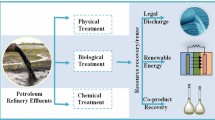
Abstract
As we advance towards “a non-toxic environment”, there is increased action in the soil, surface, and groundwater remediation activities in response to the world’s environmental quality objectives. Crude oil is a pollutant whose entrance into the soil, surface, and groundwater environments has elicited profound negative impacts as harbinger of soil, water, and air pollution. The effects of oil spillage in the environments are unprecedented and cannot be ignored. It is necessary to decontaminate the polluted ecosystem after a spill since they are potent immunotoxicants and carcinogens, which can cause kidney diseases, cancer, and liver damage. Bioremediation, a technology that exploits the various capabilities of microorganisms to degrade or convert organic pollutants to innocuous products through mineralisation, has become the process of choice in the quest to remove soil contaminants. The bioremediation technology is deemed efficient, is low cost, does not require any technical skills to function, and mostly does not impact the ecosystem negatively. Although the efficacy of the bioremediation treatment is inhibited by the properties of the pollutants, the soil matrix, and the ecological factors, it remains the process of choice for most environmentalist. This article reviews the bioremediation process, highlighting the use of adsorption and photocatalysis as the most popular strategies applied in the reduction of pollutants in contaminated water bodies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world’s total dependence on crude oil as a source of clean energy is a stimulus to large-scale exploration, refining, transportation, and usage of crude oil-derived products (Khudur et al. 2015). Each step in the processing and usage of crude oil is accompanied by a measure of the oil spill with resultant environmental pollution. The entrance of crude oil, industrial pollutants and domestic effluents into the ecosystem has adversely affected humans, plants and animal health as most contaminants are carcinogenic accounting for over 0.005 billion deaths annually (Saranya et al. 2016; Varjani and Upasani 2016; Muhammad and Harmin 2018; Oluyoye et al. 2019). The magnitude of environmental pollution from crude oil-related activities, industrial sewage effluents, domestic contaminants, and the global health impacts by pollutants is a common problem that has generated the need to search for environmental low-cost clean-up techniques to reduce, degrade, or remove the pollutants.
The total estimated sites of crude oil contamination all over the world are significant (Hassan et al. 2016), resulting in the alteration of the ecological balance of the contaminated ecosystem. Even though crude oil is not classified as a hazardous chemical, it is considered persistent, including the derivatives that can bioconcentrate and bioaccumulate in food chains (Shaker and Almukhtar 2016).
Crude oil is a widespread environmental contaminant, and their presence has elicited different analytical and remediation methods (Smith et al. 2015). The conventional techniques to remediate the environments tainted by petroleum hydrocarbon incorporate many technologies and include the ex situ traditional removal of contaminated soil to a landfill (M’rassi et al. 2015), onsite incineration of pollutants, soil washing, pump-and-treat operations, and the in situ thermal treatment, chemical oxidation, the use of reactive barriers, bioremediation, and phytoremediation (Mueller and Nowack 2010). Some of these models are replete with drawbacks; nevertheless, they offer the cheapest method for remediating contaminated soils; however, negative public opinion and perception towards them have resulted in the development of other treatment options. Consequently, other better technologies to destroy the pollutant or transform it into a harmless product are being sought. Bioremediation provides a good clean-up strategy for crude oil-polluted soils, but bioremediation may not be feasible when applied to sites with high concentrations of chemicals that are toxic to microorganisms making experimentation with other clean-up methodologies desirable. Solvent extraction supplemented with bioremediation and adsorption coupled to photocatalysis is an attractive approach to decontaminate polluted ecological units. Adsorption and photocatalysis with bioremediation are inexpensive approaches to treat xenobiotic in all environmental media, and they are known to generate little or no residues with a low carbon footprint.
This review attempts to present and discuss the remediation of crude oil-contaminated environments as well as contamination by the emerging contaminants (CECs). The review highlighted the importance of environmental remediation and reviewed some treatment options while paying more attention to the fabrication of flagship photocatalysts that are prospective in environmental remediation. Remediation methods such as extraction with organic solvents, subcritical fluid extraction, adsorption, photocatalysis, and bioremediation through biostimulation were discussed.
Environmental contaminants
Generally, environmental threats are not limited to crude oil and derived products (lubricating, automotive, hydraulic, and fuel oils) alone. Environmental pollution problems are compounded by our activities through the cumulative use of panoply of chemical substances, particularly in the pharmaceutical industries and agriculture.
These have given rise to the appearance of a detectable level of a broad range of inorganic and organic obnoxious materials in the environment with a severe consequence on the receiving ecosystem (Violette et al. 2015; Guo et al. 2016; Dzionek et al. 2016). The contaminants are classified as inorganic ions, organic chemicals, pathogens, aqueous organic waste (pesticides, e.g. thiophanate methyl) (Sharma et al. 2018a, b, c), organic liquids, and inorganic compounds (heavy metals), some of which are toxic to microorganisms, a primary agent in pollutants degradation (Jianlong et al. 2019). Other xenobiotics often referred to as contaminants of emerging concerns (CEC) are, by nature, designed to be recalcitrant and to interact with human and animal biochemistry has consistently gained entrance into the environment. The pollutants mostly enter the ecosystem through wastewater from production plants and include pesticides and their metabolites (Violette et al. 2015), pharmaceutical and personal care products (PPCPs) (Suave 2014; Ebele et al. 2017; Sharma et al. 2018a, b, c), food additives and industrial wastes such as nanometal (Jianfel et al. 2019) that are problematic to the environment. The emerging pollutants are not commonly monitored but have the potential to enter the environs with adverse ecological impact on the recipient ecosystem (Violette et al. 2015; Petrie et al. 2015).
Other CECs including the endocrine-disrupting compounds, analgesic, antibiotics, antiepileptics, antiseptics, represent a severe environmental hazard that has elicited much interest because of their incomplete removal when subjected to treatment by adsorption (Sharma et al. 2018a, b, c; Lesley and Yeomin 2019). Severally, conventional remediation methods and treatment technologies have shown limited effectiveness in the degradation of some pollutants (Mueller and Nowack 2010). Though many microorganisms can degrade different pollutants, the regular treatment scheme for industrial sewage typically involves aerobic biological processes which are also proven ineffective, because of poor biodegradability when used on some contaminants. The global challenge now is how to evolve more appropriate methods of control and removal without affecting the environment negatively (Violette et al. 2015).
Nanotechnology used in conjunction with water treatment strategies including coagulation and sedimentation (Okoh et al. 2019), adsorption (Ali 2014), membrane filtration (Ang et al. 2015), photocatalysis and biological degradation (Sharma et al. 2018a, b, c; Kumar et al. 2018; Jianlong et al. 2019) holds promises for efficiency in the treatment of persistent pollutants. Studies are being undertaken to establish a better way to tackle the potential health hazards, even though the polluting nature and health risk of some of the contaminants are not yet understood. It has been reported that pollutants removal through physical, chemical, and biological methods or a combination thereof constitutes the only options to eliminate them from the environment (Mayur et al. 2019).
The use of adsorption and a combination of aerobic treatment with chemical oxidation processes such as photocatalysis dominate other methods of reduction of wastewater-dissolved pollutants (Abebe et al. 2018). A significant interest has also arisen to develop novel photocatalytic technology covering an extensive range of environmental applications such as water remediation and environmental clean-up of petroleum hydrocarbon spills and other pollutants (Pawar et al. 2018; Sharma et al. 2018a, b, c).
Remediation technologies
There are three basic strategies, including elimination or alteration, extraction or separation, and immobilisation of contaminants, that are employed separately or in conjunction with decontaminate polluted sites. The treatment technologies applied in situ or ex situ to contaminated media and that could eliminate contaminants through the alteration of their structure consist of thermal, biological, and chemical technologies. Treatment technologies for extraction or separation strategy include thermal desorption, air stripping, soil washing, and adsorption, while immobilisation technologies consist of stabilisation, solidification, and placement in landfills. Immobilisation has five basic techniques, such as adsorption, flocculation, binding on a surface, entrapment, and encapsulation.
