto solid sorbents surface in three stages: 1. transport of pollutants from balk solutions to the adsorbent; 2. adsorption on the surface of any particles; and 3. transport within the absorbent particle (Barakat 2011). The mechanism of adsorption is shown in Fig. 6.
Different factors affect the adsorption process, such as pH, temperature, types of adsorbates and adsorbents, the concentration of pollutants, the presence of other pollutants, contact time, surface functional group and other atmospheric and experimental conditions. Sometimes, prefiltration is required to avoid the unwanted effect of suspended particles, oils and greases in water during adsorption (Ali 2010). The adsorption process is especially suited for wastewater treatment and more so in the case of high concentration but a low volume of wastewater. Watonabe and Ogawa (1929) first demonstrated the application of activated carbon heavy metals adsorption. The adsorption process is effective to remove soluble as well as insoluble organic pollutants. The removal efficiency can be up to 99.9% as reported in the literature (Ali et al. 2012). As a result, researchers considered that adsorption is the greatest universal method as it can remove various types of inorganic as well as organic pollutants from wastewater. It is economically viable for the treatment of a large volume of wastewater due to low construction, maintenance and operation cost of the adsorption equipment (Gupta et al. 2012). The adsorption method has the benefit to remove analytes instead of creating potentially more hazardous metabolites (Rivera-Utrilla et al. 2009; Putra et al. 2009). Many industries like dyes, textiles, paper and plastic discharge different types of dyes in the water. These dyes are detrimental to aquatic life (O’Neill et al. 1999). The removal of these color substances is very difficult because the dyes are intractable organic molecules, inert to aerobic digestion, stable under light, heat and oxidizing agents (Ravi Kumar et al. 1998). Adsorption shows the best results for the removal of various kinds of dyes (Ho and McKay 2003; Derbyshire et al. 2001; Jain et al. 2003).
Different conventional water treatment techniques like pre-/post-chlorination, coagulation/flocculation/filtration, cannot efficiently remove cyanobacterial toxins from wastewater (Lambert et al. 1996; Huang et al. 2007; Momani et al. 2008). For example, the flocculation treatment can eliminate cyanobacterial cells from water by an appropriate concentration of flocculent. But the treatment increases the concentration of cyanobacterial toxins by cell lysis, which cannot be removed by this treatment (Lepistö et al. 1994). Huang et al. (2007) reported successful removal of cyanobacterial toxins by the adsorption process (Huang et al. 2007).
Adsorption has advantages over conventional techniques, which are shown below (Volesky 1999):
-
Cheap Adsorbent is often made from abundant agricultural or waste materials, which makes adsorbents cheap.
-
Metal selective The metal sorbing capability of different kinds of biomass adsorbents can be dependent on metal ions as well as the nature of biomass, composition in the solution, preparation and physicochemical treatment of biomass.
-
No sludge generation Unlike other techniques, no secondary sludge generation occurs with adsorption.
-
Regenerative The adsorbent can be regenerated and used for several cycles of adsorption.
-
Metal recovery It is possible to recover metal (pollutants) ions after being sorbed.
Major operating variables of adsorption
Based on the review of the latest published research on the application of adsorption process for water treatment, major operating variables are discussed below.
Effect of initial concentration and contact times on adsorption of pollutants
The initial concentration and contact time influence the metal ion (Cu2+) adsorption shown in Fig. 7. At pH 6.0 and adsorbent dose 2 g/L, the adsorption experiment was carried out at initial concentration varying from 25 to 100 mg/L. The figure shows that the metal ions adsorption increased simultaneously with the increase in contact time at all concentration, and after 3 h, the adsorption became constant. It indicates that the contact time was not dependent on metal ion concentration. The optimum uptake period of Cu2+ adsorption was about 3 h. The adsorption of Cu2+ increased from 11.92 to 36.40 mg/g as the initial concentration changing from 25 to 100 mg/g (Anirudhan and Sreekumari 2011).
Comparison of experimental contact time data and the fittings to pseudo-first-order and pseudo-second-order kinetic models for the adsorption of Cu (II) onto AC at different concentrations (Anirudhan, and Sreekumari 2011)
But the different scenario was found, when Lv et al. (2011) studied different initial concentrations (10–60 mg/L) of Cr(VI) for removal by nanocomposite. The complete removal was found at concentrations 10, 20 and 40 mg/L. However, at a concentration of 60 mg/L, the removal capacity decreased to 40% as shown in Fig. 8. The reason might be the lack of available sites for adsorption in the constant amount of nano-zerovalent particles for high Cr(VI) concentration (Lv et al. 2011).
Effect of initial Cr(VI) concentration on Cr(VI) removal (pH, 7.0; nZVI-to-MWCNTs mass ratio, 1:2; ZVI amount 0.1 g/L) (Lv et al. 2011)
Effect of pH on adsorption
pH has a direct effect on pollutant removal. The adsorbent should be stable under working pH conditions. For example, the efficiency of Cr(VI) removal reduced from 100% to 91% with increasing pH from 5.0 to 9.0. The reason is electrostatic repulsion between nano-zerovalent iron multiwalled carbon nanotube (composites) and dichromate ions. The positive zeta potential of nano-zerovalent iron multiwalled carbon nanotube (nanocomposites) at pH < 7.5 accelerated attraction of negatively charged Cr contaminant and resulted in the highest removal efficiency and kinetics. When pH raised above the pH (point of zero charges), negatively surface charge accelerated electrostatic repulsion (Lv et al. 2011; Pradhan et al. 1999; Das et al. 2000).
The influence of pH was further investigated with activated carbon at pH range (2–9) to remove Cu (II) and Hg(II) as shown in Fig. 9. The increase in the pH value of the solution increased the pollutant removal percentage. The point of zero charges of activated carbon (adsorbent) was 5.2. Below pH 5.2, the surface of the activated carbon remains positive due to the presence of H+; as a result, electrostatic repulsion occurs between adsorbent and metal cations. On the other hand, above pH 5.2 the surface charge of the adsorbent becomes negative and electrostatic attraction occurs between adsorbent and cationic pollutants. The optimum value of pH for the removal of Cu (II) and Hg(II) was observed to be 6 and 7, respectively (Anirudhan and Sreekumari 2011).
Influence of pH on the adsorption of pollutants (Hg and Cu) by activated carbon. [condition: adsorbent dose 2 g/L; temperature 30 °C; equilibrium time 3 h; concentration 25 mg/L (Anirudhan and Sreekumari 2011)
Effect of ionic strength on adsorption
The ionic strength (i.e., electrolyte concentration) of a solution influences the binding of adsorbate and adsorbent. It influences the interface potential and width of the double layer around the adsorbent. Generally, the outer sphere complexes are more susceptible to ionic strength disparities than inner-sphere complexes of the adsorption process. For example, an experiment was performed in three different ionic strength solutions (0 M, 0.05 M and 0.1 M) containing Cr(VI). At strength 0 M, the removal efficiency reached above 95% by 2 h; when it raised to 0.05 M, the rate of Cr(VI) removal was enhanced and within 1 h it showed above 95% removal efficiency. However, the removal efficiency decreased to 81% within 2 h, due to the increase in ionic strength (0.1 M). The moderate ionic strength (0.5 M) might strengthen the adsorbent capacity for the high removal of dichromate ions. On the other hand, high ionic strength would have the opposite effect (Lv et al. 2011). Hayes and Leckie (1987) postulated that the effect of electrolyte concentrations on adsorption can replicate the adsorption type (Hayes and Leckie 1987).
Adsorption isotherms are used to investigate the solute–solvent interaction, adsorption capability of adsorbent and accumulation degree of adsorbate on the adsorbent surface (Anirudhan and Sreekumari 2011). The two most common isotherms Langmuir and Freundlich are used extensively in the literature to elucidate the application of various adsorbents (Mor et al. 2007; Arslan and Pehlivan 2007). Langmuir isotherm describes the monolayer formation of adsorbent on a surface which contains a finite number of binding sites, without any lateral interaction between adsorbed molecules (Arslan and Pehlivan 2007). On the other side, Freundlich isotherm describes the multilayer adsorption having interaction among adsorbed molecules (Anirudhan and Sreekumari 2011).
Effect of adsorbent
The efficacy of adsorption depends on the porosity, surface area and pore size of the adsorbent (Singh and Kaushal 2013; Estevinho et al. 2007). To be an effective adsorbent, the pore size of the adsorbent and the diameter of the adsorbate molecule should be comparable to each other (Tinge et al. 1987). The characteristics of good adsorbents are high adsorption volume and quick separation of a large amount of solutions, selectivity, renewability, high porosity resulting large surface area, chemical and physical stability, low diffusion resistance, very low solubility in the respective contacting fluid, sorbent preservation and its properties, high hardness and compressive strength to prevent crushing and erosion, high confrontation to biofouling to extend long life, no side reactions, suitable for both batch and continuous processes, compatibility and cost-effective (Singh and Kaushal 2013; Ali et al. 2012; Mayadevi 1996; Mohanty et al. 2006; Mei et al. 2010). Ali et al. (2012) also gave importance to the presence of a high volume of carbon or oxygen in the adsorbent moiety for better adsorption.
The most commonly used adsorbents for wastewater treatment are clay (El-Guendi et al. 1995), activated alumina (Singh and Pant 2004), silica gel (Do 1998), limestone (Aziz et al. 2008), activated carbon from different raw materials (Aggarwal et al. 1999; Rao et al. 2009; Kadirvelu et al. 2001; Mohan et al. 2000), zeolite (Ming and Dixon 1987), and chitosan (Crini 2006), etc. The adsorbents are discussed in the following section.
Clays
Clay materials got attention and used as adsorbent due to their abundance, low cost, high adsorption and ion exchange properties (Crini 2006). In recent years, clay materials have been applied for the removal of inorganic and organic pollutants. Various researchers studied clay particles for dyes removal from wastewater (El-Guendi et al. 1995; Alkan et al. 2004; Ozdemir et al. 2004; Gürses et al. 2004; Alkan et al. 2005). Different types of clay materials like smectites (saponite, montmorillonite, etc.), kaolinite, mica (illite), serpentine, pyrophyllite (talc), vermiculite and sepiolite are used for pollutant removal purposes. Among them, montmorillonite clay exhibits the highest surface area and cation exchange efficiency (Shichi and Takagi 2000). The adsorption abilities of clay materials are due to the net negative charge which came from the chemical structure of minerals. Clay materials can adsorb positively charged species due to this negativity. Besides, their high sorption behavior is due to the high surface area which can go up to 800 m2/g and high porosity (Alkan et al. 2004; Cadena et al. 1990). In 1996, El-Guendi investigated the removal performance of natural clays and activated clays for cationic dye such as Basic Blue 69 and Basic Red 22 from waste aqueous solution. Natural clays showed 390 mg/g and 365 mg/g adsorption capacities (maximum, qmax) for Basic Blue 69 and Basic Red 22, respectively. The adsorption efficiency increased by 23% for Basic Blue 69 and 13% for Basic Red 22 after activation of natural clays with H2O2 (El-Guendi 1996). Like other adsorbent materials, clay materials can be modified to improve their ability to remove pollutants. Ozdemir and his group (2004) modified sepiolite with quaternary amines and applied to adsorb different types of azo-reactive dyes. This experiment exhibited substantially improved adsorption of the pollutants (Ozdemir et al. 2004). Though clay materials are considered as low-cost adsorbent, the removal efficiency of clays for heavy metals is not adequate (Babel and Kurniawan 2003).
Zeolites
Zeolites are microporous in structure and can be found both naturally as silicate minerals and prepared synthetically like magnetically modified zeolite, bio-zeolite, etc. (Ming and Dixon 1987; Adebajo et al. 2003; Nah et al. 2006; Bai et al. 2010). Clinoptilolite is the most abundant zeolite in nature (Ming and Dixon 1987). They are also used as an adsorbent due to high ion exchange capability (Adebajo et al. 2003). Babel and Kurniawan (2003) and Bose et al. (2002) reported high selectivity of clinoptilolite (natural zeolites) for some specific heavy metal ions like Pb2+(2.4 mg/g), Cd2+(1.6 mg/g), Zn2+(0.5 mg/g) and Cu2+(1.64 mg/g). Magnetically modified zeolite prepared by Nah et al. (2006), exhibited high adsorption capabilities for the Pb2+ ion and good chemical inertness in the pH range 5–11. In 2010, Bai et al. applied bio-zeolite for wastewater treatment containing pyridine and/or quinoline. This adsorbent is comprised of mixed bacteria and reformed zeolites. Bacteria degraded the organic pollutant, and the modified zeolite removed the ammonium ion derived from pyridine and quinoline degradation (Bai et al. 2010). Although zeolites originate from low-cost natural resources, their selectivity and competitive adsorption to various ions make their use limited. Moreover, these materials do not show the good capability to adsorb anionic ions and organics. Further modification of zeolite is needed to improve its efficiency to adsorb organics and anions and can be done via acid wash, ion exchange and/or surfactant functionalization (Wang and Peng 2010). Zeolites show low permeability and require external support during column operations (Calzaferri et al. 2000).
Activated alumina
A highly porous, granular crystalline gel used as an adsorbent (Singh and Pant 2004) due to having a high surface area (Do 1998) varies from 200 to 300 m2 g−1. Researchers have used alumina due to its amphoteric properties (Naiya et al. 2009) for the removal of dyes (Huang et al. 2007) as well as other ions such as cadmium, led, arsenate and arsenite (Naiya et al. 2009). Activated alumina was also investigated for defluoridation of water. At neutral pH (pH = 7), adsorption efficiency was found to be 1450 mg/kg (Ghorai and Pant 2004). This defluoridation occurred by nonspecific adsorption. But alumina fluoro complexes were formed due to the presence of aluminum ions in the treated water at pH < 7. However, the regeneration of activated alumina can lower its removal efficiency of pollutants.
Silica gel
Various workers also used silica gel as an adsorbent for its comparatively higher surface area (250 to 900 m2 g−1) than alumina (Do 1998). It also has some other advantages like local availability and high thermal resistance (Jal et al. 2004; Sharma et al. 2003). Some researchers also modified this material by salinization techniques to enhance pollutant removal efficiency (Jal et al. 2004; Sharma et al. 2003). Preliminary researches reported that various elements from wastewater, such as Pb, Cd, Zn, Cu, Fe and Mn, could be effectively removed by functionalized silica gels (Jal et al. 2004; Sharma et al. 2003). Though the adsorption of basic dyes onto silica was significant, the high price of silica restricts its wide applications as adsorbent (McKay et al. 1999; Woolard et al. 2002); moreover, the regeneration of adsorbent is a complicated process (Seshadri and Kettrupt 1982). Silica shows high sensitivity toward alkaline solutions that limit the usage in the media of pH higher than 8 (Ahmed and Ram 1992).
Chitosan
Chitosan, a cationic amino polysaccharide biopolymer, gets extra attention from researchers due to its sorption capacity of dyes and heavy metals at very low concentrations (ppm or ppb levels) (Crini 2006). Among other biomaterials, chitosan provides high reactivity, better chemical inertness and selectivity toward pollutants (Ravi Kumar 2000; Varma et al. 2004; Guibal 2004). The structural flexibility, presence of hydroxyl group and primary amino groups in the chemical structure favor the formation of a stable configuration for adsorption of pollutants (metal ions) (Babel and Kurniawan 2003). Natural chitosan has been modified to improve the adsorption capability for different types of pollutants (Ravi Kumar 2000). Zhu et al. (2012) synthesized xanthate-modified magnetic chitosan and used it for adsorption of Pb(II), Cu (II) and Zn (II). The highest adsorption efficiency was found to be 76.9, 34.5 and 20.8 mg/g for Pb(II), Cu (II) and Zn (II), respectively. Chitosan also has some disadvantages in wastewater treatment. Chao et al. (2004) stated that chitosan has a low attraction for cationic (basic) dyes. The powder/flake-form chitosan is responsible for pressure drop in the sorption column which eventually lowers the adsorption efficiency. Also, chitosan is acid sensitive which triggers the necessary physical and chemical modification of chitosan powder before using in acid condition (Crini 2006).
Activated carbon
Activated carbon, among the conventional adsorbents, is the most prevalent and extensively used adsorbent for wastewater treatment all over the world (Babel and Kurniawan 2003). The US Environmental Protection Agency (EPA) has referred to the activated carbon-based adsorption as one of the best technologically feasible methods (Derbyshire et al. 2001). The credit goes to Raphael von Ostrejko, who first developed commercial activated carbon and got patent in 1900 and 1901. The most common organic precursors are wood, olive stones, anthracite coal, bituminous coal, lignite, peat shell, and almond and coconut shell. The carbon contents of those materials vary from 40 to 90% (w/w) (Bansal et al. 1988; Holden 1982).