Soil washing with organic solvents and aqueous solutions
Soil washing often called mechanical scrubbing or attrition is a water-based technique for treating soils unearthed from contaminated lands which could be done in situ or ex situ. Hydrophobic organic pollutants including pesticides and crude oil as well as fuel residues and a diverse array of contaminants like heavy metals also may be eliminated from the soil using soil washing with organic solvents (Murena and Gioia 2009). Li et al. (2012) studied and applied solvent extraction method to remove petroleum hydrocarbon from polluted soil by using a hexane–acetone solvent mixture and demonstrated that solvent extraction was effective in crude oil removal from soils. Soil washing (in situ) can also be done by sedimentation method in which the soil particles are separated hydraulically based on their particle size (Saranya et al. 2016).
Solvent extraction with subcritical fluid
Current remediation methods are limited when treating certain types of contaminated soils. Supercritical fluid carbon dioxide at a pressure of about 7.4 MPa is a common liquid for the extraction of soil contaminants (Saldana et al. 2005). In this method of decontamination, the pollutants liquefy into the solvent through the changes in temperature and pressure. In supercritical water extraction, water is heated and pressurised and applied as a solvent instead of the usual organic chemicals. The supercritical fluid extraction can be used to remove organic contaminants such as petroleum hydrocarbons and inorganic pollutants, including heavy metals.
Remediation by adsorption
One underlying technology with advantages on ease of operation and comparatively low cost in ecosystem decontamination is the adsorption process. Adsorption is a surface phenomenon and widely used in the treatment of water, air emissions, and in the elimination of heavy metals from contaminated media (Wang et al. 2019). The adsorption process is described through equilibrium isotherm which occurs when an adsorbate meets the adsorbent for a period. Freundlich, Langmuir, and BET isotherms are the regularly used isotherm models with Freundlich and Langmuir isotherms having broad applicability. The basic adsorbents in the treatment of water and wastewater include activated carbon, metal oxides, carbon nanotubes, zeolite, clay, mesoporous silica, polymeric resin, metal–organic frameworks, and several agro-based wastes (Sahu et al. 2019). The adsorption process gives sufficient and steady results but produces sludge with a high concentration of nutrients as a significant drawback. The sludge containing nutrients requires proper treatment and disposal. This limitation is overcome by the integration of adsorption technology with photocatalysis under solar and visible-light irradiation which combines to oxidise and hydrolyse the resultant solution to innocuous level (Liu et al. 2010).
Lignocellulosic adsorbents can be chemically modified by adding cationic groups through quaternisation to enhance their absorption capacities towards anion such as phosphate and sulphate. Quaternisation involves the reaction of biopolymers with quaternary ammonium compounds. Kumar et al. (2018) prepared and applied the novel quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nanojunction for visible-light degradation of sulphamethoxazole from a contaminated aqueous environment. Zarrabi et al. (2014) achieved the removal of toxic phosphate anions from contaminated water by treating with pectin-based quaternary amino anion exchanger.
Similarly, wastewater contaminated with lead was purified by Okoye et al. (2010) through the application of activated carbon adsorption. TPH and PAHs may be removed using activated carbon and matrix-modified organoclay adsorbents, while the adsorption technology can also reduce arsenic concentrations to less than 10 mg/l. Adsorption is a surface phenomenon, although the phenomena may not be entirely dependent on the surface area of the adsorbing media alone. Yasinta et al. (2018) studied the elimination of the hazardous anion in water through adsorption and concluded that the rate of adsorption rarely depends on the surface area of the adsorbent instead on a combination of factors including pH, ionic strength, pore volume, and particle size.
Nanoparticles (nanobioremediation)
The potential of nanoparticles (NPs) made from a variety of bulk materials to revolutionise the remediation technology is enormous. Nanotechnology is characterised by the use of small manufactured particles at the nanoscale (< 100 nm) and by the particle structures. The nanoparticles are extensively used to restore contaminated soil and polluted waters. Nanoparticles can occur naturally or produced from specialised materials and processes or occur as accidental by-products of industrial processes (Mueller and Nowack 2010). Nanoparticles, especially biosynthetic nanoparticles, are increasingly being used and thriving as effective sorbents of pollutants. Environmental applications include indoor and outdoor air cleaning, water and wastewater purification, soil and groundwater remediation. Soil, air, and water remediation with nanomaterials guarantee effective and cheaper approaches to environmental clean-up due to the improved reactivity of nanoparticles and the prospect of in situ treatment. A thermally activated persulphate could effectively degrade the TCS in soils (Oturan and Aaron 2014). Nanoparticles in soil remediation include nanoscale zero-valent iron often employed in the degradation of halogenated organic compounds. Nanoscale calcium peroxide applied to the soil to remove organics, including crude oil products such as gasoline and nanoscale metal oxides, is also useful in the adsorption of metals from the environment.
Environmental remediation by photocatalysis
Studies show that adsorption does not degrade pollutants and photocatalysts will not degrade the pollutant without adsorbing it to the surface. The adsorption process is, therefore, tied to the photocatalyst for rapid elimination of contaminants (Sahu et al. 2019). The principle of photocatalysis involves the transfer of the contaminant from the water to the surface of the photocatalyst where they are adsorbed with the implication that the high surface area of the catalyst will provide active sites for the reaction. The contaminants are degraded to CO2 and H2O, which then desorbs from the surface of the catalyst creating holes for other reactions to occur. Photocatalysis is a radiation-induced redox reaction on a semiconductor surface (photocatalyst) in which the catalyst is activated by solar energy or by ultraviolet radiation to accelerate a chemical reaction. The process uses light energy, water, and oxygen from the air to generate reactive hydroxyl radicals (–OH) with a high oxidation potential of 2.80 V for rapid oxidation–reduction of contaminants. Pure semiconductors are usually used as catalysts, but the fast recombination of generated electron–hole pairs often results in a low photoquantum yield.
Most pure semiconductors respond only to UV light due to the wide band gap, thereby affecting their practical application in photocatalysis. Efforts to suppress the electron–hole pair recombination and extend the semiconductor’s response to the visible-light region are being sought. Nanomaterial-based photocatalysts provide a large surface area to volume ratio to enhance adsorption of the pollutants and surface reactivity. Photocatalysts are an integral part of the advanced oxidation processes (AOPs) which have been extensively explored to remove the non-biodegradable and highly stable compounds in air and water (Oturan and Aaron 2014). The wide-scale use of photocatalysts is challenging, and it lacks large-scale practical application in environmental decontamination, and the process is also limited by low efficiency.
Photocatalyst fabrication
The challenge to fabricate high-quality, prepared nanosemiconductor materials relying solely on the individual semiconductor while eliminating the problem of fast electron–hole recombination, agglomeration, and lack of visible-light absorption is significant. nTiO2 and nZnO are benchmark photocatalysts for a broad class of organic compounds and microorganisms degradation in the UV range. nTiO2 has an advantage over other semiconductors as a photocatalyst because it is chemically stable, possesses large surface area, non-toxic, and is low cost (Baudys et al. 2015; Jianlong et al. 2019). But the wide energy band gap (3.0–3.2 eV) implies that it can only be excited by the UV light, whereby less than 5% of the irradiated solar energy is effectively utilised (Tong et al. 2012). nTiO2 and nZnO suffer from fast recombination speed of electron–hole pairs which limits photocatalytic activity, thereby prompting the need for alternatives. TiO2 could be modified through anion doping and heterostructuring, to make it usable in the visible-light region. The high cost of the traditional methods of nanoparticles production resulted in the search for cheaper pathways of synthesis by involving microorganisms and plant extract (Sharma et al. 2018a, b, c).