Preparation and surface modification
The activated carbons are prepared by controlled pyrolytic decomposition of the precursors (Fig. 10) (Fitzer et al. 1971). During carbonization, different low molar mass volatiles, light aromatics and hydrogen gas are released (Hucknall 1985). The resulted fixed carbonaceous char contains tarry pyrolysis residue, which blocks the pores of the char. The resulted char can be activated by physical or chemical treatment (Ali et al. 2012). The physical treatment requires high temperature and steam, CO2 and air as an activator (Lizzio et al. 1990). In chemical treatment, different activators such as ZnCl2, H3PO4, H2SO and KOH are impregnated into the precursors. The final material is carbonized followed by an activation step in a single action by two different temperatures (Smíšek and Černý 1970). Generally, the required temperature for chemical activation is 400–800 °C (Ali et al. 2012). This catalytically activated sample further needs a post-activation treatment to remove the residual catalyst. The advantages of chemical treatment over physical treatment are (1) it needs low temperature and (2) the yield is high because the burning of char can be avoided.
The heating rate for carbonization and activation is the prime process parameter, which influences pore structure consisting of macro-, meso- and micropore, surface area and surface functional group (Rodriguez-Reinoso 1986; Wigmans 1986; Bhatnagar et al. 2013). The key functional groups of the activated carbon are carboxyl, carbonyl, phenols, lactones and quinines. These functional groups arise by thermal treatment, activation process and post-chemical treatment on the activated carbon surface. The properties and concentration of functional groups are modified by appropriate chemical or thermal treatment to enhance the adsorption efficiency of activated carbon (Bhatnagar et al. 2013). Different researchers reported that activated carbon comprises both cationic and anionic ions (Lukens 2007). During basic treatment, the increasing trend of net cationic ions is due to a decrease in the number of anionic ions on the activated carbon surface (Lukens 2007). Generally, surface modification is done after the activation process. The alteration can be performed by various approaches that include base treatment, acid treatment, microwave treatment, impregnation treatment, plasma treatment, ozone treatment and surfactant treatment (Bhatnagar et al. 2013). Some of the surface modification processes are discussed below:
Acid treatment
It oxidizes the surface of the porous activated carbon as well as improves its hydrophilic properties (Shen et al. 2008). During the acid treatment, huge percentages of oxygen-containing functional groups appear on the carbon surface by replacing hydroxide groups and thus increase cation exchange properties (Ahn et al. 2009). The acidic treatment removes mineral elements from activated carbon (Shen et al. 2008). Generally, nitric acid and sulfuric acid are most widely used for acid treatment (Bhatnagar et al. 2013). Here, metal ions form complexes due to strong interaction with the negatively charged acid groups (electrostatic adsorbate–adsorbent interaction) by replacing (H+) ions from the oxidized carbon surface (Bhatnagar et al. 2013). The adsorption method depends on pH competing for metals. The capacity of activated carbon to adsorbed metallic compounds is closely related to the number of surface functional groups (Ahn et al. 2009). Jia and Thomas (2000) oxidized activated carbon by nitric acid treatment and reported the incorporation of acidic oxygen functional group (carboxylic acid group, phenol and quinine groups). This surface-modified activated carbon shows cation exchange behavior over long range of pH values as well as exhibited multifunctional nature (Jia and Thomas 2000). However, this oxidation treatment sometimes decreases the surface area and reduces carbon porosity (Alvarez-Merino et al. 2005).
Base treatment
This treatment creates a positive surface charge of the activated carbon which adsorbs negative pollutants in higher amounts (Menendez et al. 1996; Faria et al. 2004; Shaarani and Hameed 2011). Here, activated carbon is treated at an elevated temperature in inert, hydrogen or ammonia atmosphere (Faria et al. 2004; Shaarani and Hameed 2011). The basic surface properties are due to the development of basic nitrogen functionalities like amines, amides, protonated amides, pyridine-type structures, etc. (Mangun et al. 2001; Jansen and Vanbekkum 1995; Raymundo-Pinero et al. 2003). This surface-functionalized activated carbon adsorbs organic dye molecule by electrostatic forces or dispersive interaction. The dispersive forces arise due to the delocalization of π electrons, which are present at Lewis basic sites in the basal planes of a carbon atom and free electrons of the dye molecules present in the aromatic rings (Bhatnagar et al. 2013). Przepiorski (2006) modified the activated carbon by gaseous ammonia at 400 to 800 °C. The resulted adsorbent shows 29% more adsorption capacity for phenol than untreated one (Przepiorski 2006). Shaarani and Hameed (2011) also modified the surface through ammonia and revealed that the adsorption capacity for 2,4-dichlorophenols increased from 232.56 to 285, 71 mg/g.
Impregnation
The impregnation technique involves the fine distribution of chemical/metal particles in activated carbon pores. Iron (Vaughan Jr and Reed 2005), copper (Yeddou et al. 2011), silver (Miyanaga et al. 2002) and aluminum (Tchomgui-Kamga et al. 2010) are deposited on the activated carbon using the impregnation process. Huang and Vane in 1989 synthesized iron impregnated activated carbon for arsenic removal. They reported a tenfold increase in arsenic removal by treated activated carbon than untreated activated carbon. The enhancement of adsorption was due to the adsorption of ferrous ion and the formation of arsenate complexes (Huang and Vane 1989). The main advantages of impregnated carbon include catalytic properties optimization of activated carbon by enhancing its inherent catalytic oxidation properties and promote synergism between activated carbon and the impregnated material.
The adsorbents produced by these aforementioned methods possess highly porous morphology with a high surface area of 500 to 2000 m2 g −1 (Carrott et al. 1991). The adsorption on activated carbon usually happens through van der Waals forces. Different types of activated carbon are commercially available in the market, such as powdered activated carbon (PAC), granular activated carbon (GAC), activated carbon pellet and activated carbon fiber (ACF). Among them, granular activated carbon is mostly used due to its adaptation to continuous interaction with pollutants and no need of additional step to remove the carbon from the bulk (Najm et al. 1991). Activated carbon is extensively used as adsorbents to remove various types of dyes (Al-Degs et al. 2001; Pelekani and Snoeyink 2000), organic and inorganic pollutants such as metal ions (Gabaldón et al. 2000) phenols (Carrott et al. 2005), pesticides (Hu et al. 1998), humic substances (Lee et al. 1983), chlorinated hydrocarbons (Urano et al. 1991), polychlorinated biphenyls (Pirbazari et al. 1992), detergents (Malhas et al. 2002), organic compounds which cause taste and odor (Lalezary et al. 1986) and many other chemicals and organisms (Carrott et al. 2000).
Removal of inorganic pollutants by activated carbon
Rao et al. synthesized activated carbon from Ceiba pentandra hulls and used it for Hg2+ removal. At pH 6, this activated carbon showed higher adsorption capacity (25.88 mg/g). The increased adsorption capacity is because of the presence of sulfur groups on the surface of activated carbon. Since Hg2+ is a soft Lewis acid, according to Pearson theory, it will interact with surface sulfur groups (soft bases). The adsorption isotherm is well fitted to the Freundlich model and adsorption kinetics followed pseudo-second-order models. Here, the adsorption equilibrium state is achieved by the hydrophilic character (Rao et al. 2009) and also by the affinity of activated carbon to mercury species. Kadirvelu et al. 2004 prepared activated carbon from waste materials of the sago industry by treating with H2SO4 and (NH4)2S2O8. The adsorption capacity of Hg2+ was observed to be 55.6 mg/g at pH 5.0. The adsorption process obeyed Langmuir equilibrium isotherm model (Kadirvelu et al. 2004).
Arsenic (As) adulteration in pure drinking water leads to serious health problems. EPA recommended the arsenic level for drinking water 0.01 mg/L (Bohlen 2002). During the last few decades, activated carbon is widely applied to remove arsenic from contaminated water (Budinova et al. 2009; Mondal et al. 2008a; b; Navarro and Alguacil 2002; Mohan and Pittman 2007; Kalderis et al. 2008, Daus et al. 2004). Asadullah et al. (2014) prepared activated carbon from jute stick and loaded with iron by impregnation method. They used it for separation from polluted water. The presence of iron oxide on activated carbon initiates the oxidation of As (III) into As (V) which can more strongly be adsorbed on the surface (Mondal et al. 2007, 2008a; Fierro et al. 2009). The iron-loaded chemically activated carbon reduced as concentration to 3 µg/L when the initial concentration was 100 µg/L and pH 7 (Asadullah et al. 2014). Iron oxide-loaded activated carbon removed 99.90% As (V) within 5 min of the batch adsorption process. This highest adsorption capacity was observed 27.78 mg/g at pH 7. The adsorption process well fitted both Langmuir and pseudo-second-order kinetic models (Yurum et al. 2014). In 2011, Kocabas and Yurum used iron-loaded red mud for arsenic removal. At pH 2.0, the adsorbent showed a maximum capacity of 11.64 mg/g (Kocabas and Yurum 2011).
Alslaibi et al. (2013) synthesized microwave-irradiated activated carbon from olive stone and investigated its performance for Cd2+ removal for aqueous solution. Microwave treatment provides lesser holding time for heating than the conventional heating method. This olive stone-activated carbon showed an adsorption capacity of 11.72 mg/g for Cd2+ removal. The adsorption followed Langmuir isotherm, i.e., monolayer adsorption occurred on the activated carbon surface. The value well fitted with pseudo-second-order kinetics and directs chemisorption which is the rate-controlling reaction step during Cd2+ removal (Alslaibi et al. 2013). Activated carbon synthesized from coconut buttons was investigated through batch adsorption process to remove heavy metal ions like Pb2+, Hg2+ and Cu2+. The activated carbon was synthesized by steam activation at 400 °C. The adsorbent shows good adsorption at pH 6.0 for Pb2+, Cu2+ and Hg2+ at pH 7.0. The monolayer adsorption capacity for Pb2+, Hg2+ and Cu2+ was found to be 92.72, 78.84 and 73.60 mg/gm, respectively, at 30 °C. The adsorption isotherm best fitted with the Freundlich model and indicates a heterogeneous surface. The adsorption obeys the pseudo-second-order kinetics model. The adsorption process was influenced by the concentration of metal ions, contact time, solution pH, ionic strength and adsorbent concentrations (Anirudhan and Sreekumari 2011).
Removal of organic pollutants by activated carbon
Activated carbon has also been extensively used to eliminate different classes of industrial dyes from water. McKay used activated carbon filtrasorb type (1.4–2.8 mm) to remove dyestuffs from water and observed outstanding adsorption for acidic, basic and dispersed dyes compared to direct dyes (Mckay 1982). Mendez-Diaz et al. (2010) carried out adsorption of imidazole, sulfonamides and trimethoprim on activated carbon and found almost 90% removal of pollutants. Similar research was studied by Kim et al. (2010) where they investigated trimethoprim adsorption as a batch or continuous process and found above 90% removal efficiency.
Nowadays, pharmaceutically activated compounds and endocrine-disrupting compounds have emerged as novel contaminants in environmental water (Cabrita et al. 2010). Most of them are not biodegradable and cannot be eliminated completely from water by conventional water treatment processes (Villaescusa et al. 2011; Domínguez et al. 2011). Various natural materials such as hydrous oxides (Gu and Karthikeyan 2005), soils (Figueroa and MacKay 2005), clays (Pils and Laird 2007) and silica (Bui and Choi 2010) have been used for adsorption of pharmaceutical pollutants from wastewater. Activated carbon shows promising roles for the efficient removal of these pollutants. It can remove the pharmaceuticals without generating any toxic by-products (Dutta et al. 1999). Baccar et al. (2012) synthesized activated carbon from agricultural waste (olive-waste cakes) at a laboratory scale and used it for adsorption study of four common pharmaceutically activated compounds (diclofenac, naproxen, ketoprofen and ibuprofen) (Baccar et al. 2012). At 25 °C, the adsorbent shows maximum adsorption volumes of 56.17, 39.52, 24.69 and 10.83 mg/g for diclofenac, naproxen, ketoprofen and ibuprofen, respectively.
The adsorption process follows Langmuir and pseudo-second-order adsorption kinetics. The authors also investigated pH and temperature influence on the adsorption of these pollutants. Increasing pH gradually decreases the adsorption of these four pharmaceutically activated compounds (Baccar et al. 2012). Trimethoprim is a common pharmaceutical product used extensively as human and veterinary medicine. It is poorly metabolized by the animal body, and approximately 60% of trimethoprim is discharged in the environmental water in the original form (Lindberg et al. 2004; Hirsch et al. 1999; Molu and Yurdakoc 2010). In 2012, Liu and his co-workers investigated the removal efficiency of four kinds of oxyacids of phosphorus-activated lotus stalk for trimethoprim (Liu et al. 2012). These oxyacids were H3PO4, H4P2O7, HPO3 and H3PO3. All the prepared activated carbon contains more acidic oxygen functionalities. The order of sorption capacity of trimethoprim by activated carbon was activated carbon–H4P2O7 > activated carbon–H3PO4 > activated carbon–H3PO3 > activated carbon-HPO3. The adsorption process followed both the Freundlich and Langmuir models, which indicates that both chemisorption and physisorption mechanisms took place simultaneously on the surface of the activated carbon (Liu et al. 2012) (Table 3).
Challenges of conventional adsorbent
Some major disadvantages of conventional adsorbents are given below.
Cost
The activated carbons are commonly used to remove various kinds of pollutants, but sometimes their applications are restricted because of high-quality activated carbon and its higher cost (Babel and Kurniawan 2003). On the other hand, low-quality adsorbent has generally shown low adsorption capabilities, so large volumes of materials will be required for water purification (Aksu and Kabasakal 2004). For improvement in removal performance, activated carbon is treated with different complexing agents. This additional cost makes the use of activated carbon less attractive in small-scale industries (Babel and Kurniawan 2003). Both physical and chemical activations have some shortcomings, such as physical activation needs a longer time as well as high temperature for activation. Chemical activation requires long washing for the removal of chemical agents.
Exhaustion
During the treatment of wastewater, the adsorbent (activated carbon) becomes exhausted and loses its competence to further adsorb the pollutants. Different techniques like thermal, chemical, oxidation and electrochemical are used for the regeneration of exhausted adsorbent for further use in wastewater. This regeneration process also adds extra cost as well as lowers the adsorption efficiency compared to activated carbon (Taiwo and Adesina 2005; Zhou and Lei 2006).
Surface modification
The surface modification also introduces some shortcomings like the cost involved in the surface modification process, leaching of impregnated metals, regeneration of surface-modified activated carbon and limitation in column, pilot or full-scale study of real wastewaters, surface water and groundwater treatment (Bhatnagar et al. 2013). The presence of other metal ions reduces the adsorption capacity of activated carbon for specific pollutants (Makeswari and Santhi 2013). This process only transfers pollutants from liquid to solid phase, where further treatment is required for complete removal (Homem and Santos 2011).
Separation of adsorbent
Convenient removal of adsorbent from treated wastewater is another concern for promising adsorbent. The traditional adsorbents are hard to remove after treatment; moreover, the conventional separation processes like centrifugation, precipitation, filtration and chromatography induce malformation like deformation and inactivation of an adsorbent. This operation adds extra cost and time to water purification. Therefore, economically feasible and effective separation techniques are in urgent demand (Li et al. 2011; Liu et al. 2011).
Removal efficiency
Generally, activated carbon cannot reduce the concentration of the pollutants at ppb levels. It adsorbs only a few milligrams of pollutant per gram of absorbent. Moreover, activated carbon shows poor adsorption for inorganic pollutants due to its nonpolar surface (Mohan and Pittman 2006). In the case of activated carbon adsorption, the adsorption equilibrium achieved very slowly due to slow pore diffusion (Lu and Chiu 2006; Deng and Bai 2004). In Fig. 11, activated carbon shows less than 1% adsorption, whereas non-functionalized multiwall carbon nanotubes show a greater ability to adsorb Cr(VI) like 98% adsorption. The lower adsorption efficiency of activated carbon might be due to the filling of pores by water. As a result, Cr(VI) cannot retain the solid surface of activated carbon (Nxumalo 2006).
The influence of contact time on the amount of Cr(VI) adsorbed by each adsorbent (Nxumalo 2006)
The above points indicate that the application of activated carbon in wastewater treatment also encounters various challenges. Carbon fibers were developed as second-generation carbonaceous adsorbents to solve the above-mentioned problems. These adsorbents show higher adsorption kinetics than activated carbon due to the highly porous structure, resulting in a shortening of the diffusion time of pollutants to adsorption sites. Carbon nanotube, an excellent third-generation carbonaceous adsorbent, was synthesized in 1991 with hollow and layered structures. The adsorption sites are located both on the outer and on the inner layer surface of this nanostructure (Liu et al. 2013).