Requirements for efficient photocatalysis are met by synthesising novel materials through the incorporation of semiconductors with various functional components to form a composite catalyst of high efficiency. This will have the dual advantage of efficient contaminant degradation and power generation (microbial fuel cell technology) from organic/inorganic compounds using microorganisms as biocatalysts (Ghasemi et al. 2013). Different photocatalysts production methods exist which includes modifying with carbon nanostructures and hydrogenated metal oxides, integrating with equivalent band gap materials, noble metal deposition, and dye sensitisation, but most researchers choose the coupling of semiconductor metal oxides of related band gaps as the preferred method. Advances in photocatalysis are realised through the discriminatory control of the nanomaterials morphology, doping with heteroatoms, heterojunction construction, and porous material support. These approaches are summarised through:
Heteroatom doping (metal cation and non-metal anion)
Free radicals are keys to organic degradation giving the doped photocatalyst high stability. Heteroatoms are introduced into the lattice of corresponding semiconductors as a dopant in which the doped metal cations act as an electron–hole trap (Kudo et al. 2007). Transition metal ions, such as Fe3+, Co+3, Mo+5, Ru+3, Ag+, are used to provide a measure of new energy levels as electron donors or acceptors. The doping of semiconductors with suitable non-metallic anions will also improve their inherent electronic structure (Liu et al. 2010). Oxidation technologies based on non-radical activation mechanism such as doping a carbon nanotube (CNT) with nitrogen are fundamental to designing metal-free catalyst with high performance and stability (Duan et al. 2015). Asahi et al. (2001) discovered that the photocatalytic activity of TiO2 and its optical absorption in visible-light irradiation could be improved through the substitution doping of non-metal elements like nitrogen and carbon. Kumar et al. (2018) exploited the characteristic large surface area and the reduced charge recombination rate of some photocatalyst materials to develop a metal-free coal-char/polymeric-g-C3N4/RGO nanohybrids particle to degrade ciprofloxacin (CIF) and β-estradiol (ESD) while converting CO2 into CH4, CO, and O2. By ensuring sufficient contact between POPD-CdS heterojunction and the imprinted layer, Peng et al. (2019) utilised the enhanced selectivity of a magnetic catalyst modified with POPD-CdS heterojunction embedded, imprinted layer to effectively and selectively degrade ciprofloxacin while suppressing the secondary pollution resulting from CdS photocorrosion. To effectively remove oil and grease from wastewater, Shivaraju et al. (2016) fabricated a coated N-doped TiO2 photocatalytic polyscales under sunlight as alternative driving energy using the sol–gel technique. Nitrogen heteroatoms in carbon nanotube are significant in phenol oxidation with PMS by enhancing the pathways beneficial to phenol degradation. Mengjuan et al. utilised peroxymonosulphate activated with expanded graphite-loaded CoFe2O4 particles to degrade sulphamethoxazole in soil (Mengjuan et al. 2019).
Heterojunction construction
The spatial separation of electron–hole pairs to improve photocatalysis performance of semiconductors is assured by constructing heterojunctions to form band alignment (Jianlong et al. 2019). Heterojunction photocatalysts are fabricated by creating two phases in the same semiconductor. The p-n heterojunction is a common type of heterostructure in which electrons and holes migrate in different directions, effectively separating the electrons and holes, thereby suppressing recombination (Li et al. 2016). Heterojunction construction will increase reactivity, but the fear of toxicity has limited its use of bimetallic and trimetallic nanophotocatalyst in Europe (Mueller and Nowack 2010). Sharma et al. (2018a, b, c) synthesised La/Cu/Zr trimetallic nanoparticles (TNPs) and applied it to remove ampicillin antibiotic from aqueous media effectively.
Morphology modification of photocatalysts.
The arrangement of atoms on the surface of a catalyst directly controls the catalytic reactivity. The photocatalytic degradation effectiveness of a pollutant is affected by many factors with the photocatalysts’ activity playing a pivotal role. The focus of every catalytic remediation study is how to improve the activity of the photocatalysts which could be enhanced through morphology control, including the reduction of thickness and building increasing the efficiency of charge transfer (Li et al. 2016). To fully exploit the visible-light-responsible semiconductors such as Ag2O, BiVO4, Cu2, g-C3N4, which can be excited by visible light and are more amenable to solar utilisation, morphology control needs to be studied. According to Sharma et al. (2018a, b, c), various strategies, including the large surface area of material, low thickness, and the hierarchical and hollow structure, are necessary to increase the light absorption and accessibility of photocatalysts. Sharma et al. (2018a, b, c) cross-linked guar gum with soya lecithin to form nanohydrogel sheets which was employed to remove thiophanate methyl from the aqueous solution of concentration 25 ppm, while arsenite oxidase–chitosan nanoparticle conjugates could be applied to improve the biotransformation of arsenic (Awual et al. 2012).
Bioremediation
Although the search for efficient methods to decontaminate the ecosystem polluted by the emerging contaminants like PPCP, polychlorinated naphthalenes (PCNs), antibiotics, triclosan, most studies on bioremediation are focused on hydrocarbons because of the frequency of crude oil pollution of the soil, surface, and groundwater (Firmino et al. 2015). Several investigations on the technologies desirable for the remediation of contaminated ecosystems, are ongoing, and significant conclusions have been drawn from such studies with attendant technologies including bioremediation (Smith et al. 2015; Jianlong et al. 2019), phytoremediation (Moubasher et al. 2015; Yavari et al. 2015; Wafa et al. 2019), methods involving chemical decomposition, chemical oxidation, and soil washing (Gang et al. 2016). Other technologies have also been put to use which included the high-temperature incineration model as thermal remediation (Li et al. 2009) and electrokinetics (Mena et al. 2015; Yongsong et al. 2018). Also, solvent, supercritical fluid, and ultrasonic extraction (Li et al. 2012; Saranya et al. 2016), land farming (Silva-Castro et al. 2015) are all crude oil impacted soils remediation methods that are increasingly being put to use. Nonetheless, despite the efforts, most of these methods of remediation are technologically sophisticated and extraordinarily costly and lack public acceptance (Smith et al. 2015; Abo-State et al. 2018). Bioremediation is a cost-effective and promising biotechnology approach, increasingly being studied and implemented, which offers the possibility to destroy or render various contaminants harmless including petroleum hydrocarbon and even some contaminants of emerging concern by natural biological activity. It has an advantage over other methods to detoxification or degradation of environmental pollutants (Firmino et al. 2015). Bioremediation is a controlled process of organic substances degradation, relying on the inherent capacity of the soil microorganisms to degrade the environmental contaminants (Agamuthu et al. 2013). The use of microbes to decontaminate crude oil impacted soils is adjudged to be efficient and effective and an alternative to the traditional methods. Although the cost of bioremediation treatment is enormous, the huge operating cost is compensated by a reduction in clean-up time. Moreover, unlike the conventional techniques that transfer contaminants from one medium to another bioremediation eradicates pollutants by converting them to CO2 and water.
The selection of an appropriate site-specific remediation technology and performance criteria is challenging in the quest for environmental clean-up. The issue of approach is addressed by looking at the physical, biological, and chemical processes encountered in soil decontamination. A conceptual approach based on information employed at waste sites includes identifying, quantifying, and controlling contaminant sources, the nature of pollutants, type of environment, and also considering the clean-up required for the soil medium to protect human health and environment (Smith et al. 2015).
Crude oil as a soil contaminant
Crude oil and products are recognised as a significant contributor to health and environmental hazard, especially in areas of intense human activities. The frequency and the extent of soil contamination by crude oil and petroleum products is a pervasive problem that is universally felt, and the consequences are extremely high and a dangerous threat to human, animals, and plant health (Oluyoye et al. 2019). Crude oil contamination in the soil can affect the soil physical and chemical properties such as the maximum surface temperature (Azubuike et al. 2016). The entrance of crude oil in the soil makes the environment, anaerobic by blocking the diffusion of air, which affects the soil microbial communities (Sutton et al. 2013). The aromatic hydrocarbons (BTEX) are compounds with one or more fused aromatic rings found in crude oil which entrance into the ecosystem gives much concern as they are carcinogenic or may be converted into carcinogens by microbial actions when crude oil is spilled. Crude oil in mangrove soil causes complete mortality of the mangrove vegetation (Lin and Mendelssohn 2012) and inhibits seed germination by creating a nutrient deficiency, which may lead to stunted plant growth or death on contact.
When crude oil is spilled on land, it prevents water absorption by making the soil to become hydrophobic repelling water (Brown et al. 2017), and when dropped on the grass and agricultural lands, it tends to choke off plant life. Spilled crude oil could be held in voids in the soil while forming a large bank of residual saturation, which might result to high contamination of groundwater if not removed (Hohener and Ponsin 2014; Dzionek et al. 2016). Crude oil in the soil can also increase the soil total organic carbon and change soil pH values. The level of soil contamination and the remediation measures taken determines how long the impacted soils remain unsuitable for crop growth. The sustainability of the soil is vital because we mostly rely on it for our sustenance. It is, therefore, essential that the soil quality and fertility are monitored and maintained. Crude oil-contaminated sites represent a dark side of many communities and a significant environmental challenge in most of the oil-producing area of Nigeria. Local soil contamination and groundwater pollution are majorly associated with the operations and activities of the oil companies.
The fate of crude oil in the soil
Several studies have examined the outcome of hydrocarbon in soil and other ecosystems and recognised crude oil as a substrate that supports microbial growth, being an object and a product of microbial activities (Brown et al. 2017). When crude oil enters the environment, it is subjected to several degradation changes that contribute to the loss or alteration (M’rassi et al. 2015; Whelan et al. 2015). According to Dzionek et al. (2016), microorganisms, which are widely distributed in the ecosystem, utilise the hydrocarbons as a source of energy and the retention of the petroleum hydrocarbon pollutant in the soil is governed by the structural complexity of soils and the environmental conditions. If the conditions for biodegradation are ideal, the hydrocarbon could be entirely mineralised to innocuous products in which some portions of the crude oil mass will volatilise, and some parts will solubilise as components of soil vapour and groundwater. The volatilisation and solubilisation tend to make the remaining mass of crude oil denser and less mobile. The partially degraded hydrocarbon is incorporated into the soil as part of the organic matter forming asphalt crust that is more challenging to biodegrade (Murygina et al. 2016.; Brown et al. 2017). The weathering processes alter the properties of the contaminants in such a way that it affects the methods of decontaminating the polluted environment. Bringing the contaminants and the soil microbes in close contact is imperative to enhance the bioavailability of the substrates to the degrading microbial communities for maximum remediation of the ecosystem (Shaker and Almukhtar 2016). Other important crude oil properties that affect degradability include the API gravity, viscosity, and soil conditions including temperature, soil pH, moisture content, soil texture, sorption, bioavailability, contaminant concentration, and the abundance of microbial toxins (Atlas 1981).
A variety of physicochemical influences such as (1) chemical processes, e.g. hydrolysis, oxidation, and reduction, (2) physical or transport processes and features, e.g. advection, evaporation, leaching, dispersion and diffusion, volatilisation, (3) biological processes, e.g. biodegradation, and toxicity, and (4) combined environmental factors are the essentials upon which the behaviour of crude oil pollutant in the environment depends. Other factors such as the chemical composition of the crude, the quantity released, the physical state, volatility, pH, and biochemical oxygen demand (BOD) affect the rate and degree of biodegradation.
Bioremediation as a clean-up technology
There are several approaches to clean up the contaminated environment, but the biological treatment is the most robust, accessible, and cost-effective strategy (Chen et al. 2015; Suvi et al. 2016). Bioremediation built on the science of biodegradation is environmentally acceptable and effective remediation method that exploits the abilities of bacteria to completely remove pollutants from such environment or degrade them into less harmful forms through mineralisation (Agamuthu et al. 2013). Bioremediation provides a complete transformation or removal of the organic compound even at low concentration, and it is adjudged the best clean-up method for environment contaminated by crude oil. According to Wolejko et al. (2016) the microbiological decontamination of oil-contaminated environment is an efficient, economical alternative to the physicochemical treatment.
The use of enhanced bioremediation technology is necessary to remove a specific contaminant that is readily degraded by bacteria or the addition of nutrients to facilitate the degree and rate of decomposition (Kalliola et al. 2016). Enhanced bioremediation, a process in which indigenous or inoculated microorganism degrades organic compounds, encompasses a range of technologies that differs concerning their inputs (Ivshina et al. 2015). Although the science of bioremediation is not complicated, it requires a considerable measure of experience and expertise to design and implement a remediation programme. Therefore, advances in science and engineering are critical to manipulate, design, and use different input parameters to enhance the rate of biodegradation. The efficiency of the bioremediation treatment is inhibited by the properties of the contaminants, the soil matrix, including the environmental factors (Chen et al. 2015).
Consequently, the assessment and selection of a bioremediation strategy will require a detail of the contaminated sites, the soil factors, and the soil matrix which has a considerable influence on the degradation of total petroleum hydrocarbon from it (Song et al. 2006). To achieve optimum biodegradation, the parameters must be optimised to reduce the treatment time of the systems by accelerating the rate achieved using a variety of technologies. Adequate knowledge of what constitutes the influential factors in the bioremediation process is necessary for the decision to assist the biodegradation efficacy.
Bioremediation technologies for pollutants removal from the soil
The need to adopt an effective remediation technique led to the development of several physicals, chemical, thermal, and biological technologies (Rene et al. 2012). The most common technologies though with a limited degree of success as they rarely result in comprehensive clean-up when employed in contaminated soil include mechanical, burying, evaporation, and dispersion (Al-Mansoory et al. 2017). Generally, they are expensive and often result in incomplete decomposition of pollutants as they merely transfer the contaminants from one ecosystem to another (Ivshina et al. 2015). Moreover, because of the limitations of the physiochemical methods, it became essential that other technologies are developed to overcome their inadequacies. A considerable amount of the literature has reported that bioremediation technologies are alternative to these methods. Bioremediation is accessible and cost-effective biotechnology strategy to degrade crude oil and other pollutants in the soil to harmless substances with no attendant negative environmental effect (Varjani et al. 2015; Pal et al. 2017; Farag et al. 2018).
Bioremediation techniques are inherently destructive technique, easily implemented at low cost, adequately inexpensive (Pal et al. 2017) and directed towards stimulating the growth of microorganisms that is using the crude oil as food and energy source by creating favourable environments for the organisms to thrive (Abo-State et al. 2018). Biodegradation of crude oil by the natural population of microorganisms in soil is an effective primary mechanism through which the petroleum hydrocarbon is eliminated from the soil. Numerous researchers, including (Bian et al. 2015), have extensively reviewed the necessities for optimum bacterial growth and the degradation trajectories for petroleum hydrocarbons and other pollutants in the soil. Additionally, the influence of soil parameters and crude oil physical interactions such as mass transport, sorption, and desorption (Abdel-Shafy and Mansour 2016) on the remediation rate is also well documented in the literature too. Various remediation techniques to clean up the environment are summarised and presented in Tables 1 and 2.
Bioremediation strategies
The objective of bioremediation treatment is to degrade contaminants to an innocuous species. Bioremediation utilises the natural role of microorganism in the contaminated media to transform or mineralise inorganic pollutants to a level where they will no longer put human, animal, or plant to harm. Over the years, several treatment strategies have been proposed and developed for petroleum hydrocarbon-contaminated sites, and several new and promising approaches are under development. The most frequently used technique in bioremediation involves the enhancement of the activities of microorganism through stimulation with nutrients (biostimulation) (Simpanen et al. 2016), controlling the environmental parameters or through the addition of external organisms (bioaugmentation) (Suja et al. 2014; Dzionek et al. 2016). These treatment methods are classified into two basic categories: in situ and ex situ treatment based on where the contaminated materials are treated. Ex situ technologies refer to procedures that remove contaminants to a separate treatment facility, while in situ bioremediation technologies are used for treatments of pollutants in the place of contamination, and it is considered to minimise material handling and reduction in costs (Frascari et al. 2015). The biostimulation technique is categorised as biological, chemical, or physical, covered for contaminated soils, surface, and groundwater.