Nanoadsorbents
Nanotechnology has successfully introduced nanoadsorbents for wastewater treatment, which has emerged as an enormous growing and enthralling area of interest (Förstner and Wittmann 1985). Generally, nanotechnology is related to the creation, processing, characterization and application of materials at the nanoscale (1–100 nm) in diverse areas (Biswas et al. 2017, 2019; Mamalis 2007; Stander and Theodore 2011; Padmanabhan et al. 2019; Golieskardi et al. 2019; Ragurajan et al. 2018; Rangari et al. 2017, 2019; Nuge et al. 2020). Nanomaterials own exceptional properties, which are absent in their corresponding bulk materials. These properties are conductivity, selectivity, and catalytic, magnetic and optical properties and high surface area per mass ratio (Lu et al. 2019). Nanomaterials are chemically active due to large surface areas with a large number of active surface sites. Carbon nanotubes, ordered mesoporous carbon (OMC), graphene, carbon nanofibers, titania nanotubes and their modifications are some of the popular nanomaterials that have been used in wastewater treatment. The higher concentration of surface defects arises from large surface area and facilitates interaction with pollutants. Sometimes, nanomaterials are coated with other coating agents to enhance their adsorption properties. For example, when TiO2 was coated with carbon nanotubes (CNTs), the composite showed larger surface area as well as high removal efficiency than their precursor in the as-received form (Hurt et al. 2006; Ilisz et al. 2004). Photocatalytic degradation of pollutants carried out by using TiO2-based nanomaterials results in non-toxic end products (Mahmoodi et al. 2007). These nanomaterials possess outstanding absorbing capabilities and chemical stability (Chen et al. 2005).
Nanomaterials as adsorbent
The structural properties (porosity, surface area, pore size and pore volume), chemical composition and thermal stability of nanoparticles depend on the synthesis procedure and operating conditions. Solgel, ion sputtering, impregnation, co-precipitation, spray pyrolysis, thermal spraying, arc discharge, chemical vapor deposition (CVD), mechanical alloying/milling, laser ablation, thermal plasma synthesis, catalytic growth and electrodeposition are the common methods applied for nanoparticle synthesis (Liu and Zhang 2007; Sharma et al. 2009). The solgel method gets extra attention for the synthesis of nanoparticle due to its low cost, homogenous product formation, high purity and environmental friendliness (Zeng et al. 1998). Different types of nanomaterials are synthesized and used as an adsorbent for pollutant removal, for example, alumina, TiO2, Al2O3, Fe3O4, carbon nanotube (CNT), graphene, mesoporous carbon, MnFe2O4, nano-zerovalent iron, magnetite, etc. (Sharma et al. 2009). The role of nanoadsorbent to remove inorganic, organic and biological pollutants is discussed below with recently reported data.
Removal of inorganic pollutants by nanoadsorbents
Zerovalent iron nanoparticle, iron oxide nanoparticle, titanium oxide, aluminum oxide, etc., are commonly used for metal pollutants removal from wastewater. Zerovalent iron has been reported for adsorption of different metal ions like arsenic, chromium, cadmium, lead, selenium, silver and zinc (Kanel et al. 2005; Ponder et al. 2000a; Sturchio et al. 1997).
Arsenic (As)
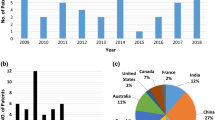
Arsenic adulteration of groundwater is a major problem in whole around the world. The prevalence is the highest in Bangladesh, where about 35 million people are affected by arsenic contamination. A study carried on arsenic affected area/villages in Bangladesh reported that almost 90% of groundwater contained a higher concentration of arsenic than the permissible limit. Various water treatment techniques have been used for arsenic removal, but none of them is effective in community level due to high cost and handling problems (Khan et al. 1997; Dhar et al. 1998; Chatterjee et al. 1995; Das et al. 1995). Nanoadsorbents like akaganeite, magnetite, maghemite and zerovalent iron nanoparticle were used for the removal of arsenic pollutant from drinking water (Deliyanni et al. 2006; Chowdhury and Yanful 2010; Machado et al. 2006; Kanel et al. 2006; Li and Zhang 2007). At pH 7.5, Deliyanni et al. (2003) reported removal of 120 mg/gm as (v) from the solution by akaganeite nanoparticle (2.6 nm). After adsorption, nanoadsorbents can be separated by membrane filtration (0.45 μm) (Deliyanni et al. 2006). Hristovski et al. (2007) also studied the As (v) removal by 16 metal oxide nanoparticles in fixed bed columns, including TiO2, NiO, Fe2O3 and ZrO2. Except for ZrO2, all the nanoparticles showed ~ 98% removal capacity. The adsorption data fitted well with Freundlich adsorption isotherm (Hristovski et al. 2007). Zhu et al. (2009) impregnated non-zerovalent iron on activated carbon and used it to adsorb arsenic pollutant As (III). At pH 6.5, the composite removed approximately 1.997 mg/g in 2.0 mg/L arsenic solution. The authors proposed this adsorbent as an ideal candidate to treat arsenic-polluted drinking water (Zhu et al. 2007). Velickovic et al. (2012) and Peng et al. (2005) used iron (III) oxide comprised of ethylene diamine-functionalized multiwall carbon nanotubes and ceria nanoparticles supported on carbon nanotube, respectively, for As (V) removal. Tang et al. (2013) successfully produced superparamagnetic ultrafine magnesium ferrite (Mg0.27Fe2.50O4) nanoadsorbent which shows superior arsenic adsorption performance on both As (III) and As (V). Its adsorption efficiency of As (III) and As (V) was found to be 127.4 mg/g and 83.2 mg/g, respectively, at pH 7.0. The hydroxyl group played a major role in arsenic adsorption. They found that the arsenic adsorption capacity of the as-synthesized nanoadsorbent was higher than Fe2O3 nanoadsorbent. Moreover, this nanoadsorbent (Mg0.27Fe2.50O4) could be easily removed by the external magnetic field and could be regenerated and reused for arsenic removal (Tang et al. 2013). Zhang et al. produced a series of iron oxide–graphene oxide composites to remove arsenate from water. They found that water with arsenate concentration at 51.14 ppm, the composites adsorb more than 95% arsenate, results in an absorption capacity of 23.78 mg arsenate/g of composite in the pH range of 4–9 (Zhang et al. 2010). Yu et al. synthesized magnetic iron oxide/graphene oxide with high iron oxide loading (51% by wt.). They reported significantly high adsorption capacities 54.18 and 26.76 mg g−1 for As (III) and As (V), respectively. In general, graphene-based adsorbent materials show improved adsorption capacities because they offer a large surface area and inhibit the agglomeration of the deposited nanoparticles (Yu et al. 2015).
Chromium (Cr)
Chromium contamination is generally initiated by steel mill waste, erosion of natural waste, electroplating wastewater and dye industries. The U.S. EPA fixed the discharge limit of chromium to 0.1 mg/L in surface water. Among various valence states of chromium, only trivalent, Cr(III) and hexavalent Cr(VI) forms are important for the environmental point of view. The hexavalent form exhibits more toxicity than its trivalent form (Browning 1969; Kowalski 1994; Singh and Singh 2002). Ponder et al. (2001) removed Cr(VI) by applying nano-size zerovalent iron (Ferragels) which is 10 − 30 nm in diameter. They fabricated the nanoadsorbent using a borohydride reducing agent to reduce aqueous iron salt. The authors explained pseudo-first-order reaction kinetics of the hexavalent chromium adsorption process. Here, Cr(VI) is reduced to Cr(III) by nano-size zerovalent iron. The author showed that the reduction rate of chromium was 7–12 times faster by prepared nano-size zerovalent iron than the equivalent weight of iron powder (Ponder et al. 2000a, b).
In 2005, Hu et al. synthesized and used maghemite (γ-Fe2O3) nanoparticles (diameter around 10 nm) to remove and recover Cr(VI) from wastewater. The highest adsorption performance of maghemite for Cr(VI) removal was 19.2 mg/g of maghemite at pH 2.5, and the saturation time for this method was only 15 min. The adsorption of Cr(VI) on the maghemite was because of electrostatic interactions and ion exchange (Hu et al. 2005). Hu et al. (2007) synthesized a group of magnetic nanoparticles of about 20 nm by chemical co-precipitation method. The synthesized magnetic nanoadsorbents are CoFe2O4, CuFe2O4, MgFe2O4, MnFe2O4, NiFe2O4 and ZnFe2O4. The major advantages in adsorption trailed by magnetic parting are rapidness, efficacy and easiness of the process. The author found adsorption equilibrium within 1 h for Cr(VI) by all kinds of nano-ferrites. At a similar primary Cr concentration (1000 mg/L), among all the ferrite nanoparticles, MnFe2O4 nanoparticles showed the highest Cr adsorption efficacy (99.5%) within the shortest adsorption time. This is because of the high surface area of MnFe2O4 (180 m2/g). The removal efficacy was extremely pH-dependent, and the optimum adsorption occurred at pH 2. The effects of contact time and pH on chromium removal by various ferrites are shown in Fig. 12. The figure shows that the adsorption capacities of magnetic nanoadsorbent followed the order: CoFe2O4 < NiFe2O4 < CuFe2O4 < ZnFe2O4 < MgFe2O4 < MnFe2O4 (Hu et al. 2007).
The effect of contact time and pH on chromium removal by various ferrites; a absorption versus time and b absorption versus pH (Hu et al. 2007)
For the first time, Lv et al. (2011) used nanoscale zerovalent iron multiwalled carbon nanotube nanocomposite for Cr(VI) removal. In this composite, nano-zerovalent iron nanoparticles (20–80 nm) remain dispersed on the surface or into the network of multiwalled carbon nanotube (Fig. 13a). The study reported that this composite exhibited a 36% higher efficacy for Cr(VI) removal than bare nano-zerovalent iron or nano-zerovalent iron-activated carbon composite. The reason might be bare nano-zerovalent iron formed inert oxide film as it exposed to air or water. This oxidation layer prevents further reactions with contaminants. Coupling with multiwalled carbon nanotube prevents this oxide film formation on the surface and allowed more adsorption. This composite also completely removed Cr(III) from aqueous solution. At concentration 1.09 mg/L, the composite removed completely Cr(III) at 15 min. The removal process depends on ionic strength, initial concentration of pollutants, pH and presence of other anions. The experiment was allowed to run for 4 h and found complete removal of dichromate ions and other anions at their high concentrations (Lv et al. 2011). Figure 13b shows that after 2 h of reaction some polygon shape appeared on the nano-zerovalent iron multiwalled carbon nanotube. This is possible because of the co-precipitation of chromate and iron ions (Lv et al. 2011).
a SEM and TEM images of nZVI supported on MWCNTs before adsorption, b SEM images of nZVI supported on MWCNTs after reaction (adsorption) with Cr(VI) for 2 h. The polygon shape appeared on the composite (Lv et al. 2011)
Chandra et al. (2010) synthesized magnetite (Fe3O4)–graphene composite. The composite showed adsorption capability of 5.5 mg/g of composite that is higher than the unsupported Fe3O4. Goharshadi et al. used functionalized graphene oxide to remove Cr(VI) ions. The functionalized graphene oxide has a high concentration of epoxy and hydroxyl groups, which assist in adsorption. The adsorption capability was found to be 1.66 mg/g of graphene.
Cadmium (Cd)
Karimi (2013) reported the synthesis of magnetite nanorods (average diameter 60 nm) by the electrochemical method in pulsed conditions. He used this nanoparticle as an adsorbent for various metal pollutants removals such as Cd2+, Cu2+, Fe2+, Ni2+, Pb2+, Zn2+, etc. The maximum adsorption capacities of this nanoadsorbent for Cd2+, Fe2+, Pb2+, Zn2+, Ni2+ and Cu2+ were 88.39, 127.01, 112.86, 107.27, 95.42 and 79.10 mg/g, respectively (Karami 2013). Like the removal of As(V) and Cr(VI), synthetic akaganeite nanocrystals (average crystallite size 2.6 nm) also efficiently removed Cd (II) from aqueous waste solutions. This nanoadsorbent showed 17.1 mg/g adsorption ability for Cd (II) ions. The sorbent capacity can be improved by increasing the temperature and pH, whereas it decreased by increasing the electrolyte ions in solution. Here, the adsorption of Cd (II) took place by a weak chemisorption process. The adsorption data fitted both Freundlich and Langmuir isotherms and obeyed those two models (Deliyanni and Matis 2005).
Recently, biodegradable and renewable organic polymers are used for coating nanoadsorbents which have been broadly used for the separation of heavy metal pollutants. These organic polymers originate from industrial wastes, agricultural wastes and biomass (Abdel-Halim and Al-Deyab 2011). Gong et al. (2012) used shellac, a biorenewable and biodegradable resin with ample hydroxyl and carboxylic groups that provided the possibility of chelating heavy metal ions. The authors coated the iron oxide nanoadsorbent with this shellac polymer and examined its capacity for Cd2+ removal from waste solution. The adsorption capacity of the coated nanoadsorbent increased with increasing pH. At pH 8.0, it showed the highest adsorption capability of (18.80 mg/g). After adsorption, the coated nanoadsorbent can be easily regenerated by organic acid. Though the adsorption capability was not high enough, there is still much room for development such as optimizing the synthesis parameters to coat a thicker shellac layer. Another advantage of this coated nanoadsorbent was that it can be used in a high saline solution for Cd2+ removal. At 3.0% sodium chloride solution, this adsorbent showed 10.10 mg/g adsorption capacity. Adsorption mechanisms followed electronic attraction as well as chemical adsorption (Gong et al. 2012).
Pacheco et al. (2006) synthesized aluminum–silica nanoparticles by the solgel process. They used this nanoadsorbent for Cd2+ removal from wastewater and stated 96.4% adsorption on Si–Al nanoparticles. This nanoadsorbent has various kinds of functional groups on their surfaces, such as alkoxy, hydroxyl and oxy groups, and metallic ions (Cd2+) that interact with one group by electrostatic attraction as well as by ionic exchange to another group (Pacheco et al. 2006).
Mercury (Hg2+)
Mercury is harmful to the human body. It can cross the blood–brain barrier. Mercury poisoning can damage the cardiovascular system, kidney and bones as well as cause neuronal disorder (Miretzky and Cirelli 2009; Clarkson 1993). Pan et al. (2012) fabricated mercapto-functionalized nanoscale Fe3O4 and carefully investigated the adsorptive characteristics of this nanoadsorbent for removal of Hg2+. At the optimized condition, i.e., 308 K, and at pH 3.0, this functionalized nanoadsorbent showed the highest adsorption capacity toward Hg2+ (522.9 mg/g) with an initial concentration of 500gm/L. The adsorption of Hg(II) reached saturation within 60 min. The results fitted well to Freundlich isotherm and pseudo-second-order kinetic isotherm, and the process was endothermic (Pan et al. 2012). In 2013, Zhang et al. synthesized SiO2-coated Fe3O4 nanoparticle. The coatings protectedFe3O4 nanoparticles from oxidation at low pH conditions, and Fe3O4 core is comprised of superparamagnetic and separate wastes which are magnetic in nature. Further, they used 3-mercaptopropyltrimethoxysilane, for modifying coated Fe3O4 nanoparticle to prepare a unique mercaptopropyl-modified sorbents to remove mercury from solution. The highest adsorption capacity obtained from Langmuir fitting was 148.8 mg/g at pH 6.5. The researchers also found significant mercury removal efficiency (110 mg/g) from natural wastewater samples by this adsorbent (Zhang et al. 2013).
Recently, carbon-based nanomaterials like graphite oxide attracted researchers for its potential application as adsorbent (Seredych et al. 2011). Kyzas et al. (2014) synthesized two composites: (1) graphite oxide nano-filled with chitosan and (2) graphite oxide–magnetic chitosan. The groups evaluated the performance of untreated graphite oxide and two as-prepared composites to remove Hg2+ from aqueous solution. At pH 6 and 25 °C, the maximum adsorption capability for graphite oxide, graphite oxide nano filled with chitosan and graphite oxide–magnetic nanocomposite was 187, 381and 397 mg/g, respectively. The results supported the Langmuir isotherm well, and the adsorption mechanism followed chemisorption type. The authors evaluated the study by varying the parameters, i.e., pH, contact time and temperature (Kyzas et al. 2014).
Copper (Cu2+)
Amino-functionalized magnetite nanoparticles were investigated for Cu2+ removal from aqueous solution. The adsorption equilibrium achieved within 5 min of the adsorption process. The equilibrium data satisfactorily fitted by Langmuir isotherm, and the maximum adsorption was found to be 25.77 mg/g at pH 6 and 298 K (Mei et al. 2010). Here, the fast adsorption indicates that adsorption happened primarily on the adsorbent surface (Liu et al. 2008). The sorption mechanism between Cu2+ and NH2 groups on the amino-functionalized magnetite nanoparticles was the chemisorption process. By 0.2 mol/L HCl solution, the amino-functionalized magnetite nanoparticles can be regenerated within one minute and retained the original metal removal capacity (Mei et al. 2010). Nanogeothite (α-FeOOH, average particle size 10–15 nm) and nanohematite (α-Fe2O3, average particle size 75 nm) were synthesized by the co-precipitation method (Fig. 14). The surface area of both the particles was 71.49 m2/g and 24.82 m2/g, respectively. It was found that both nanogeothite and nanohematite showed good adsorption capability of 149.25 and 84.46 mg/g, respectively for Cu2+. Both the adsorption processes were befitting to Langmuir isotherm, and the adsorption onto nanomaterials was a spontaneous process (Chen and Li 2010).
TEM images of a nanogeothite and b nanohematite (Chen and Li 2010)
Gum aerobic modified magnetic (Fe3O4) nanoadsorbent was developed for the removal of Cu2+ ions from various solutions. The interaction between gum aerobic and magnetic nanoadsorbent took place via the carboxylic groups present in the structure of gum aerobic and the hydroxyl groups present in Fe3O4. This surface modification led to the formation of the particle with a diameter range of 13–64 nm. The author compared the adsorption capacity of both gum aerobic modified magnetic nanoadsorbent and naked magnetic nanoadsorbent. The maximum adsorption capacities were 38.5 and 17.6 mg/g, for modified magnetic nanoadsorbent and naked magnetic nanoadsorbent, respectively. The modified magnetic nanoadsorbent adsorbed copper ions via complexation with the amine group of gum aerobic, whereas naked magnetic nanoadsorbent used surface hydroxyl groups for complexation with copper ions. Both adsorption data fitted to Langmuir isotherm (Banerjee and Chen 2007).
Zhao et al. prepared few-layered graphene oxides via the modified Hummers method. They studied the effect of operating conditions like pH and ionic strength to remove Cd2+ and Co2+ ions from water. They found that the presence of an oxygen-containing group on the graphene surface was the key to the adsorption of the ions. The adsorption capacity at near-neutral pH for Cd2+ removal was 106.3 mg/g. They also calculated the adsorption of Cd2+ on graphene which is endothermic, and the process is spontaneous (Zhao et al. 2011). In a separate study, Xu et al. also prepared graphene oxide layers for the removal of Cd2+ ions from water. The maximum adsorption capacity was found to be 44.64 mg/g at pH 4.00 with a loading of 2 g/L.
Mubarak et al. showed the comparison of the removal capacity of acid-functionalized multiwall carbon nanotubes (MWCNTs) and biochar for the Cd2+ from water. The introduction of functional groups like hydroxyl and carbonyl and the carboxylic acid act as anchor points for the cations. The highest Cd2+ adsorption of f-MWCNTs was found to be 83.33 mg/g, and it was 62.5 mg/g for the magnetic biochar at pH near 7 (Mubarak et al. 2015). Ramana et al. synthesized silver-coated MWCNT using the reduction method. The maximum adsorption capacity for Cd2+ removal was found to be 54.92 mg/g at the neutral pH (Ramana et al. 2013). Although graphene oxide and CNTs have shown good adsorption capacities, still there remains a great concern over their elution to the system. It has been shown that biochars can stabilize them by acting as host in the nanocomposite. Biochars have large pore volumes and surface areas. Therefore, biochar–CNT and biochar–graphene composite were tested for Cd2+ removal. Liu et al. fabricated carbon nanomaterial–biochar nanocomposites (SG–PySA–CNT and SG–PySA–GO) via pyrolysis of sweetgum biomass pretreated with carbon nanotubes (CNTs) and graphene oxide (GO). The adsorption capacity for Cd2+ removal was found to be 10.2 mg/g, which is low compared to the previously reported values. Several modifications of graphene oxide have been reported. Deng and Bai prepared iron oxide–graphene oxide nanocomposite for the simulation’s removal dyes and Cd2+. In a mono-component system, the maximum sorption capacity in ultrapure water for Cd (II) was 91.29 mg/g (Deng and Bai 2004).
Lead (Pb)
Corrosion of domestic plumbing and natural erosion are the prime effects of Pb contamination. Nanoscale zerovalent iron was successfully used by Ponder et al. (2001) for Pb(II) removal. Here, Pb(II) is reduced to Pb (0). The iron nanoadsorbent showed 30 times higher removal efficiency than from iron powder on a (Fe) molar basis (Ponder et al. 2000a, b). Wang et al. (2007) treated MWCNTs by nitric acid and used these functionalized MWCNTs for Pb(II) adsorption. After treatment, the acid-functionalized MWCNTs become more negatively charged because of the formation of –OH and –COOH functional groups and showed adsorption capacity up to 91 mg/g at pH 3.5. The adsorption is due to electrostatic interactions (Wang et al. 2007). The hexagonal arrangements of carbon atoms in its graphite structure and its large pore size enable the high adsorption of Pb(II) by carbon nanotubes. This adsorption might happen in various places such as interstitial pore space between the grove edge of its boundary bundles, tube handle or outer layer surfaces (Stafiej and Pyrzynska 2007).
Some researchers used single adsorbent for multiple metal ion adsorption. Polymer-based hybrid nanoparticle sorbents (ZrPS-001) were synthesized by Zhang et al. (2008) and applied for adsorption of different metal ions (cadmium, lead and zinc) from aqueous solution. After the treatment, water met the WHO requirements as drinking water standards (Zhang et al. 2008). Another research group developed nanoadsorbent (2,4-dinitrophenyl hydrazine immobilized on sodium dodecyl sulfate-coated nanoalumina) and studied its feasibility for removal of metal cations like lead, cadmium, chromium, cobalt, nickel and manganese from water. The adsorbent showed the highest removal for lead, chromium and cadmium ions. The adsorption data matched well with Freundlich and Langmuir models (Afkhami et al. 2010).
Nano-hydroxyapatite (NHA), which is a major inorganic segment of human bone (Michael et al. 2016a, b), could also play a significant role in wastewater treatment. Nano-hydroxyapatite (Sairam et al. 2008), polypyrrole (PPy)/Fe3O4 magnetic nanocomposites (Bhaumika et al. 2011) and Fe–Al–Ce nanoadsorbent (Chen et al. 2009) were used for defluoridation of water. Nano-hydroxyapatite showed a defluoridation capacity of 1845 mg/kg. The ion exchange and adsorption processes were the main mechanisms (Sairam et al. 2008). Kassaee et al. (2011) investigated the removal efficiency of iron nanoparticle for nitrate ions. The author found that each experiment showed almost 76% reduction of nitrate ions after 12 h, above 90% after 24 h, and approximately 100% after 72 h (Kassaee et al. 2011).
Huang et al. fabricated graphene nanosheets (GNSs) via vacuum-assisted low-temperature exfoliation and applied to adsorb Pb2+ ions from an aqueous solution. They observed that Pb2+ ion adsorption depended on the solution pH and increased with it. They concluded that the Lewis basicity of GNSs is enhanced due to heat treatment under vacuum condition and favors simultaneous adsorption of Pb2+ ions and protons by GNSs. GNSs showed a desorption capacity of up to 35.7 mg/g at pH 3.5 (Huang et al. 2011). Fang et al. prepared magnetic chitosan–graphene composite and observed that the presence of Fe3O4 in the composites enables quick extraction after saturated adsorption completed. The unique characteristics of MCGO such as high surface area, abundant functional groups and the high volume of sp2 carbon not only enhance its adsorption behavior but also help to immobilize and disperse the GO. The magnetic adsorbent exhibited high stability and eco-friendly having high Pb2+ ions adsorption and desorption capacity of 76.94 mg/g and 90.3%, respectively. This behavior makes them an excellent candidate to remove Pb2+ ions from agricultural and industrial wastewater (Fan et al. 2013).
Vilela et al. reported very innovative graphene-based microdots made from graphene sheets. Those microdots behaved like self-propelled structures to capture, transfer and remove heavy metals (i.e., lead) and its subsequent recovery for recycling purposes. Their structure is comprised of nanoscale graphene oxide, nickel and platinum in multilayers fashion, offering various functionalities. For example, the graphene oxide outer layer surface attracts lead, the inner layer of platinum decomposes hydrogen peroxide fuel for self-propulsion, whereas the middle layer of nickel permits external magnetic control of the microdots. Mobile GOx-microbots showed excellent efficiency to remove lead compared to non-mobile GOx-microbots. They exhibited excellent removal capability of lead and water pollutants from 1000 ppb down to 50 ppb in 60 min. Furthermore, the saturated microdots can be regenerated and reused by removing lead from the surface (Vilela et al. 2016).
Nanoadsorbents for wastewater treatment
Removal of organic pollutants using nanoadsorbents
Naoadsorbents are also used for organic pollutants removal like different types of dyes, pesticides and hydrocarbons. Belessi et al. (2009) prepared mesoporous TiO2 and used it for commercial azo dye Reactive Red 195 removal in the liquid phase at room temperature under dark condition. The mean synthesized particle size was ~ 8.8 nm. The nanoadsorbent exhibited the highest adsorption percentage (87.0 mg/g) at pH 3.0 and a temperature of 30 °C. The authors considered the influence of pH, dye concentration and adsorbent dose to remove dye molecules. The equilibrium data showed an excellent fit to Langmuir and pseudo-second-order kinetic models. The adsorption process showed high electrostatic attractions between the positively charged surface of the adsorbent and anionic dye (Belessi et al. 2009). Surfactant-coated magnetic nanoparticles have been used for 2-hydroxyphenyl extraction from wastewater (Bahaj et al. 2002). The perchloroethylene undergoes reduction by chemisorption on zerovalent iron nanoparticle in anaerobic conditions (Joo and Cheng 2006; Tratnyek et al. 2003).
Du et al. (2008) prepared chitosan nanoparticles by the ionic gelation method. They used a batch adsorption process to remove eosin Y (a model anionic dye) by this nanoadsorbent. Experimental results followed Langmuir isotherm, and the adsorption capability was found 3.333 g/g. The process was influenced by contact time, dye concentration, pH and temperature (Du et al. 2008). The Fe3O4 nanoparticles–carboxymethylated chitosan conjugate (anionic magnetic nanoadsorbent) was synthesized by the carbodiimide activation process. This conjugate was studied to remove acid dyes, such as orange G and acid green. In both cases, it is found that the adsorption capability reduced with increasing solution pH. The maximum adsorption capability for Orange G and acid green was 1883 and 1471 mg/g, respectively. Here, the adsorption route followed the pseudo-second-order kinetic model and the Langmuir model (Chang and Chen 2005). Due to hydrophobic nature, high surface area, hollow and layered structure, carbon nanotubes show a strong attraction to organic materials, especially to nonpolar organic materials, such as polycyclic aromatic hydrocarbons, benzene derivatives, phenolic compounds, organic dyes (Nas et al. 2019a, b), naphthalene (Gotovac et al. 2007), phenanthrene and pyrene (Yang et al. 2006). More interested readers are referred to a recent review by Apul et al. (2016).
Recently, degradation of methyl parathion (MP), methyl orange (MO), methylene blue (MB), sodium dodecylbenzene sulfonate (SDBS) and bisphenol A (BPA) in aqueous solution has been investigated using three different grades of acid-functionalized CNTs of different tube diameters in the presence of microwave radiation. The effect of irradiation time, initial concentration of the organic compound, CNT doses, microwave power and initial pH was studied. CNTs having diameter ranges from 10 to 20 nm showed the highest catalytic behavior under microwave treatment. Further, complete degradation was obtained using 10–20 nm CNTs within 7.0-min irradiation when 25 mL solution (25 mg/L), 1.2 g/L catalyst dose, 450 W, 2450 MHz and pH = 6.0 were applied. The concept of “hot spot” was believed to be behind the remarkable degradation by CNTs in the presence of microwave.
Graphene-based materials have been extensively used to remove organic dyes and oil from water. The high aspect ratio of π electronic surface favors the adsorption of organic contaminants on the graphene surface and their eventual removal from water (Nas et al. 2019a, b; Wang et al. 2016). Methyl orange, methylene blue, phenol, aromatic compounds, hydroquinone and phenol have been adsorbed on graphene. Graphene was decorated with flowerlike TiO2 microspheres using a simple surfactant-assisted hydrothermal process. Poly(vinyl pyrrolidone) was used as a surfactant. The composite was used to degrade rhodamine B, and its adsorption capacity was compared with the TiO2 microsphere and Degussa P-25. Excellent removal was achieved using the composite.
Removal of biological pollutants
Cyanobacteria are common habitants of water. These bacteria produce microsystems (nonribosomal peptides) which also contaminate water. So far, conventional technology faces difficulties in removing this pollutant completely from the water. Carbon nanotubes showed 98% removal capacity of this pollutant from water, which is four times higher than did granular activated carbon (GGC) (Yan et al. 2006). Nanoadsorbent is reported to remove viruses from infected water. A comparative study was carried out on four commercial nanoparticles of iron oxide to remove bacteriophage phiX174. Among them, α-Fe2O3 showed the most efficiency (100% adsorption) at low initial virus concentration. The adsorption method followed both Langmuir and Freundlich adsorption isotherms. The authors also studied the effect of ionic strength, presence of cations and anions on the adsorption process. The presence of anion reduced the adsorption, whereas the reverse scenario was found in the presence of cation. The mechanism for adsorption of the virus on iron oxide nanoparticle was due to electrostatic interaction (Shen et al. 2010).
Singh et al. (2011) synthesized surface-modified magnetic nanoparticles (Fe3O4) by a soft chemical method. They prepared three types of surface-functionalized nanoparticles—functionalized Fe3O4 nanoparticles with (1) carboxyl group (succinic acid), (2) amine group (ethylenediamine) and (3) thiol group (2,3-dimercaptosuccinic acid). These nanoparticles successfully removed both toxic heavy metal ions (As3+, Co2+, Cr3+, Cd2+, Cu2+, Ni2+, Pb2+, etc.) and bacterial pathogens (Escherichia coli) from water. In the case of a pathogen, the hydrophilic magnetic particles damaged the cell wall partially and/or completely, infiltrate into the lipid bilayer component of the membrane and disrupt its structural integrity (Fig. 15) (Singh et al. 2011).
TEM images of aE. coli (control) and bE. coli obtained after incubating these bacteria with carboxyl magnetic nanoparticle (MNP) (Singh et al. 2011)
In comparison with other low-cost adsorbents, nanoparticles show low contact time (1.0–15.0 min) and low adsorbent dose (µg/L) and can work in various conditions of pH (3–9) during the adsorption process. These parameters ensure the fast adsorption capacity of nanoadsorbent (Ali et al. 2012) (Table 4).
Regeneration and separation of nanoadsorbents
Regeneration of nanoparticle offers two aspects in nanoadsorbent application, i.e., reusability of nanoadsorbent and recovery of valuable metal species. Various research groups investigated the reusability of nanoadsorbents for pollutant removal. Some of the findings are briefly discussed here. Peng et al. (2005) reported ceria nanoparticle-coated carbon nanotube and used it for arsenate removal. After being exhausted, the nanoadsorbent was regenerated by 0.1 M NaOH with a desorption efficiency of 94% (Peng et al. 2005). Through regeneration, carbon nanotubes become economically attractive for wastewater treatment. Zhou et al. (2009) modified the Fe3O4 by utilizing chitosan and α-keto glutaric acid to remove Cu (II). After the complete removal of metal pollutants, this nanoadsorbent was regenerated by using Na2EDTA. This regenerated nanoadsorbent reused without varying its removal efficiency (Zhou et al. 2009).
Maghemite nanoparticles (diameter around 10 nm) were successfully applied for adsorption of different metal pollutants, namely Cr(VI), Cu (II) and Ni (II). This nanoadsorbent got saturated within 10 min. After successive adsorption, the exhausted nanoadsorbent was treated with 0.01 M of NaOH and 0.05 M of HCl for Cr(VI), Cu (II) and Ni (II) desorption, respectively. The removal efficiency was highly dependent on solution pH. After five consecutive adsorption–desorption processes, the nanoadsorbents (maghemite) adsorption capacity remains almost constant (Hu et al. 2006).
In 2003, Deliyanni et al. used akaganeite-type nanocrystals for sorption of As (V). After each regeneration of nanoadsorbent, the capacity decay was 25–30% of its initial capacity. Through this method, only 75% of metals were recovered (Deliyanni et al. 2003). Through the regeneration of nanoadsorbent, the adsorption process for water treatment becomes more economical. Various separation processes are applied for nanoadsorbent separation, such as centrifugation (Novak et al. 2001), ultracentrifugation (Tsao et al. 2009), gel electrophoresis (Hanauer et al. 2007), diafiltration (Sweeney et al. 2006) and fractional crystallization. Due to the small particle size, the centrifugation process needs higher centrifugal force and time for sedimentation (Tsao et al. 2009). Among them, membrane filtration is a promising one, which provides continuous operation with small chemical use (Qu et al. 2013a, b). Diafiltration, a membrane-based method, provides a convenient way for the separation of water-soluble nanoparticles by a single step (Sweeney et al. 2006). It is a continuous flow process. As a result, materials cannot build up on the membrane surface like traditional ultrafiltration, thus preventing membrane fouling. Due to simple equipment and reusability of the membrane, diafiltration is cost-effective, suitable and versatile (Sweeney et al. 2006).