In situ bioremediation
In situ or on-site strategy is defined as those techniques in which the contaminants are treated at the site of pollution where the soil is unearthed with minimal disturbance. Application of the in situ method is dependent on the availability of oxygen, the nature of the soil, and the depth of penetration of the contaminants into the soil (Angelucci and Tomei 2016). If contaminants are recalcitrant, bioaugmentation with adapted or specially designed microbial inoculants is a useful alternative (Tomei and Daugulis 2013; Dzionek et al. 2016). Adopting the in situ technology option is good as excavation, and transportation of contaminated materials is avoided, but achieving uniform remediation is challenging because of soil heterogeneity (Simarro et al. 2013; Vogt and Richnow 2014). Technologies of note in in situ bioremediation are summarised in Table 1. Emerging methods in use for the clean-up of crude oil-contaminated soils include microbial fuel cells, nanoremediation, genetic engineering, and photoheteromicrobial system.
Ex situ bioremediation
In the ex situ technology approach, impacted media are physically excavated or pumped from the contaminated site to another location for treatment and subsequently brought back to the site after treatment in record time. If the groundwater is contaminated, it is also removed along with the soil for treatment. Advantages of the ex situ methods include the uniformity of treatment, easy monitoring, and the possibility of screening and homogenisation of the contaminants (Tomei and Daugulis 2013; Dzionek et al. 2016). However, the cost of excavation and transport is high. Existing ex situ remedial options for contaminated soil including dig-and-dump (landfills and engineered landfills), slurry bioreactors, incineration, oxidation, adsorption, ion exchange, soil washing, and pyrolysis, are as summarised in Table 2. In most cases, the physicochemical and biological technologies are integrated for better clean-up of polluted sites.
The bioremediation process of crude oil-polluted soils
Whereas biodegradation refers to a controlled process in which organic substances are degraded through the actions of soil microorganisms to other less hazardous materials, conversely, bioremediation is much about the restoration of contaminated environment through the efforts of bacteria or fungi to degrade, remove, immobilise, or alter contaminants as seen from the standpoint of biodegradation (Mosa et al. 2016). Biodegradation is a natural weathering process in which microorganisms degrade organic molecules to less harmful products such as fatty acids, water, and CO2 (Mosa et al. 2016). Bioremediation is the hastening of the process of biodegradation either through the addition of external microbial populations that is not indigenous to the soil (Dzionek et al. 2016). Alternatively, through the biostimulation of indigenous bacteria communities by the addition of nutrients and by manipulating the environmental media such as soil pH, temperature, soil moisture content, and aeration, the bioremediation process could be enhanced (Adams et al. 2017; Acharya et al. 2019). Bioremediation technologies abound, including such techniques as biostimulation, bioaugmentation, bioventing, bioreactor, phytoremediation, and composting. Bioremediation is not always a win all solution in every remediation events; the use is limited by the types of contaminants, and it is not yet a matured technology. Although bioremediation has excellent potential in dealing with crude oil contaminant, the length of time needed to eradicate the pollutants from a contaminated environment is long, and the level of contaminants removal achievable may not always meet the standard desired by the regulating authorities (Sharma 2012; Yan et al. 2016).
Factors affecting the bioremediation of crude oil-contaminated soil
In the effort to harness the capacity of soil bacteria to rid the environment of contaminants, a careful selection of soil organisms and the sustenance of the optimal soil conditions necessary and favourable to their growth, are imperative (Dzionek et al. 2016). The optimal rate biodegradation could be achieved and sustained by ensuring that all the factors that favour rapid contaminant degradation are optimal (Atlas 1981). Studies by various remediation practitioners show that the high molecular weight, aromatic, and branched hydrocarbons are not easily degraded unlike the lighter, straight-chained, and saturated hydrocarbons. Similarly, several authors have demonstrated that application of the traditional methods such as tilling, sprinkling with water, and addition of organic nutrients such as cow dung, straw, pig manure, and inorganic fertiliser could effectively decontaminate the polluted environment (Roy et al. 2015). In spite of this, many environmental restrictive factors have been acknowledged to affect the rate of crude oil degradation in soil, and the most important of these factors is optimised to guarantee a safer environment. The optimised factors include microorganism types, nutrients availability, soil pH, temperature, moisture content of the soil, oxygen availability, other soil properties, and the contaminant concentration (Firmino et al. 2015). The environmental factors interactively affect the rate of biodegradation, and usually, the rate responds to the most limiting factor (Smith et al. 2015).
Several contributors to this topic have acknowledged that the addition nutrients accelerates the rate of removal of crude oil from the contaminated environment. It could, therefore, be stated that the major requirements for an effective biodegradation process are an energy source (nutrients) and a carbon source (crude oil). In summary, (a) the natural ability of the microorganisms at the site, (b) characteristics of the crude oil, (c) availability of nutrient, and (d) the soil factors, are significant in limiting the degradation of crude oil.
Effect of soil texture on crude oil remediation
The soil is a habitat to crude oil-degrading microorganisms and is classified into four distinct classes such as sand, clay, silt, and coarse materials and also contains moisture and air. The matrix of the soil influences the removal of petroleum hydrocarbon from it. Some characteristics of the soil that determines how favourable a microbial degradation process will proceed include texture, permeability, pH, nutrients, water-holding capacity, and availability of oxygen. The size and the number of the soil’s pore spaces are essential and are dependent on the soil type and degree of compaction. Clay soils generally have a higher degree of pore spaces than sandy soil but may not allow the passage of nutrients efficiently because of the physical size of the pores. Typically, a large amount of oil is held in voids in the soil, forming a large bank of residual saturation. The concentration of the spilt oil in soil voids may result in continual contamination of groundwater if not removed on time. The soil type, the sorptive surfaces, available soil organic matter, and intrinsic bioavailability of the crude oil fraction, are the most critical consideration in the appraisal of the suitability of a bioremediation method (Garcıa-Delgado et al. 2015).
Biostimulation
Biostimulation involves the manipulation of abiotic factors to optimise the conditions that are essential for indigenous microbes to remove contaminants or the stimulation of the degrading abilities of microorganisms by the introduction of rate-enhancing nutrients. The biostimulation option is only adopted when there are indigenous microorganisms with degradation ability, but the rate is slow and needed to be beefed up. Studies show that the addition of nutrients to the polluted media stimulates biodegradation by increasing microbial biomass which dramatically enhances the rate of crude oil removal. However, the optimal nutrient concentrations and types necessary for active degradation of contaminants vary significantly concerning the soil condition (Zhu et al. 2001). It is noted that excessive concentration of nutrient in the ecosystem might induce toxic response as well (Zhu et al. 2001).
Amendments necessary for effective bioremediation application
The effectiveness of bioremediation technology is profoundly affected by the soil environmental characteristics highly related to the type of soil. The soil is the medium in which bioremediation will occur places emphasis on its parameters as a necessity that must be evaluated. Several soil factors have significant effects on the degradation of crude oil. The biodegradation of soil’s pollutant could be enhanced by making these environmental factors optimum for effective clean-up.
Soil pH
The pH of the soil is highly variable and ranges from 2.5 to as high as 11 and significantly affects the biodegradability of hydrocarbons. The suitability of a pH range in any bioremediation work is site-specific, and this is influenced by the complicated relationship between the organism, the contaminants, and the properties of the soil. The pH is highly variable over a wide range, and it affects solubility in soil, and subsequently, the availability of various constituents of the soil. The optimal bacterial activity in soil is improved when the pH is close to 8. At this range, the fungi are more tolerant of the soil’s condition than bacteria (Atlas 1981). Numerous remediation studies have shown that the optimum pH for accelerated contaminant degradation is generally in the range of pH 6.5–8.5, and a pH of 7.8 was nearly optimum in most soils. pH affects permeability, influences the dissolution of soil metals, the growth of microorganisms, and determines nutrients accessibility (Ben 2003). The soil pH is continually monitored for deviations from optimum and subsequently adjusted by adding chemical reagents. If the soil is acidic, lime is added to raise the pH, while a high pH is brought down by adding aluminium sulphate or sulphur.
Soil moisture content
The soil’s moisture content is essential in bioremediation treatment. The estimation of moisture content and the maintenance at the optimal level is essential in soil remediation studies. The volume of water held by the soil is assessed during soil characterisation, and it influences the bioremediation technology by controlling the movement of air (Ben 2003). Water is the medium through which nutrients and other organic constituents needed for degradation pass into the microbial cell, and waste products move out of the cell.
The microorganisms involved in the remediation process are more active at a soil moisture content within the optimum range (Song et al. 2006). If the contaminated soil is saturated with water, the passage of oxygen will be inhibited; conversely, in dry conditions, microbial activity will slow down or halt the biodegradation process. The desired range of moisture will permit the passage of oxygen for microbial respiration between 70 and 80% of the soil’s water-holding capacity. According to Ben Banipaul, a soil is said to be at field capacity when soil micropores are filled with water to facilitate the diffusion of soluble substrate and macropores are filled with air which makes O2 diffusion into the soil easier (Ben 2003) and the water-holding capacity is dependent on the type of soil. Table 3 culled from the work of Ben (2003) provides a general soil moisture characteristic necessary for biodegradation in two common soils. If the moisture content of the field is maintained at optimum levels, studies by Ben Banipaul show that clay soil decontamination rate is higher than sandy soil.
Oxygen supply
An adequate supply of oxygen is an essential and necessary condition for biodegradation to occur. Microorganism employs 3–4 parts of dissolved oxygen to oxidise one portion of hydrocarbon to water and carbon dioxide. However, degradation can still occur when oxygen is deficient in the aerobic treatment of hydrocarbon-contaminated soils. The supply of oxygen is limited as the soil gets saturated with water above the optimum, and the oxygen is expended faster than it can be replaced resulting in an anaerobic condition. The soil needs to be tilled to enhance the circulation of air to accelerate contaminant removal from soil.
Monitoring crude oil biodegradation
The massive oil spill in the ecosystem has prompted increased research on techniques available to monitor crude oil degradation an effort being limited by methodology. The traditional method of monitoring biodegradation relies on sampling and analysis, but this method is replete with errors. Consequently, attention is gradually shifting to the geophysical method due to their non-invasive nature, spectral, and cost-effectiveness (Heenan et al. 2013). One such approach is the spectral induced polarisation method (SIP). In the soil and marine environment, the SIP method is sensitive to biogeochemical changes occurring because of microbial oil degradation. The rate of crude oil pollutant degradation in soil may be indirectly measured by the respirometric techniques (Song et al. 2006). Most researchers engaged in bench-scale laboratory studies used carbon dioxide evolution to evaluate the rate and extent of biodegradation. In the application of this technique, a respirometer equipped with sensors and biometer flasks is employed to enumerate the microbial respiration rates. Respirometry is a proven technique in the determination of biodegradation rate in water, but not proved for the soil (Ben 2003). Another method that serves as a measure to evaluate the degree of remediation achieved is by following the concentrations of particular oil constituents in the spilt environment.
The components of crude oil degrade at different rates, with the lighter hydrocarbon quickly deteriorated even by abiotic processes leaving the heavier constituents such as the cycloalkane which are resistant to attack by microorganism to persist in the soil. The overall degradation rate of oil is not feasible, but we can use the method of TPH a term used to describe a mixture of a chemical originating from crude oil for evaluation resulting in a sum parameter that does not give the concentration of any specific compound. The TPH technique includes the gravimetric gas chromatography/flame ionisation detection (GC/FID) and infrared (IR) spectroscopic methods. Other methods include gas chromatography/mass spectrometry (GC/MS) and thin-layer flame ionisation (TLC/FID) methods (Zhu et al. 2001).
Kinetics of crude oil biodegradation
Granting that the decontamination of the soils through bioremediation has been subjected to numerous studies, but yet not much information is on the public domain with respect to the kinetics of the bioremediation process. Handy information on the kinetics of crude oil biodegradation under different environmental conditions is still imperative for evaluating the potential fate of targeted pollutants while assessing the efficacy of bioremediation methods in use (Zhu et al. 2001). For contaminated soil of unknown biodegradability, applying laboratory investigation to study the degradation kinetics of that soil contaminant is necessary to evaluate the duration of treatment. The rate and the extent to which microorganisms will remove organic compounds from the soil could be expressed mathematically to estimate the time for remediation. So in the quest to find out the rate of degradation of contaminants in the environment, it is necessary that the variables required for the rate of degradation determination are included in the rate equation. The soil factors, nutrients concentration, soil moisture content, soil pH, and temperature are the likely key parameters that should be introduced into the model to predict the degradation rates of crude oil in the environment. Numerous kinetic rate studies on crude oil degradation have been conducted under laboratory conditions, but few of such studies have been done under field conditions. Song et al. developed a base model for crude oil degradation in soil built on carbon dioxide accumulation. Investigators on the Exxon Valdez programme established a rate of crude oil remediation model from multiple regression analysis for remediation field studies (Zhu et al. 2001). Venosa et al. (1996) also compared oil biodegradation rate obtained from bench-scale laboratory studies with that of the field studies and concluded that the rate of the targeted component degradation in the field was lower than that of the laboratory.
Conclusion
As the world steers towards “a non-toxic environment”, there is an intensification of action in the air, soil, surface, and groundwater remediation activities in response to the environmental quality objectives. The world’s pollution concerns are addressed through various treatment technologies such as adsorption, photocatalysis adsorption used with photocatalysis. Others including bioremediation, burning, pump and treat, and efforts are to improve the efficacy of these methods.
A significant evolution in bioremediation practices, based on integrated scientific principles, has also been witnessed over the years. Although the bioremediation technology is economical and straightforward, easily deployed, efforts to extrapolate information on degradation rates from laboratory and bench-scale studies to field-scale practices are hindered by tremendous diversity in measurement techniques and the heterogeneity of the soil. Moreover, again, bioremediation may be cost-effective, but one major disadvantage of bioremediation is that it is limited to only those groups of contaminants that are biodegradable. The use of engineering modelling techniques will also help the remediation public to comprehend the dynamics structure of microbes and help to transform bioremediation from a little practice into a science.
Future perspectives
Except for a few limiting factors, bioremediation technology can invigorate a contaminated environment effectively. However, a combination of the right microorganism and control of the proper field parameter will be a step towards achieving a higher and acceptable rate of biodegradation. However an element of unpredictability still exists in the attempts to achieve desired success in bioremediation. A concerted effort by researchers and innovators in this direction and a combination of technologies will give the bioremediation industry a quantum leap. Important features such as morphological architecture, the choice of semiconductor materials, and surface properties of photocatalysts should be considered in the design and selection of photocatalyst material.