Recently, nanomaterials are immobilized on different support like resin, membranes to avoid the separation. But this immobilization reduces their efficiency (Qu et al. 2013a, b). Generally, magnetic nanomaterials provide extra benefit over nonmagnetic nanoparticles, because these magnetic nanoadsorbents provide a magnetic field for effective and efficient separation of inorganic pollutants. After being exhausted, this magnetic nanoadsorbent can be removed from the matrix by applying an external magnetic field. After separation, the harmful components can be desorbed by acidic or basic treatment, while these nanoadsorbents can be recovered and recycled without varying their initial efficacy (Kurniawan et al. 2006a; Ngomsik et al. 2005).
Future prospects of nanoadsorbents
Generally, the adoption efficiency and feasibility of any innovative technique depend on two factors: cost minimization and involvement of potential toxicity.
Cost and market opportunities Though nanoadsorbents offer excellent efficiency in water treatment in some cases, its production and application are costly (Qu et al. 2013a, b). They proposed some approaches to overcome the cost issue. These are (1) using low-purity nanomaterials without compromising adsorption efficiency. For example, Lee et al. (2010) prepared amino fullerene photocatalysts from fullerene soot rather than ultrapure C60 and saved average 90% production cost with minimal (< 10%) loss of effectiveness, (2) long-term use of nanoadsorbent may compromise their cost-effectiveness and (3) the treatment cost can be minimized by regeneration and reusing nanomaterials (Qu et al. 2013a, b).
Recently, nanoadsorbent's synthesis increases, due to high demands for clean water treatment. In 2003, approximately € 2.28 million was utilized for environmental remediation (Li et al. 2006). According to a recent technical market report, the value of nanomaterials for environmental remediation in 2004 and 2005 was €3.9 and €9.11 million, respectively. In 2009, the market predicted to reach almost double (€20 billion). Day by day, the world population is increasing. By the next 50 years, the world population growth is expected to double with the existing population. To meet the demand of this huge population, water purification needs more investment. In 2015, the market value for nanomaterials related to environmental remediation would increase 250% (€ 1 trillion), and by 2020 it is expected to reach €1.6 trillion (Li et al. 2006; German Federal Ministry of Education and Research 2009). The increasing trend of nanotechnology products may open a potential market in environmental industries both domestic and international levels and can provide job opportunities among the increasing population. For example, at present European Union (EU) alone made €227 billion from nanotechnology related to environmental applications, which is accounted for 2.2% of its total domestic materials, and created 3.4 million employment (Roco 2003). This increasing trend of nanomaterials in the environmental application in positive not only in water treatment but also in economic competitiveness.
Nanotoxicity Nanoparticles attracted researchers for their efficient capacity to remove water pollutants. But due to its new identities in the water system, it might be a new concern soon. It may release in the environment from the synthesis process, application or disposal management. The special attributes such as small size and shape and the high surface area of nanomaterials are responsible for their toxicities also (Ali et al. 2012). For example, titanium dioxide (TiO2) is a biologically inert chemical and has no toxicity effect in rat’s lung at a single instilled dose (5 mg/rat) (Ferin and Oberdorster 1985). On the other hand, nano-size TiO2 particles could be responsible to produce more pulmonary toxicity compared to their bulk counterparts (Ali et al. 2012).
The enhancement of the surface area provides a potential number of reactive groups on the particle surface. These properties make them chemically and biologically reactive, which consequences more reactive oxygen species (ROS) production. This reactive oxygen species is the primary mechanism of nanotoxicity. Due to its small size, it gets easy excess in the living systems. It is reported that nanoparticles may initiate oxidative stress, inflammation, DNA mutation, major structural injury to mitochondria and cell death (Savić et al. 2003; Li et al. 2003; Geiser et al. 2005; Ali et al. 2012).
So far, there is a lack of specific tests or systems developed for ascertaining non-toxicity. So, the safe and careful strategy should be undertaken during handling and deposal. In 2003, the Environmental Protection Agency (EPA, USA) launched a research plan to study the harmful effect of nanoparticles on the environment. At this moment, there is no specific guideline for dealing with nanoparticles. In 2010, Schmidt-Ott et al. mention some facts regarding nano-handling and disposal (Schmidt-Ott et al. 2010). For eco-friendly synthesis and application, the following precautions can be taken for minimizing the nanotoxicity (Table 5) (Schmidt-Ott et al. 2010; Freeland et al. 2012).
Conclusion
With the expansion of industrialization and agricultural activities meeting the demand for continued population growth in the world, water pollution has become a major global concern for the last few decades. Techno-economic assessment of various water treatment methods suggests that the adsorption process can be an efficient tool for water purification. However, the conventional adsorbents inherit some limitations such as high cost of adsorbent, exhaustion, poor separation efficiency, costly regeneration and so on. Recently, nanoadsorbents have received tremendous momentum in the water treatment process because of their unique properties like high surface/volume ratio with active sites, selectivity, catalytic and magnetic properties, etc. The exotic properties of nanoadsorbents and their incorporation with current technologies have added a new dimension in the revolutionization of the water treatment process around the world. So far, a wide range of nanoadsorbents has already been used successfully to remove inorganic, organic and biological pollutants. The regeneration and separation of magnetic nanoadsorbents after treatment make the process more user-friendly (i.e., convenient and cost-effective). The adsorbents show excellent results in the batch adsorption process. However, more pilot and industrial-scale studies are essentially important to evaluate the overall efficiency of large-volume water treatment. Though the economic value of nanoadsorbents for environmental remediation is well recognized, still it requires appropriately safe and suitable strategies to avoid harmful effects (e.g., toxicity, etc.) of nanoadsorbents in the water treatment process.
References
Abdel-Halim ES, Al-Deyab SS (2011) Removal of heavy metals from their aqueous solutions through adsorption onto natural polymers. Carbohyd Polym 84(1):454–458
Adebajo MO, Frost RL, Kloprogge JT, Carmody O, Kokot S (2003) Porous materials for oil spill cleanup: a review of synthesis and absorbing properties. J Porous Mat 10(3):159–170
Afkhami A, Saber-Tehrani M, Bagheri H (2010) Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2,4-dinitrophenylhydrazine. J Hazard Mater 181(1–3):836–844
Aggarwal D, Goyal M, Bansal RC (1999) Adsorption of chromium by activated carbon from aqueous solution. Carbon 37(12):1989–1997
Ahmed MN, Ram RN (1992) Removal of basic dye from wastewater using silica as adsorbent. Environ Pollut 77:79–86
Ahn CK, Kim YM, Woo SH, Park JM (2009) Removal of cadmium using acid-treated activated carbon in the presence of nonionic and/or anionic surfactants. Hydrometallurgy 99:209–213
Aksu Z, Kabasakal E (2004) Batch adsorption of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep Purif Technol 35:223–240
Al-Bastaki N (2004) Removal of methyl orange dye and Na2SO4 salt from synthetic wastewater using reverse osmosis. Chem Eng Process 43(12):1561–1567
Al-Degs Y, Khraisheh MAM, Allen SJ, Ahmad MNA (2001) Sorption behavior of cationic and anionic dyes from aqueous solution on different types of activated carbons. Sep Sci Technol 36(1):91–102
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Sep Purif Rev 39(3–4):95–171
Ali I, Aboul-Enein HY (2004) Chiral pollutants: distribution, toxicity and analysis by chromatography and capillary electrophoresis. Wiley, Chichester
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183
Ali I, Mbianda XY, Burakov A, Galunin E, Burakova I, Mkrtchyan E, Tkachev A, Grachev V (2019) Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ Int 1(127):160–180
Alkan M, Demirbaş Ö, Doğan M, Özdemir Y, Özmetin C (2004) Sorption of acid red 57 from aqueous solutions onto sepiolite. J Hazard Mater 116:135–145
Alkan M, Celikcapa S, Demirbas O, Dogan M (2005) Removal of reactive blue 221 and acid blue 62 anionic dyes from aqueous solutions by sepiolite. Dyes Pigm 65(3):251–259
Alslaibi TM, Abustan I, Ahmad MA, Foul AA (2013) Cadmium removal from aqueous solution using microwaved olive stone activated carbon. J Environ Chem Eng 1:589–599
Alvarez-Merino MA, Lopez-Ramon V, Moreno-Castilla C (2005) A study of the static and dynamic adsorption of Zn(II) ions on carbon materials from aqueous solutions. J Colloid Interface Sci 288(2):335–341
Anirudhan TS, Sreekumari SS (2011) Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J Environ Sci-China 23(12):1989–1998
Apul O, Hoogesteijn N, Ladner D, Westerhoff P (2016) Adsorption of phenanthrene by superfine powdered activated carbon and electrospun polystyrene nanofiber composites. In: Abstracts of papers of the American Chemical Society, vol 252. Amer Chemical Soc., Washington, DC
Aroua MK, Zuki FM, Sulaiman NM (2007) Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafiltration. J Hazard Mater 147(3):752–758
Arslan G, Pehlivan E (2007) Batch removal of chromium(VI) from aqueous solution by Turkish brown coals. Bioresour Technol. 98(15):2836–2845
Asadullah M, Jahan I, Ahmed MB, Adawiyah P, Malek NH, Rahman MS (2014) Preparation of microporous activated carbon and its modification for arsenic removal from water. J Ind Eng Chem 20(3):887–896
Ayoob S, Gupta AK, Bhat VT (2008) A conceptual overview on sustainable technologies for defluoridation of drinking water and removal mechanisms. Crit Rev Environ Sci Technol 38:401–470
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour Technol 99(6):1578–1583
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97(1–3):219–243
Baccar R, Sarra M, Bouzid J, Feki M, Blanquez P (2012) Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem Eng J 211:310–317
Bahaj AS, James PAB, Moeschler FD (2002) Efficiency enhancements through the use of magnetic field gradient in orientation magnetic separation for the removal of pollutants by magnetotactic bacteria. Sep Sci Technol 37(16):3661–3671
Bai Y, Sun Q, Xing R, Wen D, Tang X (2010) Removal of pyridine and quinoline by bio-zeolite composed of mixed degrading bacteria and modified zeolite. J Hazard Mater 181(1–3):916–922
Banerjee SS, Chen DH (2007) Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. J Hazard Mater 147(3):792–799
Bansal RP, Donnet JP, Stoeckli F (1988) Active carbon. Marcel Dekker, New York
Barakat MA (2008) Removal of Cu (II), Ni (II), and Cr (III) ions from wastewater using complexation–ultrafiltration technique. J Environ Sci Technol 1(3):151–156
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4(4):361–377
Belessi V, Romanos G, Boukos N, Lambropoulou D, Trapalis C (2009) Removal of Reactive Red 195 from aqueous solutions by adsorption on the surface of TiO2 nanoparticles. J Hazard Mater 170:836–844
Bhatnagar A, Hogland W, Marques M, Sillanpaa M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511
Bhattacharyya KG, Sarma A (2003) Adsorption characteristics of the dye, Brilliant Green, on Neem leaf powder. Dyes Pigm 57(3):211–222
Bhaumika M, Leswifia TY, Maity A, Srinivasu VV, Onyango MS (2011) Removal of fluoride from aqueous solution by polypyrrole/Fe3O4 magnetic nanocomposite. J Hazard Mater 186:150–159
Biswas MC, Jeelani S, Rangari V (2017) Influence of biobased silica/carbon hybrid nanoparticles on thermal and mechanical properties of biodegradable polymer films. Compos Commun 1(4):43–53
Biswas MC, Tiimob BJ, Abdela W, Jeelani S, Rangari VK (2019) Nano silica-carbon-silver ternary hybrid induced antimicrobial composite films for food packaging application. Food Packag Shelf Life. 1(19):104–113
Blackburn JW (1999) Electrodialysis applications for pollution prevention in the chemical processing industry. J Air Waste Manag 49(8):934–942
Blanco M, Monteserín C, Angulo A, Pérez-Márquez A, Maudes J, Murillo N, Aranzabe E, Ruiz-Rubio L, Vilas JL (2019) TiO2-doped electrospun nanofibrous membrane for photocatalytic water treatment. Polymers 11:747. https://doi.org/10.3390/polym11050747
Bohlen A (2002) States move forward to meet new arsenic standard. Southwest Hydrol May/June:18–19
Bose P, Bose MA, Kumar S (2002) Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc and cyanide. Adv Environ Res 7(1):179–195
Britto JM, Rangel MC (2008) Processos avançados de oxidação de compostos fenólicos em efluentes industriais. Quím Nova 31:114–122
Browning E (1969) Chromium in toxicity of industrial metals, 2nd edn. Butterworths, London
Budinova T, Savova D, Tsyntsarski B, Ania CO, Cabal B, Parra JB, Petrov N (2009) Biomass waste-derived activated carbon for the removal of arsenic and manganese ions from aqueous solutions. Appl Surf Sci 255(8):4650–4657
Bui TX, Choi H (2010) Influence of ionic strength, anions, cations, and natural organic matter on the adsorption of pharmaceuticals to silica. Chemosphere 80(7):681–686
Cabrita I, Ruiz B, Mestre AS, Fonseca IM, Carvalho AP, Ania CO (2010) Removal of an analgesic using activated carbons prepared from urban and industrial residues. Chem Eng J 163(3):249–255
Cadena F, Rizvi R, Peters RW (1990) Feasibility studies for the removal of heavy metals from solution using tailored bentonite, in hazardous and industrial wastes. In: Proceedings of the twenty-second mid-atlantic industrial waste conference, Drexel University, pp 77–94
Calzaferri G, Bruhwiler D, Megelski S, Pfenniger M, Pauchard M, Hennessy B, Maas H, Devaux A, Graf U (2000) Playing with dye molecules at the inner and outer surface of zeolite L. Solid State Sci 2(4):421–447
Carrott PJM, Carrott MMLR, Roberts RA (1991) Physical adsorption of gases by microporous carbons. Colloid Surf 58(4):385–400
Carrott PJM, Carrott MMLR, Cansado IPP, Nabais JMV (2000) Reference data for the adsorption of benzene on carbon materials. Carbon 38(3):465–474
Carrott PJ, Mourao PA, Ribeiro Carrott MM, Goncalves EM (2005) Separating surface and solvent effects and the notion of critical adsorption energy in the adsorption of phenolic compounds by activated carbons. Langmuir 21(25):11863–11869
Chang YC, Chen DH (2005) Adsorption kinetics and thermodynamics of acid dyes on a carboxymethylated chitosan-conjugated magnetic nano-adsorbent. Macromol Biosci 5(3):254–261
Chao AC, Shyu SS, Lin YC, Mi FL (2004) Enzymatic grafting of carboxyl groups on to chitosan—to confer on chitosan the property of a cationic dye adsorbent. Bioresour Technol 91:157–162
Chatterjee A, Das D, Mandal BK, Chowdhury TR, Samanta G, Chakraborti D (1995) Arsenic in Groundwater in Six districts of West Bengal, India: the biggest Arsenic calamity in the world. Part I. Arsenic species in drinking water and urine of The affected people. Analyst 120:643–650
Chandra V, Park J, Chun Y, Lee JW, Hwang IC, Kim KS (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 4(7):3979–3986
Chen GH (2004) Electrochemicals technologies in wastewater treatment. Sep Purif Technol 38(1):11–14
Chen YH, Li FA (2010) Kinetic study on removal of copper(II) using goethite and hematite nano-photocatalysts. J Colloid Interfaces Sci 347(2):277–281
Chen YS, Crittenden JC, Hackney S, Sutter L, Hand DW (2005) Preparation of a novel TiO2-based p-n junction nanotubes photocatalyst. Environ Sci Technol 39:1201–1208
Chen L, Wu H, Wang T, Jin Y, Zhang Y, Dou X (2009) Granulation of Fe–Al–Ce nano-adsorbent for fluoride removal from drinking water by spray coating on sand in a fluidized bed. Powder Technol 193:59–64
Choi K-J, Son H-J, Kim S-H (2007) Ionic treatment for removal of sulphonamide and tetracycline classes of antibiotic. Sci Total Environ 387:247–256
Chowdhury SR, Yanful EK (2010) Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. J Environ Manage 91(11):2238–2247
Christie RM (2007) Environmental aspects of textile dyeing. Woodhead, Boca Raton
Clarkson TW (1993) Mercury: major issues in environmental health. Environ Health Perspect 100:31–38
Comninellis C, Kapalka A, Malato S, Parsons SA, Poulios I, Mantzavinos D (2008) Advanced oxidation processes for water treatment: advances and trends for R&D. J Chem Technol Biotechnol 83:769–776
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30(1):38–70
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97(9):1061–1085
da Silva FJG, Gouveia RM (2020) Global population growth and industrial impact on the environment. In: Cleaner production. Springer, Cham, pp 33–75
Dabrowski A (2001) Adsorption–from theory to practice. Adv Coll Interface Sci 93(1–3):135–224
Das D, Chatterjee A, Mandal BK, Samanta G, Chakraborti D, Chanda B (1995) Arsenic in groundwater in six districts of West Bengal, India: the biggest arsenic calamity in the world. Part II. Arsenic concentration in drinking water, hair, nail, urine, skin-scale and liver tissue (biopsy) of the affected people. Analyst 120:917–924
Das DD, Mahapatra R, Pradhan J, Das SN, Thakur RS (2000) Removal of Cr(VI) from aqueous solution using activated cow dung carbon. J Colloid Interfaces Sci 232(2):235–240
Daus B, Wennrich R, Weiss H (2004) Adsorption of polluting substances on activated carbons prepared from rice husk and sugarcane bagasse. Water Res 38:2948–2954
De Witte B, Dewulf J, Demeestere K, Van Langenhove H (2009) Ozonation and advanced oxidation by the peroxone process of ciprofloxacin in water. J Hazard Mater 161(2–3):701–708
Delee W, O’Neill C, Hawkes FR, Pinheiro HM (1998) Anaerobic treatment of textile effluents: a review. J Chem Technol Biot 73(4):323–335
Deliyanni EA, Matis KA (2005) Sorption of Cd ions on to akaganeite type nanocrystals. Sep Purif Technol 45:96–102
Deliyanni EA, Bakoyannakis DN, Zouboulis AI, Matis KA (2003) Sorption of As(V) ions by akaganeite-type nanocrystals. Chemosphere 50(1):155–163
Deliyanni EA, Nalbandian L, Matis KA (2006) Adsorptive removal of arsenites by a nanocrystalline hybrid surfactant-akaganeite sorbent. J Colloid Interface Sci 302(2):458–466
Deng SB, Bai RB (2004) Removal of trivalent and hexavalent chromium with aminated polyacrylonitrile fibers: performance and mechanisms. Water Res 38(9):2424–2432
Derbyshire F, Jagtoyen M, Andrews R, Rao A, Martin-Gullon I, Grulke E (2001) Carbon materials in environmental applications. In: Radovic LR (ed) Chemistry and physics of carbon, vol 27. Marcel Dekker, New York, pp 1–66
Dhar RK, Biswas BK, Samanta G, Mandal BK, Chowdhury RT, Chanda CR, Basu G, Chakraborti D, Roy S, Kabir S (1998) Groundwater arsenic contamination and sufferings of people in Bangladesh may be the biggest arsenic calamity in the world. In: International conference on arsenic pollution of groundwater in Bangladesh: causes, effects and remedies, Dhaka, Bangladesh
Dickert C (2007) Kirke Othmer encyclopedia chemical technology. Wiley, New York
Do DD (1998) Adsorption analysis: equilibria and kinetics. Imperial College Press, London
Domínguez JR, González T, Palo PE, Cuerda-Correa M (2011) Removal of common pharmaceuticals present in surface waters by Amberlite XAD-7 acrylic-esterresin: influence of pH and presence of other drugs. Desalination 269:231–238
Donlan B, Colgan S, Sheils L (2009) Innovation for a green economy: Environmental technology for a win-win story. Wexford, Ireland
Du WL, Xu ZR, Han XY, Xu YL, Miao ZG (2008) Preparation, characterization and adsorption properties of chitosan nanoparticles for eosin Y as a model anionic dye. J Hazard Mater 153(1–2):152–156
Dutta M, Dutta NN, Bhattacharya KG (1999) Aqueous phase adsorption of certain beta-lactam antibiotics onto polymeric resins and activated carbon. Sep Purif Technol 16(3):213–224
El-Guendi MS (1996) Adsorption kinetics of cationic dyestuffs on to natural clay. Adsorp Sci Technol 13:295–303
El-Guendi MS, Ismail HM, Attyia KME (1995) Activated clay as an adsorbant for cationic dyestuffs. Adsorpt Sci Technol 12:109–117
Elsayed AE, Osman DI, Attia SK, Ahmed HM, Shoukry EM, Mostafa YM, Taman AR (2019) A study on the removal characteristics of organic and inorganic pollutants from wastewater by low cost biosorbent. Egypt J Chem 63(4):16–17
Estevinho BN, Martins I, Ratola N, Alves A, Santos L (2007) Removal of 2,4-dichlorophenol and pentachlorophenol from waters by sorption using coal fly ash from a Portuguese thermal power plant. J Hazard Mater 143(1–2):535–540
Fan L, Luo C, Sun M, Li X, Qiu H (2013) Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids Surfa B Biointerfaces 103:523–529
Faria PC, Orfao JJ, Pereira MF (2004) Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res 38(8):2043–2052
Ferin J, Oberdorster G (1985) Biological effects and toxicity assessment of titanium dioxides: anatase and rutile”. Am Ind Hyg Assoc J 46:69–72
Fierro V, Muniz G, Gonzalez-Sanchez G, Ballinas ML, Celzard A (2009) Arsenic removal by iron-doped activated carbons prepared by ferric chloride forced hydrolysis. J Hazard Mater 168(1):430–437
Figueroa RA, MacKay AA (2005) Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environ Sci Technol 39(17):6664–6671
Fitzer E, Mueller K, Schaefer W (1971) The chemistry of the pyrolytic conversion of organic compounds to carbon. Marcel Dekkar, New York
Foo KY, Hameed BH (2010) Detoxification of pesticide waste via activated carbon adsorption process. J Hazard Mater 175(1–3):1–11
Förstner U, Wittmann GTW (1985) Metals in the aquatic environment. Springer, New York
Franklin LB (1991) Wastewater engineering: treatment. Disposal and reuse. McGraw Hill, Inc., New York
Freeland J, Hulme J, Kinnison D, Mitchell A, Veitch P, Aitken R, Hankin S, Poland C, Bard D, Gibson R, Saunders J (2012) Working safely with nanomaterials in research & development. The UK Nano Safety Partnership Group, London
Freeman HM (1998) Standard handbook of hazardous waste treatment and disposal, 2nd edn. McGraw-Hill, New York
Gabaldón C, Marzal P, Seco A, Gonzalez JA (2000) Cadmium and copper removal by a granular activated carbon in laboratory column systems. Sep Sci Technol 35:1039–1053
Garciamontano J, Ruiz N, Munoz I, Domenech X, Garciahortal J, Torrades F, Peral J (2006) Environmental assessment of different photo-Fenton approaches for commercial reactive dye removal. J Hazard Mater 138(2):218–225
Gaston V (1979) International regulatory aspects for chemicals, vol 1. CRC Press Inc, New York
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C 9(1):1–12
Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P (2005) Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect 113(11):1555–1560
German Federal Ministry of Education and Research (2009) Nano-initiative action plan 2010. WAZ Druck, Druisburg
Ghorai S, Pant KK (2004) Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem Eng J 98:165–173
Gjessing ET, Kallovist T (1991) Algicidal and chemical effect of UV-radiation of water containing humic substances. Water Res 25(4):491–494
Golieskardi M, Satgunam M, Ragurajan D, Hoque ME, Ng AMH, Shanmuganantha L (2019) Advanced 3Y-TZP bioceramic doped with Al2O3 and CeO2 potentially for biomedical implant applications. Mater Technol 34:480–489. https://doi.org/10.1080/10667857.2019.1578912
Gong J, Chen L, Zeng G, Long F, Deng J, Niu Q, He X (2012) Shellac-coated iron oxide nanoparticles for removal of cadmium(II) ions from aqueous solution. J Environ Sci (China) 24(7):1165–1173
Gotovac S, Song L, Kanoh H, Kaneko K (2007) Assembly structure control of single wall carbon nanotubes with liquid phase naphthalene adsorption. Colloids Surf A Physicochem Eng Asp 300(1–2):117–121
Gu C, Karthikeyan KG (2005) Interaction of tetracycline with aluminum and iron hydrous oxides. Environ Sci Technol 39(8):2660–2667
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38(1):43–74
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal–a review. J Environ Manag 90(8):2313–2342
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling-an overview. RSC Adv 2(16):6380–6388
Gürses A, Karaca S, Dogar C, Bayrak R, Acikyildiz M, Yalcin M (2004) Determination of adsorptive properties of clay/water system: methylene blue sorption. J Colloid Int Sci 269:310–314
Hage R, Lienke A (2006) Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew Chem Int Ed 45(2):206–222
Hanauer M, Pierrat S, Zins I, Lotz A, Sonnichsen C (2007) Separation of nanoparticles by gel electrophoresis according to size and shape. Nano Lett 7(9):2881–2885
Hansen HK, Ottosen LM, Kliem BK, Villumsen A (1997) Electrodialytic remediation of soils polluted with Cu, Cr, Hg, Pb and Zn. J Chem Technol Biot 70(1):67–73
Hayes KF, Leckie JO (1987) Modeling ionic-strength effects on cation adsorption at hydrous oxide-solution interfaces. J Colloid Interfaces Sci 115(2):564–572
Helmer R, Hespanhol I (1997) Water pollution control—a guide to the use of water quality management principles, 1st edn. E & FN Spon, London, p 526
Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225(1–2):109–118
Ho YS, McKay G (2003) Sorption of dyes and copper ions onto biosorbents. Process Biochem 38(7):1047–1061
Holden MJ (1982) Manufacture and Uses of Activated Carbon. Effluent Water Treat 22(1):27–35
Homem V, Santos L (2011) Degradation and removal methods of antibiotics from aqueous matrices–a review. J Environ Manag 92(10):2304–2347
Hoque ME, Philip OJ (2011) Biotechnological recovery of heavy metals from secondary sources—an overview. Mater Sci Eng C 31:57–66
Hoque ME, Peiris AM, Rahman SMA, Wahab MA (2018) New generation antibacterial nanofibrous membrane for potential water filtration. Curr Anal Chem 14(3):278–284
Hristovski K, Baumgardener A, Westerhoff P (2007) Selecting metal oxide nanomaterials for arsenic removal in fixed bed columns: from nanopowders to aggregated nanoparticle media. J Hazard Mater 147:265–274
Hu JY, Aizawa T, Ookubo Y, Morita T, Magara Y (1998) Adsorptive characteristics of ionogenic aromatic pesticides in water on powdered activated carbon. Water Res 32(9):2593–2600
Hu J, Chen G, Lo IMC (2005) Removal and recovery of Cr(VI) from waste-water by maghemite nanoparticles. Water Res 39:4528–4536
Hu J, Chen G, Lo ICM (2006) Selective removal of heavy metals from industrial wastewater using maghemite nanoparticles: performance and mechanisms. J Environ Eng ASCE 32(7):709–715
Hu J, Lo IMC, Chen GH (2007) Comparative study of various magnetic nanoparticles for Cr(VI) removal. Sep Purif Technol 56(3):249–256
Huang CP, Vane LM (1989) Enhancing As5 + Removal by a Fe2 + -Treated Activated Carbon. Res J Water Pollut C 61(9–10):1596–1603
Huang WJ, Cheng BL, Cheng YL (2007) Adsorption of microcystin-LR by three types of activated carbon. J Hazard Mater 141(1):115–122
Huang ZH, Zheng X, Lv W, Wang M, Yang QH, Kang F (2011) Adsorption of lead (II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 27(12):7558–7562
Hucknall DJ (1985) Chemistry of hydrocarbon combustion. Chapman and Hall, London
Hurt RH, Monthioux M, Kane A (2006) Toxicology of carbon nanomaterials: status, trends and perspectives on the special issues. Carbon 44:1028–1033
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26(4):201–221
Hutson DH, Roberts TR (1990) Environmental fate of pesticides, vol 7. Wiley, New York
Ilisz I, Dombi A, Mogyorósi K, Dékány I (2004) Photocatalytic water treatment with different TiO2 nanoparticles and hydrophilic/hydrophobic layer silicate adsorbents. Coll Surf A Physicochem Eng Asp 230:89–97
Jain AK, Gupta VK, Bhatnagar A (2003) Suhas, Utilization of industrial waste products as adsorbents for the removal of dyes. J Hazard Mater 101(1):31–42
Jal PK, Patel S, Mishra BK (2004) Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 62(5):1005–1028
Jansen RJJ, Vanbekkum H (1995) Xps of nitrogen-containing functional-groups on activated carbon. Carbon 33(8):1021–1027
Jia YF, Thomas KM (2000) Adsorption of cadmium ions on oxygen surface sites in activated carbon. Langmuir 16(3):1114–1122
John DZ (1990) Hand book of drinking water quality: standards and controls. Van Nostrand Reinhold, New York
Joo SH, Cheng IF (2006) Nanotechnology for environmental remediation. Springer, New York
Joss A, Zabczynski S, Gobel A, Hoffmann B, Loffler D, McArdell CS, Ternes TA, Thomsen A, Siegrist H (2006) Biological degradation of pharmaceuticals in municipal wastewater treatment: proposing a classification scheme. Water Res 40(8):1686–1696
Kadirvelu K, Thamaraiselvi K, Namasivayam C (2001) Adsorption of nickel (II) from aqueous solution on to activated carbon from coir pith. Sep Purif Technol 24:497–505
Kadirvelu K, Kavipriya M, Karthika C, Vennilamani N, Pattabhi S (2004) Mercury(II) adsorption by activated carbon made from sago waste. Carbon 42(4):745–752
Kalderis D, Koutoulakis D, Paraskeva P, Diamadopoulos E, Otal E, del Valle JO, Fernández-Pereira C (2008) Adsorption of polluting substances on activated carbon prepared from rice husk and sugarcane bagasse. Chem Eng J 144:42–50
Kalfa OM, Yalcinkaya O, Turker AR (2009) Synthesis of nano B2O3/TiO2 composite material as a new solid phase extractor and its application to preconcentration and separation of cadmium. J Hazard Mater 166(1):455–461
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ Sci Technol 39(5):1291–1298
Kanel SR, Greneche JM, Choi H (2006) Arsenic (V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ Sci Technol 40(6):2045–2050
Karami H (2013) Heavy metal removal from water by magnetite nanorods. Chem Eng J 219:209–216
Karimi-Maleh H, Fakude CT, Mabuba N, Peleyeju GM, Arotiba OA (2019) The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J Colloid Interface Sci 15(554):603–610
Karimi-Maleh H, Shafieizadeh M, Taher MA, Opoku F, Kiarii EM, Govender PP, Ranjbari S, Rezapour M, Orooji Y (2020) The role of magnetite/graphene oxide nano-composite as a high-efficiency adsorbent for removal of phenazopyridine residues from water samples, an experimental/theoretical investigation. J Mol Liq 15(298):112040
Kassaee MZ, Motamedi E, Mikhak A, Rahnemaie R (2011) Nitrate removal from water using iron nanoparticles produced by arc discharge vs. reduction. Chem Eng J 166(2):490–495
Kato MT, Field JA, Lettinga G (1997) The anaerobic treatment of low strength wastewaters in UASB and EGSB reactors. Water Sci Technol 36(6–7):375–382
Khan AW, Ahmad A, Sayed SU, Hadi A, Khan MH, Jalil MA, Ahmed R, Faruquee MH (1997) Arsenic contamination in ground water and its effect on human health with particular reference to Bangladesh. J Soc Prev Med 16:65
Kim SH, Shon HK, Ngo HH (2010) Adsorption characteristics of antibiotics trimethoprim on powered and granular activated carbon. J Ind Eng Chem 16:344–349
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35(2):402–417
Kleiser G, Frimmel FH (2000) Removal of precursors for disinfection by-products (DBPs)—differences between ozone- and OH-radical-induced oxidation. Sci Total Environ 256:1–9
Kocabas ZO, Yurum Y (2011) Kinetic modeling of arsenic removal from water by ferric ion loaded red mud. Sep Sci Technol 46:2380–2390
Korngold E, Aronov L, Kedem O (1998) Novel ion-exchange spacer for improving electrodialysis. I. Reacted spacer. J Membrane Sci 138:165
Koter S, Warszawski A (2000) Electromembrane processes in environment protection. Pol J Environ Stud 9(1):45–56
Kowalski Z (1994) Treatment of chromic tannery wastes. J Hazard Mater 37(1):137–141