References
Abdel-Moghny T, Ramadan S, El-Sayed E, Shoukry M, Moustafa (2012) Effect of moisture on the remediation of hydrocarbon contaminated soil at El-Mina district, Upper Egypt. Chem Eng 13:1–13. https://doi.org/10.5402/2012/406598
Abdel-Shafy H, Mansour M (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, the effect on human health and remediation. Egypt J Pet 25:107–123
Abebe B, Murthy H, Amare E (2018) Summary on adsorption and photocatalysis for pollutant remediation: mini-review. J Encapsul Adsorpt Sci 8:225–255
Abo-State M, Riad B, Bakr A, Abdel-Aziz M (2018) Biodegradation of naphthalene by Bordetella avium isolated from petroleum refinery wastewater in Egypt and its pathway. J Radiat Res Appl Sci 11:1–9
Acharya K et al (2019) A quantitative structure-biodegradation relationship (QSBR) approach to predict biodegradation rates of aromatic chemicals. Water Res 157:181–190
Adams F, Niyomugabo A, Sylvester O (2017) Bioremediation of crude oil contaminated soil using agricultural wastes. Procedia Manuf 7:459–464
Agamuthu P, Tan YS, Fauziah BH (2013) Bioremediation of hydrocarbon contaminated sites using organic waste. Procedia Environ Sci 18:694–702
Ali I (2014) Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev 43:175–205
Al-Mansoory A, Idris M, Abdullah S, Anuar N (2017) Phytoremediation of contaminated soil containing gasoline using Ludwigia Octovalvis (Jacq.) in greenhouse pots. Environ Sci Pollut Res 24(13):11998–12008
Ang WL, Mohammad AW, Hilal N, Leo CP (2015) A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 363:2–18
Angelucci D, Tomei M (2016) Ex-situ bioremediation of chlorophenol contaminated soil: comparison of slurry and solid-phase bioreactors with the two-step polymer extraction–bioregeneration process. J Chem Technol Biotechnol 91:1577–1584
Asahi R, Morikawa T, Ohwaki T, Aoki KT (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Atlas RM (1981) Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev 45:180–209
Awual M et al (2012) Efficient arsenic (V) removal from water by ligand exchange fibrous adsorbent. Water Res 46:5541–5550
Azubuike C, Chikere C, Okpokwasili G (2016) Bioremediation techniques—classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol 32:180
Baudys M, Krýsa J, Zlámal M, Mills A (2015) Weathering tests of photocatalytic facade paints containing ZnO and TiO2. Chem Eng J 261:83–87
Ben B (2003) Aerobic biodegradation of oily wastes: a field guidance book for federal on-scene coordinators. US Environmental Protection Agency (EPA), Tulsa
Brown D et al (2017) Heavy hydrocarbon fate and transport in the environment. Q J Eng Geol Hydrogeol 50:333–346
Chen M et al (2015) Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv 33:745–755
Duan X et al (2015) N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal 5:553–559
Dzionek A, Wojcieszyńska D, Guzik U (2016) Natural carriers in bioremediation: a review. Electron J Biotechnol 23:28–36
Ebele A, Abou-Elwafa A, Harad S (2017) Pharmaceutical and personal care products (PPCPs) in freshwater aquatic environment. Emerg Contam 3:1–16
Farag S, Soliman N, Abdel-Fattah R (2018) Statistical optimization of crude oil biodegradation by a local marine bacterium isolate Pseudomonas sp. sp48. J Genetic Eng Biotechnol 16:409–420
Firmino P et al (2015) Understanding the anaerobic BTEX removal in continuous-flow bioreactors for ex situ bioremediation purposes. Chem Eng J 281:272–280
Frascari D, Zanaroli G, Danko (2015) In situ aerobic cometabolism of chlorinated solvents: a review. J Hazard Mater 283:382–399
Gang L, Shuhai G, Jinxuan H (2016) The influence of clay minerals and surfactants on hydrocarbon removal during the washing of petroleum-contaminated soil. Chem Eng J 286:191–197
Garcıa-Delgado C, Alfaro-Barta I, Eymar E (2015) Combination of biochar amendment and mycoremediation for polycyclic aromatic hydrocarbons immobilisation and biodegradation in creosote-contaminated soil. J Hazard Mater 285:259–266
Ghasemi M et al (2013) Nano-structured carbon as electrode material in microbial fuel cells: a comprehensive review. J Alloys Compd 580:245–255
Guo J, Sinclair C, Selby K, Boxall A (2016) Toxicological and ecotoxicological risk-based prioritisation of pharmaceuticals in the natural environment. Environ Toxicol Chem 35:1550–1559
Hassan I, Mohamedelhassan E, Yanful E, Yuan Z-C (2016) A review article: Electrokinetic bioremediation current knowledge and new prospects. Adv Microbiol 6:57–72
Heenan J et al (2013) Sensitivity of the spectral induced polarisation method to microbial enhanced oil recovery processes. Geophysics 78(5):E261–E269
Hohener P, Ponsin V (2014) In situ vadose zone bioremediation. Curr Opin Biotechnol 27:1–7
Ivshina I et al (2015) Oil spill problems and sustainable response strategies through new technologies. Environ Sci Process Impacts 17(7):11201–11219
Jianfel Y et al (2019) Efficient degradation of sulfamethoxazole by the CuO@Al2O3(EPC) coupled PMS system: optimization, degradation pathways and toxicity evaluation. Chem Eng 359:1097–1110
Jianlong G, Yifan Z, Young-Jung H, Soo-Jin P (2019) Advanced design and synthesis of composite photocatalysts for the remediation of wastewater: a review. Catalysts 9:122
Kalliola S et al (2016) The stability of green nanoparticles in increased pH and salinity for applications in oil spill-treatment. Colloids Surf A Physicochem Eng Asp 493:99–107
Khudur L et al (2015) Evaluating the efficacy of bioremediating a diesel-contaminated soil using ecotoxicological and bacterial community indices. Environ Sci Pollut Res 22:14809–14819
Kudo A, Niishiro R, Iwase A, Kato H (2007) Effects of doping of metal cations on morphology, activity, and visible light response of photocatalysts. Chem Phys 339:104–110
Kumar A et al (2018) Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano junction for visible light degradation of sulphamethoxazole from aqueous environment. Chem Eng J 334:462–478
Lászlová K, Dercova K, Horvathova H (2016) Bioremediation of PCB contaminated river sediments: role of autochthonous bacteria and efficacy of bioaugumentation on contaminants biodegradation. In: International conference on contaminated soil. Slovak Environmental Agency, Bratislava, pp 150–152
Lesley J, Yeomin Y (2019) Removal of contaminants of emerging concern by metallic organic framework nano adsorbent: a review. Chem Eng J 369:928–946
Li D, Zhang Y, Quan X, Zhao Y (2009) Microwave thermal remediation of crude oil contaminated soil enhanced by carbon fiber. J Environ Sci 21:1290–1295
Li X, Du Y, Wu G, Li Z, Li H, Sui H (2012) Solvent extraction for heavy crude oil removal from contaminated soils. Chemosphere 88(2):245–249
Li X, Yu J, Jaroniec M (2016) Hierarchical photocatalysts. Chem Soc Rev 45:2603–2636
Lin Q, Mendelssohn I (2012) Impacts and recovery of the deepwater horizon oil spill on vegetative structure and function of coastal salt marsh in the northern Gulf of Mexico. Environ Sci Technol 46(7):3737–3743
Liu G et al (2010) Titania-based photocatalysts-crystal growth, doping and heterostructuring. J Mater Chem 20:831–843
M’rassi A, Bensalah F, Gury J, Duran R (2015) Isolation and characterisation of different bacterial strains for bioremediation of n-alkanes and polycyclic aromatic hydrocarbons. Environ Sci Pollut Res Int 22:15332–15346
Mayur B et al (2019) Uptake and biodegradation of emerging contaminant sulfamethoxazole from aqueous phase using Ipomoea aquatica. Chemosphere 225:696–704
Mena E et al (2015) Biological permeable reactive barriers coupled with electrokinetic soil flushing for the treatment of diesel-polluted clay soil. J Hazard Mater 283:131–139
Mengjuan X et al (2019) Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles. Chem Eng J 369:403–413
Mosa K et al (2016) Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci 7:303
Moubasher H et al (2015) Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int Biodeterior Biodegrad 98:113–120
Mueller N, Nowack B (2010) Nanoparticles for remediation: solving big problems with little particles. Elements 6:395–400
Muhammad F, Harmin S (2018) Optimisation of diesel biodegradation by Vibrio alginolyticus using Box–Behnken design. Environ Eng Res 23(4):374–382
Murena F, Gioia F (2009) Solvent extraction of chlorinated compounds from soils and hydro de-chlorination of the extract phase. J Hazard Mater 162:661–667
Murygina V, Gaydamaka S, Gladchenko M, Zubaydullin A (2016) Method of aerobic-anaerobic bioremediation of a raised bog in Western Siberia affected by old oil pollution. A pilot test. Int Biodeterior Biodegrad 114:150–156
Okoh E, Oruabena B, Amgbari C, Nelson E (2019) A proposed multi-barrier option for removing iron and microbial contamination from Yenagoa Borehole Waters. In: Agnihotri A, Reddy K, Bansal A (eds) Environmental geotechnology, vol 31. Lecture Notes in Civil Engineering. Springer, Singapore, pp 273–289
Okoye A, Ejikeme P, Onukwuli O (2010) Lead removal from wastewater using fluted pumpkin seed shell activated carbon: adsorption modelling and kinetics. Int J Environ Sci Technol 7:793–800
Oluyoye I et al (2019) Beyond the obvious: environmental health implications of polar polycyclic aromatic hydrocarbons. Environ Int 123:543–557
Oturan M, Aaron J (2014) Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641
Pal S et al (2017) Genome analysis of crude oil degrading Franconibacter pulveris strain DJ34 revealed its genetic basis for hydrocarbon degradation and survival in oil-contaminated environment. Genomics 109:374–382
Pawar M, Topcu S, Gouma P (2018) A brief overview of TiO2 photocatalyst for organic dye remediation: case study of reaction mechanisms involved in Ce–TiO2 photocatalysts system. J Nanomater 2018:1–13
Peng J et al (2019) Enhanced selectivity for photodegrading ciprofloxacin by a magnetic photocatalyst modified with a POPD-CdS heterojunction embedded, imprinted layer. New J Chem 43(6):2610–2623
Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3–27
Rene E, Mohammad B, Veiga M, Kennes C (2012) Biodegradation of BTEX in a fungal biofilter: influence of operational parameters, the effect of shock-loads and substrate stratify cation. Bioresour Technol 116:204–213
Roy M, Giri A, Dutta S, Water MP (2015) Integrated phytobial remediation for sustainable management of arsenic in soil and water. Environ Int 75:180–198
Sahu JN et al (2019) Current perspectives and future prospects of nano-biotechnology in wastewater treatment. Sep Purif Rev 2014:1–20
Saldana M, Nagpal V, Gulgard S (2005) Remediation of contaminated soils using supercritical fluid extraction: a review. Environ Technol 26(9):1013–1032
Saranya K et al (2016) In-situ remediation approaches for the management of contaminated sites: a comprehensive overview. In: de Voogt P (ed) Reviews of environmental contamination and toxicology, vol 236. Springer, Cham, pp 1–117
Shaker Z, Almukhtar E (2016) Bioaccumulation of petroleum hydrocarbons by Melanopsis nodosa (Gastropoda) in River Tigris within the City of Baghdad. Iraq Int J Curr Microbiol Appl Sci 5(1):244–251
Sharma S (2012) Bioremediation: features, strategies and applications. Asian J Pharm Life Sci 2(2):202–213
Sharma G et al (2018a) Fabrication and characterisation of trimetallic nano-photocatalyst for remediation of ampicillin antibiotic. J Mol Liq 260:342–350
Sharma G et al (2018b) Guar gum-crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int J Biol Macromol 114:295–305
Sharma G et al (2018c) Guar-gum and its composites as a potential material for diverse applications: a review. Carbohydr Polym 199:534–546
Shivaraju H, Muzakkira N, Shahmoradi B (2016) Photocatalytic treatment of oil and grease spills in wastewater using coated N-doped TiO2 polyscales under sunlight as alternative driving energy. Int J Environ Sci Technol 13:2293–2302
Silva-Castro G et al (2015) Response of autochthonous microbiota of diesel polluted soils to land-farming treatments. Environ Res 137:49–58
Simarro R, González N, Bautista L, Molina M (2013) Assessment of the efficiency of in situ bioremediation techniques in a creosote polluted soil: change in bacterial community. J Hazard Mater 262:158–167
Simpanen S et al (2016) Bioremediation of creosote contaminated soil in both laboratory and field-scale: investigating the ability of methyl-b-cyclodextrin to enhance biostimulation. Int Biodeter Biodegr 106:117–126. https://doi.org/10.1016/j.ibiod.2015.10.013
Smith E et al (2015) Remediation trials for hydrocarbon-contaminated soils in arid environments: evaluation of bioslurry and biopiling techniques. Int Biodeterior Biodegrad 101:56–65
Song J, Paul F, Terry B (2006) Bioremediation of petroleum hydrocarbons in heterogeneous soils. University of Wyoming, Laramie
Suave S (2014) A review of what is an emerging contaminant. Chem Cent J 8:15
Suja F et al (2014) Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int Biodeter Biodegr 90:115–122
Sutton N, Maphosa F, Morillo J (2013) Impact of long-term diesel contamination on soil microbial community structure. Appl Environ Microbiol 79(2):619–630
Suvi S et al (2016) Biostimulation proved to be the most efficient method in the comparison of in situ soil remediation treatments after a simulated oil spill accident. Environ Sci Pollut Res 23:25024–25038
Tomei M, Daugulis A (2013) Ex-situ bioremediation of contaminated soils: an overview of conventional innovative technologies. Crit Rev Environ Sci Technol 43:2107–2139
Tong H et al (2012) Nano-photocatalytic materials: possibilities and challenges. Adv Mater 24:229–251
Varjani SJ, Upasani VN (2016) Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresour Technol 222:195–201
Varjani S et al (2015) Synergistic ex situ biodegradation of crude oil by halo tolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeterior Biodegrad 103:116–124
Venosa AD et al (1996) Bioremediation of experimental oil spill on the shoreline of Delaware Bay. Environ Sci Technol 30:1764–1775
Violette G et al (2015) Emerging pollutants in the environment: a challenge for water resource management. Int Soil Water Conserv Res 3:57–65
Vogt C, Richnow H (2014) Bioremediation via in situ microbial degradations of organic pollutants. Adv Biochem Eng Biotechnol 142:123–146
Wafa D, Abdul R, Jun H, Ming Z (2019) Biofuel production using thermochemical conversion of heave metal contaminated biomass harvested from phytoextraction process. Chem Eng J 358:759–785
Wang Y et al (2019) Environmental remediation applications of carbon nanotubes and graphene oxide: adsorption and catalysis. Nanomaterials 9:439
Whelan M et al (2015) Fate and transport of petroleum hydrocarbons in engineered biopiles in polar regions. Chemosphere 131:232–240
Wolejko E, Wydro U, Łoboda T (2016) The ways to increase efficiency of soil bioremediation. Ecol Chem Eng S 23(1):155–174
Xin-Yu Bian S, Maurice M, Yi-Fan L, Shi-Zhong Y (2015) Insights into the anaerobic biodegradation pathway of n-alkanes in oil reservoirs by detection of signature metabolites, s.l. Sci Rep 5(9801):2015. https://doi.org/10.1038/srep09801
Yan L, Sinkko H, Penttinen P, Lindström K (2016) Characterisation of successional changes in bacterial community composition during bioremediation of used motor oil-contaminated soil in a boreal climate. Sci Total Environ 542:817–825
Yasinta J, Victor E, Daniel M (2018) A comparative study on removal of hazardous anions from water by adsorption. Int J Chem Eng 2018:1–21
Yavari S, Malakahmad A, Sapari NB (2015) A review on phytoremediation of crude oil spills. Water Air Soil Pollut 226:1–18
Yongsong M et al (2018) Remediation of hydrocarbon-heavy metal co-contaminated soil by electrokinetics combined with biostimulation. Chem Eng J 353:410–418
Zarrabi M et al (2014) Environmental engineering and removal of phosphorus by ion-exchange resins: equilibrium. Kinet Thermodyn Stud Manag J 13(4):891–903
Zhu X, Venosa AD, Makram T, Lee K (2001) Guidelines for the bioremediation of marine shorelines and freshwater wetlands. U.S. Environmental Protection Agency, Cincinnati
Acknowledgements
I wish to acknowledge the Niger Delta University, Departments of Chemical and Civil Engineering, which made the necessary facilities for this work available.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest concerning this publication.
Additional information
Editorial responsibility: Gaurav Sharma.
Rights and permissions
About this article
Cite this article
Okoh, E., Yelebe, Z.R., Oruabena, B. et al. Clean-up of crude oil-contaminated soils: bioremediation option. Int. J. Environ. Sci. Technol. 17, 1185–1198 (2020). https://doi.org/10.1007/s13762-019-02605-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02605-y