Kulla HG (1981) Aerobic bacterial degradation of azo dyes. FEMS Microbiol Lett 12:387–399
Kurniawan TA, Chan GY, Lo WH, Babel S (2006a) Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118(1–2):83–98
Kurniawan TA, Chan GY, Lo WH, Babel S (2006b) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366(2–3):409–426
Kyzas GZ, Travlou NA, Deliyanni EA (2014) The role of chitosan as nanofiller of graphite oxide for the removal oftoxic mercury ions. Colloids Surf B 113:467–476
Lalezary S, Pirbazari M, McGuire MJ (1986) Evaluating activated carbons for removing low concentrations of taste-and odor-producing organics. J Am Water Works Assoc 78:76–82
Lambert TW, Holmes CFB, Hrudey SE (1996) Adsorption of microcystin-LR by activated carbon and removal in full scale water treatment. Water Res 30:1411–1422
Latifossglu AA, Surucu G, Evirgen M (1997) Improvements to the dewaterability of ferric sludge produced from chemical treatment of wastewaters. In: 4th international conference on water pollution, pp 733–742
Lee MC, Crittenden JC, Snoeyink VL, Ari M (1983) Design of carbon beds to remove humic substances. J Environ Eng-ASCE 109(3):631–645
Lee J, Mackeyev Y, Cho M, Wilson LJ, Kim JH, Alvarez PJ (2010) C60 aminofullerene immobilized on silica as a visible-light-activated photocatalyst. Environ Sci Technol 44(24):9488–9495
Lehr JH, Gass TE, Pettyjohn WA, DeMarre J (1980) Domestic water treatment. McGraw-Hill Book Company, New York
Lepistö L, Antikainen S, Kivinen J (1994) The occurrence of Gonyostomum semen (Ehr.) diesing in finnish lakes. Hydrobiologia 273:1–8
Li X-Q, Zhang W-X (2007) Sequestration of metal cations with zero valent iron nanoparticles—a study with high resolution X-ray photone electrone spectroscopy (HR-XPS). J Phys Chem C 111:6939–6946
Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A (2003) Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111(4):455–460
Li XQ, Elliott DW, Zhang WX (2006) Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit Rev Solid State 31(4):111–122
Li G, Zhao Z, Liu J, Jiang G (2011) Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica. J Hazard Mater 192(1):277–283
Lindberg R, Jarnheimer PA, Olsen B, Johansson M, Tysklind M (2004) Determination of antibiotic substances in hospital sewage water using solid phase extraction and liquid chromatography/mass spectrometry and group analogue internal standards. Chemosphere 57(10):1479–1488
Liu P, Zhang LX (2007) Adsorption of dyes from aqueous solutions or suspensions with clay nano-adsorbents. Sep Purif Technol 58(1):32–39
Liu Y, Liang P, Guo L (2005) Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 68(1):25–30
Liu JF, Zhao ZS, Jiang GB (2008) Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ Sci Technol 42(18):6949–6954
Liu J, Qiao SZ, Hu QH, Lu GQ (2011) Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small 7(4):425–443
Liu H, Zhang J, Bao N, Cheng C, Ren L, Zhang CL (2012) Textural properties and surface chemistry of lotus stalk-derived activated carbons prepared using different phosphorus oxyacids: adsorption of trimethoprim. J Hazard Mater 235:367–375
Liu X, Wang M, Zhang S, Pan B (2013) Application potential of carbon nanotubes in water treatment: a review. J Environ Sci (China) 25(7):1263–1280
Lizzio AA, Jiang H, Radovic LR (1990) On the kinetics of carbon (char) gasification—reconciling models with experiments. Carbon 28(1):7–19
Lu C, Chiu H (2006) Adsorption of Zn(II) with purified carbon nanotubes. Chem Eng Sci 61:1138–1145
Lu Y, Biswas MC, Guo Z, Jeon JW, Wujcik EK (2019) Recent developments in bio-monitoring via advanced polymer nanocomposite-based wearable strain sensors. Biosens Bioelectron 123:167–177
Lukens WW (2007) Modified activated carbon perchlorate sorbents. Lawrence Berkeley National Laboratory, Berkeley
Lv X, Xu J, Jiang G, Xu X (2011) Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 85(7):1204–1209
Machado LCR, Lima FWJ, Paniago R, Ardisson JD, Sapag J, Lago RM (2006) Polymer coated vermiculite–iron composites: novel floatable magnetic adsorbents for water spilled contaminants. Appl Clay Sci 31:207–215
Madaeni SS, Mansourpanah Y (2003) COD removal from concentrated wastewater using membranes. Filtr Sep 40(6):41–46
Mahamuni N, Adewuyi Y (2010) Advanced oxidation processes (AOPs) involving ultrasound for wastewatertreatment: a review with emphasis on cost estimation. Ultrason Sonochem 17:990–1003
Mahmoodi NM, Arami M, Limaee NY, Gharanjig K, Nourmohammadian F (2007) Nanophotocatalysis using immobilized titanium dioxide nanoparticle—degradation and mineralization of water containing organic pollutant: case study of Butachlor. Mater Res Bull 42(5):797–806
Makeswari M, Santhi T (2013) Optimization of preparation of activated carbon from ricinus communis leaves by microwave-assisted zinc chloride chemical activation: competitive adsorption of Ni2+ ions from aqueous solution. J Chem. https://doi.org/10.1155/2013/314790
Malhas AN, Abuknesha RA, Price RG (2002) Removal of detergents from protein extracts using activated charcoal prior to immunological analysis. J Immunol Methods 264(1–2):37–43
Mamalis AG (2007) Recent advances in nanotechnology. J Mater Process Technol 181(1–3):52–58
Mangun CL, Benak KR, Economy J, Foster KL (2001) Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 39(12):1809–1820
Marcucci M, Nosenzo G, Capannelli G, Ciabatti I, Corrieri D, Ciardelli G (2001) Treatment and reuse of textile effluents based on new ultrafiltration and other membrane technologies. Desalination 138(1–3):75–82
Marmagne O, Coste C (1996) Color removal from textile plant effluents. Am Dyestuff Rep 85:15–20
Matilainen A, Sillanpaa M (2010) Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 80(4):351–365
Mayadevi S (1996) Adsorbents for the removal of fluoride from water. Ind Chem Eng 38:155–157
McKay G (1982) Adsorption of dyestuffs from aqueous solutions with activated carbon I: equilibrium and batch contact-time studies. J Chem Technol Biotechnol 32(7–12):759–772
McKay G, Porter JF, Prasad GR (1999) The removal of dye colours from aqueous solutions by adsorption on low-cost materials. Water Air Soil Pollut 114(3–4):423–438
Meenakshi, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater 137(1):456–463
Mei HY, Chen Man C, Bo HZ (2010) Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J Hazard Mater 184:392–399
Mendez-Diaz JD, Prados-Joya G, Rivera-Utrilla J, Leyva-Ramos R, Sanchez-Polo M, Ferro-Garcia MA, Medellin-Castillo NA (2010) Kinetic study of the adsorption of nitroimidazole antibiotics on activated carbons in aqueous phase. J Colloid Interface Sci 345(2):481–490
Menendez JA, Phillips J, Xia B, Radovic LR (1996) On the modification and characterization of chemical surface properties of activated carbon: in the search of carbons with stable basic properties. Langmuir 12(18):4404–4410
Messalem R, Mirsky Y, Daltrophe N, Saveliev G, Kedem O (1998) Novel ion-exchange spacer for improving electrodialysis—II. Coated spacer. J Membrane Sci 138:171
Michael FM, Khalid M, Ratnam CT, Chee CY, Rashmi W, Hoque ME (2016a) Sono-synthesis of nanohydroxyapatite: effect of process parameters. Ceram Int 42(5):6263–6272
Michael FM, Khalid M, Ratnam CT, Rashmi W, Hoque ME, Ketabchi MR (2016b) Nanohydroxyapatite synthesis using optimized process parameters for load-bearing implant. Bull Mater Sci 39(1):133–145
Ming DW, Dixon JB (1987) Quantitative-determination of clinoptilolite in soils by a cation-exchange capacity method. Clays Clay Miner 35(6):463–468
Mirbagherp SA, Hosseini SN (2004) Pilot plant investigation on petrochemical wastewater treatment for the removal of copper and chromium with the objective of reuse. Desalination 171:85–93
Miretzky P, Cirelli AF (2009) Hg(II) removal from water by chitosan and chitosan derivatives: a review. J Hazard Mater 167(1–3):10–23
Miyanaga S, Hiwara A, Yasuda H (2002) Preparation and high bacteriostatic action of the activated carbons possessing ultrafine silver particles. Sci Technol Adv Mater 3:103–109
Mohammadi T, Razmi A, Sadrzadeh M (2004) Effect of operating parameters on Pb2 + separation from wastewater using electrodialysis. Desalination 167(1–3):379–385
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for the remediation of tri- and hexavalent chromium fromwater. J Hazard Mater B137:762–811
Mohan D, Pittman CU Jr (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142(1–2):1–53
Mohan D, Gupta VK, Srivastava SK, Chandra S (2000) Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids Surf 177:169–181
Mohanty K, Das D, Biswas MN (2006) Preparation and characterization of activated carbons from Sterculia alata nutshell by chemical activation with zinc chloride to remove phenol from wastewater. Adsorpt Sci Technol 12:119–132
Molu ZB, Yurdakoc K (2010) Preparation and characterization of aluminum pillared K10 and KSF for adsorption of trimethoprim. Micropor Mesopor Mat 127(1–2):50–60
Momani FL, Smith DW, Gamal El-Din M (2008) Degradation of cyanobacteria toxin by advanced oxidation process. J Hazard Mater 150:238–249
Moncayo-Lasso A, Sanabria J, Pulgarin C, Benitez N (2009) Simultaneous E-coli inactivation and NOM degradation in river water via photo-Fenton process at natural pH in solar CPC reactor. A new way for enhancing solar disinfection of natural water. Chemosphere 77(2):296–300
Mondal P, Balomajumder C, Mohanty B (2007) A laboratory study for the treatment of arsenic, iron, and manganese bearing ground water using Fe3 + impregnated activated carbon: effects of shaking time, pH and temperature. J Hazard Mater 144(1–2):420–426
Mondal P, Majumder CB, Mohanty B (2008a) Effects of adsorbent dose, its particle size and initial arsenic concentration on the removal of arsenic, iron and manganese from simulated ground water by Fe3+ impregnated activated carbon. J Hazard Mater 150(3):695–702
Mondal P, Majumder CB, Mohanty B (2008b) Treatment of arsenic contaminated water in a batch reactor by using Ralstonia eutropha MTCC 2487 and granular activated carbon. J Hazard Mater 153(1–2):588–599
Mor S, Ravindra K, Bishnoi NR (2007) Adsorption of chromium from aqueous solution by activated alumina and activated charcoal. Bioresour Technol 98(4):954–957
Mubarak NM, Sahu JN, Abdullah EC, Jayakumar NS, Ganesan P (2015) Microwave assisted multiwall carbon nanotubes enhancing Cd (II) adsorption capacity in aqueous media. J Indus Eng Chem 24:24–33
Nagarale RK, Gohil GS, Shahi VK (2006) Recent developments on ion-exchange membranes and electro-membrane processes. Adv Colloid Interface Sci 119(2–3):97–130
Nagata Y, Hirai K, Bandow H, Maeda Y (1996) Decomposition of hydrobencoic and humic acids in water by ultrasonic irradiation. Environ Sci Technol 30:1133–1138
Nah IW, Hwang KY, Jeon C, Choi HB (2006) Removal of Pb ion from water by magnetically modified zeolite. Miner Eng 19(14):1452–1455
Naiya TK, Bhattacharya AK, Das SK (2009) Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J Colloid Interfaces Sci 333(1):14–26
Najm IN, Snoeyink VL, Lykins BW, Adams JQ (1991) Using powdered activated carbon—a critical-review. J Am Water Works Ass 83(1):65–76
Namboodri CG, Walsh WK (1996) UV light/H2O2 system for decolorizing spent reactive dyebath wastewater. Am Dyestuff Rep 85:27–36
Namboodri CG, Perkins WS, Walsh WK (1994) Decolorizing dyes with chlorine and ozone: part I. Am Dyestuff Rep 83:17–22
Nas MS, Calimli MH, Burhan H, Yılmaz M, Mustafov SD, Sen F (2019a) Synthesis, characterization, kinetics and adsorption properties of Pt-Co@ GO nano-adsorbent for methylene blue removal in the aquatic mediums using ultrasonic process systems. J Mol Liq 296:112100
Nas MS, Kuyuldar E, Demirkan B, Calimli MH, Demirbaş O, Sen F (2019b) Magnetic nanocomposites decorated on multiwalled carbon nanotube for removal of Maxilon Blue 5G using the sono-Fenton method. Sci Rep 9(1):1
Navarro P, Alguacil FJ (2002) Adsorption of antimony and arsenic from a copper electrorefining solution onto activated carbon. Hydrometallurgy 66(1–3):101–105
Nemerow N, Dasgupta A (1991) Industrial and hazardous waste treatment. Van Nostrand Reinhold Publishing Company, New York
Nemerrow NL (1978) Industrial water pollution: origins, characteristics, and treatment. Addison-Wesley Publishing Company, Massachusetts
Ngomsik AF, Bee A, Draye M, Cote G, Cabuil V (2005) Magnetic nano- and microparticles for metal removal and environmental applications: a review. CR Chim 8(6–7):963–970
Novak JT, Muller CD, Murthy SN (2001) Floc structure and the role of cations. Water Sci Technol 44(10):209–213
Nuge T, Tshai KY, Lim SS, Nordin N, Hoque ME (2020) Characterization and optimization of the mechanical properties of electrospun gelatin nanofibrous scaffolds. World J Eng. https://doi.org/10.1108/WJE-04-2019-0119
Nxumalo EN (2006) Synthesis of monofunctionalised cyclodextrin polymers for the removal of organic pollutants from water. M.Sc. Thesis University of Johannesburg, South Africa
O’Neill C, Hawkes FR, Hawkes DL, Lourenco ND, Pinheiro HM, Delée W (1999) Colour in textile effluents—sources, measurement, discharge consents and simulation: a review. J Chem Technol Biotechnol 74:1009–1018
Oller I, Malato S, Sánchez-Pérez JA (2011) Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409:4141–4166
Ozdemir O, Armagan B, Turan M, Celik MS (2004) Comparison of the adsorption characteristics of azo-reactive dyes on mezoporous minerals. Dyes Pigm 62(1):49–60
Pacheco S, Tapia J, Medina M, Rodriguez R (2006) Cadmium ion adsorption in simulated wastewater using structured alumina-silica nanoparticles. J Non Cryst Solids 352:5475–5481
Padmanabhan VP, Narayanan SNTS, Sagadevan S, Hoque ME, Kulandaivelu R (2019) Advanced lithium substituted hydroxyapatite nanoparticles for antimicrobial and hemolytic studies. New J Chem 43:18484–18494. https://doi.org/10.1039/C9NJ03735G
Pan S, Shen H, Xu Q, Luo J, Hu M (2012) Surface mercapto engineered magnetic Fe3O4 nanoadsorbent for the removal of mercury from aqueous solutions. J Colloid Interface Sci 365(1):204–212
Park P, Lee CH, Choi SJ, Choo KH, Kim SH, Yoon CH (2002) Effect of the removal of DOMs on the performance of a coagulation–UF membrane system for drinking water production. Desalination 145:237–245
Parsons S (2004) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London
Pelekani C, Snoeyink VL (2000) Competitive adsorption between atrazine and methylene blue on activated carbon: the importance of pore size distribution. Carbon 38(10):1423–1436
Pendashteh AR, Fakhru’l-Razi A, Chuah TG, Radiah AB, Madaeni SS, Zurina ZA (2010) Biological treatment of produced water in a sequencing batch reactor by a consortium of isolated halophilic microorganisms. Environ Technol 31(11):1229–1239
Peng XJ, Luan ZK, Ding J, Di ZH, Li YH, Tian BH (2005) Ceria nanoparticles supported on carbon nanotubes for the removal of arsenate from water. Mater Lett 59(4):399–403
Pérez-Estrada LA, Maldonado MI, Gernjak W, Agüera A, Fernández-Alba AR, Ballesteros MM, Malato S (2005) Decomposition of diclofenac by solar driven photocatalysis at pilot plant scale. Catal Today 101:219–226
Pils JR, Laird DA (2007) Sorption of tetracycline and chlortetracycline on K- and Ca-saturated soil clays, humic substances, and clay-humic complexes. Environ Sci Technol 41(6):1928–1933
Pirbazari M, Badriyha BN, Kim SH, Miltner RJ (1992) Evaluating Gac adsorbers for the removal of pcbs and toxaphene. J Am Water Works Ass 84(2):83–90
Ponder SM, Darab JG, Mallouk TE (2000a) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34(12):2564–2569
Ponder TB, Smith D, Ramzy I (2000b) Lymphadenopathy in children and adolescents: role of fine-needle aspiration in management. Cancer Detect Prev 24(3):228–233
Ponder SM, Darab JG, Bucher JD, Caulder D, Craig CI, Davis L, Edelstein N, Lukens W, Nitsche H, Rao LF, Shuh DK, Mallouk TE (2001) Surface chemistry and electrochemistry of supported zerovalent iron nanoparticles in the remediation of aqueous metal contaminants. Chem Mater 13(2):479–486
Pradhan J, Das SN, Thakur RS (1999) Adsorption of hexavalent chromium from aqueous solution by using activated red mud. J Colloid Interfaces Sci 217(1):137–141
Przepiorski J (2006) Enhanced adsorption of phenol from water by ammonia-treated activated carbon. J Hazard Mater 135(1–3):453–456
Putra EK, Pranowo R, Sunarso J, Indraswati N, Ismadji S (2009) Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: mechanism, isotherms and kinetics. Water Res 43:2419–2430
Qu XL, Alvarez PJJ, Li QL (2013a) Applications of nanotechnology in water and wastewater treatment. Water Res 47(12):3931–3946
Qu X, Brame J, Li Q, Alvarez PJ (2013b) Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc Chem Res 46(3):834–843
Ragurajan D, Golieskardi M, Satgunam M, Hoque ME, Ng AMH, Ghazali MJ, Ariffin AK (2018) Advanced 3Y-TZP bioceramic doped with Al2O3 and MnO2 particles potentially for biomedical applications: study on mechanical and degradation properties. J Mater Res Technol 7:432–442. https://doi.org/10.1016/j.jmrt.2017.05.015
Rai HS, Bhattacharyya MS, Singh J, Bansal TK, Vats P, Banerjee UC (2005) Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Crit Rev Environ Sci Technol 35(3):219–238
Ramana DV, Yu JS, Seshaiah K (2013) Silver nanoparticles deposited multiwalled carbon nanotubes for removal of Cu (II) and Cd (II) from water: Surface, kinetic, equilibrium, and thermal adsorption properties. Chem Eng J 223:806–815
Rangari VK, Apalangya V, Biswas M, Jeelani S (2017) Preparation and microscopic characterization of biobased nanoparticles from natural waste materials. Microsc Microanal 23(S1):1938–1939
Rangari VK, Biswas MC, Tiimob BJ (2019) Biodegradable polymer blends for food packaging applications. Food Packag Innov Shelf-Life 11:151
Rao MM, Reddy DHKK, Venkateswarlu P, Seshaiah K (2009) Removal of mercury from aqueous solutions using activated carbon prepared from agricultural by-product/waste. J Environ Manag 90(1):634–643
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27
Ravi Kumar MNV, Sridhari TR, Bhavani KD, Dutta PK (1998) Trends in color removal from textile mill effluents. Colorage 40:25–34
Raymundo-Pinero E, Cazorla-Amoros D, Linares-Solano A (2003) The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibres. Carbon 41(10):1925–1932
Rether A, Schuster M (2003) Selective separation and recovery of heavy metal ions using water-soluble N-benzoylthiourea modified PAMAM polymers. React Funct Polym 57(1):13–21
Rivera-Utrilla J, Prados-Joya G, Sanchez-Polo M, Ferro-Garcia MA, Bautista-Toledo I (2009) Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon. J Hazard Mater 170(1):298–305
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77(3):247–255
Roco MC (2003) Broader societal issues of nanotechnology. J Nanopart Res 5(3–4):181–189
Rodriguez-Reinoso F (1986) Preparation and characterization of activated carbons. In: Proceeding of the NATO advanced study institute on carbon and coal gasification. Martinus Nijhoff Publishers, Leiden, Netherlands
Saffaj N, Loukil H, Younssi SA, Albizane A, Bouhria M, Persin M, Larbot A (2004) Filtration of solution containing heavy metals and dyes by means of ultrafiltration membranes deposited on support made of Morrocan clay. Desalination 168:301–306
Sairam SC, Viswanathan N, Meenakshi S (2008) Defluoridation chemistry of synthetic hydroxyapatite at nano scale: equilibrium and kinetic studies. J Hazard Mater 155:206–215
Sani RK, Banerjee UC (1999) Decolorization of triphenylmethane dyes and textile and dye-stuff effluent by Kurthia sp. Enzyme Microbial Technol 24:433–437
Savic R, Luo L, Eisenberg A, Maysinger D (2003) Micellar nanocontainers distribute to defined cytoplasmic organelles. Science 300(5619):615–618
Schmidt-Ott A, Butselaar-Orthlieb V, van Winsen J, Bosma D (2010) Nanosafety guidelines: preventing exposure to nanomaterials. Delft University of Technology, Delft
Scott K (1995) Handbook of industrial membranes. Elsevier Advanced Technology, Oxford
Seredych M, Rossin JA, Bandosz TJ (2011) Changes in graphite oxide texture and chemistry upon oxidation and reduction and their effect on adsorption of ammonia. Carbon 49:4392–4402
Seshadri T, Kettrupt A (1982) Synthesis and characterization of silica gel ion-exchanger bearing 2-amino-1-cyclopentene-1-dithio-carboxylic acid (ACDA) as chelating compound. Fresen Z Anal Chem 310:1–5
Shaarani FW, Hameed BH (2011) Ammonia-modified activated carbon for the adsorption of 2,4-dichlorophenol. Chem Eng J 169(1–3):180–185
Shamsadin-Azad Z, Taher MA, Cheraghi S, Karimi-Maleh H (2019) A nanostructure voltammetric platform amplified with ionic liquid for determination of tert-butylhydroxyanisole in the presence kojic acid. J Food Meas Charact 13(3):1781–1787
Sharma RK, Mittal S, Koel M (2003) Analysis of trace amounts of metal ions using silica-based chelating resins: a green analytical method. Crit Rev Anal Chem 33(3):183–197
Sharma YC, Srivastava V, Singh VK, Kaul SN, Weng CH (2009) Nano-adsorbents for the removal of metallic pollutants from water and wastewater. Environ Technol 30(6):583–609
Shen W, Li Z, Liu Y (2008) Surface chemical functional groups modification of porous carbon. Rec Patents Chem Eng 1:27–40
Shen L, Zhao B, Zhang J, Chen J, Zheng H (2010) Virus adsorption onto nano-sized iron oxides as affected by different background solutions. Huan Jing Ke Xue 31(4):983–989
Shichi T, Takagi K (2000) Clay minerals as photochemical reaction fields. J Photochem Photobiol C Photochem Rev 1:113–130
Singh U, Kaushal R (2013) Treatment of wastewater with low cost adsorbent—a review. VSRD Int J Tech Non-Tech Res 4:33–42
Singh TS, Pant KK (2004) Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Sep Purif Technol 36(2):139–147
Singh IB, Singh DR (2002) Cr(VI) removal in acidic aqueous solution using iron-bearing industrial solid wastes and their stabilisation with cement. Environ Technol 23(1):85–95
Singh S, Barick KC, Bahadur D (2011) Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J Hazard Mater 192(3):1539–1547
Smíšek M, Černý S (1970) Active carbon: manufacture properties and applications. Elsevier Publishing Company, Amsterdam
Sostar-Turk S, Simonic M, Petrinic I (2005) Wastewater treatment after reactive printing. Dyes Pigm 64(2):147–152
Stafiej A, Pyrzynska K (2007) Adsorption of heavy metal ions with carbon nanotube. Sep Purif Technol 58:49–52
Stander L, Theodore L (2011) Environmental implications of nanotechnology—an update. Int J Environ Res Public Health 8(2):470–479
Stipp SL, Hochella MF, Parks GA, Leckie JO (1992) Cd2+ uptake by calcite, solid-state diffusion, and the formation of solid-solution—interface processes observed with near-surface sensitive techniques (Xps, Leed, and Aes). Geochim Cosmochim Acta 56(5):1941–1954
Sturchio NC, Chiarello RP, Cheng L, Lyman PF, Bedzyk MJ, Qian Y, You H, Yee D, Geissbuhler P, Sorensen LB, Liang Y, Baer DR (1997) Lead adsorption at the calcite-water interface: synchrotron X-ray standing wave and X-ray reflectivity studies. Geochim Cosmochim Acta 61:251–263
Sturchio NC, Chiarello RP, Cheng L, Lyman PF, Bedzyk MJ, Qian Y, You H, Yee D, Geissbuhler P, Sun Q, Yang L (2003) The adsorption of basic dyes from aqueous solution on modified peat-resin particle. Water Res 37(7):1535–1544
Sweeney SF, Woehrle GH, Hutchison JE (2006) Rapid purification and size separation of gold nanoparticles via diafiltration. J Am Chem Soc 128(10):3190–3197
Taiwo EA, Adesina A (2005) Electrochemical regeneration of a native activated carbon. Chem Biochem Eng Q 19(3):269–273
Tang W, Su Y, Li Q, Gao S, Shang JK (2013) Superparamagnetic magnesium ferrite nanoadsorbent for effective arsenic (III, V) removal and easy magnetic separation. Water Res 47(11):3624–3634
Tchobanoglous G, Franklin LB (1991) Wastewater engineering: treatment disposal and reuse. McGraw Hill, Inc., New York
Tchomgui-Kamga E, Alonzo V, Nanseu-Njiki CP, Audebrand N, Ngameni E, Darchen A (2010) Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water. Carbon 48(2):333–343
Tinge JT, Mencke K, Drinkenburg AAH (1987) The absorption of propane and ethene in slurries of activated carbon in water-I. Chem Eng Sci 42:1899–1907
Tlili I, Alkanhal TA (2019) Nanotechnology for water purification: electrospun nanofibrous membrane in water and wastewater treatment. J Water Reuse Desalin 9:232–248. https://doi.org/10.2166/wrd.2019.057
Tratnyek PG, Scherer MM, Johnson TJ, Matheson LJ (2003) Chemical degradation methods for wastes and pollutants: environmental and industrial applications. Marcel Dekker, New York
Trivunac K, Stevanovic S (2006) Removal of heavy metal ions from water by complexation-assisted ultrafiltration. Chemosphere 64(3):486–491
Tsao TM, Wang MK, Huang PM (2009) Automated ultrafiltration device for efficient collection of environmental nanoparticles from aqueous suspensions. Soil Sci Soc Am J 73(6):1808–1816
Urano K, Yamamoto E, Tonegawa M, Fujie K (1991) Adsorption of chlorinated organic-compounds on activated carbon from water. Water Res 25(12):1459–1464
Üstün GE, Solmaz SKA, Birgül A (2007) Regeneration of industrial district wastewater using a combination of Fenton process and ion exchange e a case study. Resour Conserv Recycl 52:425–440
van der Zee FP, Villaverde S (2005) Combined anaerobic-aerobic treatment of azo dyes-a short review of bioreactor studies. Water Res 39:1425–1440
Van der Zee FP, Lettinga G, Field JA (2001) Azo dye decolourisation by anaerobic sludge. Chemosphere 44:1169–1176
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivative: a review. Carbohydr Polym 55:77–79
Vassilis IJ (2010) Ion exchange and adsorption fixed bed operations for wastewater treatment—part I: modeling fundamentals and hydraulics analysis. J Eng Stud Res 16(3):29–40
Vaughan RL Jr, Reed BE (2005) Modeling As(V) removal by an iron oxide impregnated activated carbon using the surface complexation approach. Water Res 39:1005–1014
Veličković Z, Vuković GD, Marinković AD, Moldovan M-S, Perić-Grujić AA, Uskoković PS, Ristić MD (2012) Adsorption of arsenate on iron(III) oxide coated ethylenediamine functionalized multiwall carbon nanotubes. Chem Eng J 2012(181–182):174–181
Venkata Mohan S, Lalit Babu V, Sarma PN (2007) Anaerobic biohydrogen production from dairy wastewater treatment in sequencing batch reactor (AnSBR): effect of organic loading rate. Enzyme Microbiol Technol 41(4):506–515
Villaescusa I, Fiol N, Poch J, Bianchi A, Bazzicalupi C (2011) Mechanism of paracetamol removal by vegetable wastes: the contribution of p–p interactions, hydrogen bonding and hydrophobic effect. Desalination 270:135–142
Vilela D, Parmar J, Zeng Y, Zhao Y, Sánchez S (2016) Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett 16(4):2860–2866
Volesky B (1999) Biosorption for the next century, biohydrometallurgy and the environment toward the mining of the 21st century. In: Ballester A, Amils R (eds) Internat. Biohydro metallurgy symposium proceedings, Madrid, Spain. Elsevier Sciences, Amsterdam, pp 161–170
Wang S (2008) A comparative study of Fenton and Fenton-like reaction kinetics in decolorization of wastewater. Dyes Pigm 76(3):714–720
Wang SB, Peng YL (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156(1):11–24
Wang LK, Vaccari DA, Li Y, Shammas NK (2004) Chemical precipitation. In: Wang LK, Hung YT, Shammas NK (eds) Physicochemical treatment processes, vol 3. Humana Press, New Jersey, pp 141–198
Wang H, Zhou A, Peng F, Yu H, Yang J (2007) Mechanism study on adsorption of acidified multi-walled carbon nanotubes to Pb(II). J Colloid Interface Sci 316:277–283
Wang Y, Mo Z, Zhang P, Zhang C, Han L, Guo R, Gou H, Wei X, Hu R (2016) Synthesis of flower-like TiO2 microsphere/graphene composite for removal of organic dye from water. Mater Des 5(99):378–388
Wasewar KL (2010) Adsorption of metals onto tea factory waste: a review. Int J Res Rev Appl Sci 3(3):303–322
Watonabe T, Ogawa K (1929) Activated carbon for purifying copper electrolytes. Chem Abs 24:1037
WHO (2004) Guidelines for drinking-water quality, vol 1, 3rd edn. World Health Organisation, Geneva
Wigmans T (1986) Fundamentals and practical implications of activated carbon production by partial gasification of carbonaceous materials. In: Proceedings of the NATO advanced study institute on carbon and coal gasification. Martinus Nijhoff Publishers, Leiden, Netherlands
Woolard CD, Strong J, Erasmus CR (2002) Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams. Appl Geochem 17:1159–1164
Wu JN, Doan H, Upreti S (2008) Decolorization of aqueous textile reactive dye by ozone. Chem Eng J 142(2):156–160
Xu TW (2005) Ion exchange membranes: state of their development and perspective. J Membrane Sci 263(1–2):1–29
Xu N, Hochella N, Brown MF, Parks GA (1996) Co(II) sorption at the calcite-water interface: I. X-ray photoelectron spectroscopic study. Geochim Cosmochim Acta 60:2801–2815
Yadla SV, Sridevi V, Lakshmi MVVC (2012) A review on adsorption of heavy metals from aqueous solution. J Chem Bio Phys Sci Sec D 2(3):1585–1593
Yan H, Gong A, He H, Zhou J, Wei Y, Lv L (2006) Adsorption of microcystins by carbon nanotubes. Chemosphere 62(1):142–148
Yang M (2011) A current global view of environmental and occupational cancers. J Environ Sci Health Part C Environ Carcinogenesis Ecotoxicol Rev 29(3):223–249
Yang K, Zhu L, Xing B (2006) Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ Sci Technol 40(6):1855–1861
Yeddou AR, Chergui S, Chergui A, Halet F, Hamza A, Nadjemi B, Ould-Dris A, Belkouch J (2011) Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of copper-impregnated activated carbon. Miner Eng 24(8):788–793
Yu F, Sun S, Ma J, Han S (2015) Enhanced removal performance of arsenate and arsenite by magnetic graphene oxide with high iron oxide loading. Phys Chem Chem Phys 17(6):4388–4397
Yurum A, Kocabas-Atakli ZO, Sezen M, Semiat R, Yurum Y (2014) Fast deposition of porous iron oxide on activated carbon by microwave heating and arsenic (V) removal from water. Chem Eng J 242:321–332
Zeng WM, Gao L, Guo JK (1998) A new sol-gel route using inorganic salt for synthesizing Al2O3 nanopowders. Nanostruct Mater 10(4):543–550
Zhang Q, Pan B, Pan B, Zhang W, Jia K, Zhang Q (2008) Selective sorption of lead, cadmium and zinc ions by a polymeric cation exchanger containing nano-Zr(HPO3S)2. Environ Sci Technol 42(11):4140–4145
Zhang K, Dwivedi V, Chi C, Wu J (2010) Graphene oxide/ferric hydroxide composites for efficient arsenate removal from drinking water. J Hazard Mater 182(1–3):162–168
Zhang SX, Zhang YY, Liu JS, Xu Q, Xiao HQ, Wang XY, Xu H, Zhou J (2013) Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem Eng J 226:30–38
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45(24):10454–10462
Zhou MH, Lei LC (2006) Electrochemical regeneration of activated carbon loaded with p-nitrophenol in a fluidized electrochemical reactor. Electrochim Acta 51(21):4489–4496
Zhou YT, Nie HL, White CB, Hea ZY, Zhua LM (2009) Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α–ketoglutaric acid. J Colloid Interface Sci 330:29–37
Zhu HJ, Jia YF, Wu X, Wang H (2009) Removal of arsenite from drinking water by activated carbon supported nano zero-valent iron. Huan Jing Ke Xue 30(6):1644–1648
Zhu YH, Hu J, Wang JL (2012) Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J Hazard Mater 221:155–161
Zinkus GA, Byers WD, Doerr WW (1998) Identify appropriate water reclamation technologies. Chem Eng Prog 94(5):19–31
Zularisama AW, Ismaila AF, Salim R (2006) Behaviours of natural organic matter in membrane filtration for surface water treatment—a review. Desalination 194:211–231
Acknowledgements
This research did not receive any specific grant from any funding agency in the public, commercial or not-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Editorial responsibility: Fatih ŞEN.
Rights and permissions
About this article
Cite this article
Ali, M.E., Hoque, M.E., Safdar Hossain, S.K. et al. Nanoadsorbents for wastewater treatment: next generation biotechnological solution. Int. J. Environ. Sci. Technol. 17, 4095–4132 (2020). https://doi.org/10.1007/s13762-020-02755-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02755-4