Abstract
Children are more vulnerable to indoor pollutants as they spend most of their time at homes or in schools. The exposure to organic air pollutants in these microenvironments can have an adverse impact on their health and performance. Exposure to the volatile organic pollutants in nine schools located in different areas of Kuwait was investigated. The schools which offer formal education to different age-groups were selected for this study, and these schools were located in different urban areas characterized as industrial, vehicular, and residential. Samples were taken from schools for 8 and 24 h during weekdays and weekends. All VOCs were collected by canister passive sampling and analyzed using an Entech pre-concentrator and gas chromatography/flame ionization detector. Seventy-two VOCs were investigated and classified into four groups: aliphatics, aromatics, oxygenated, and halogenated. The concentration of VOCs indoors and outdoors was determined, and the indoor-to-outdoor (I/O) ratio was calculated. This I/O ratio was used as to assess the indoor air quality of the schools monitored. In general, the oxygenated group concentration was higher indoors than outdoors. The variation in the concentration of VOCs was influenced by the characteristics of the area sampled. Some schools were found to have a high indoor contribution, while others had a prevalent outdoor contribution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In this modern world, high importance has been placed on the quality of life, food, air, and water. People spend most of their time indoors in a range of microenvironments such as workplaces, homes, restaurants, and schools Thus, at all these places, indoor air quality (IAQ) is an important determinant in terms of human health. In schools, students from different age-groups can be critically affected by exposure to various air pollutants. The time spent at school can have a direct impact on health and performance. The extent of exposure to organic air pollutants is strongly dependant on the activities and the emissions that takes place in these indoor spaces. However, there is a strong influence of outdoor air pollutants toward the quality of air indoors (Ohura et al. 2006). Exposure to volatile organic compounds (VOCs) is of critical concern as many of these compounds are either carcinogenic or can have adverse health effects on humans. VOCs are categorized as hazardous air pollutants (HAPs) under Title I, Part A, Section 112 (CAA 1991) (Sexton et al. 2004). Schools have been a topic of interest for the investigation of VOC emissions and its human health impact in many studies (Lee et al.,2006; Godwin and Batterman 2007; Agostinho et al. 2012; Stafford 2015). Apart from schools, homes and workplaces have also been evaluated in several studies to examine indoor, outdoor, and personal exposure to VOCs. (Pekey and Arslanbaş 2008). Many studies have explored indoor to outdoor correlations (de Gennaro et al. 2013) on the personal exposure to these organic pollutants; some of these studies have shown a direct indoor influence (Geiss et al. 2011), while others found an outdoor air correlation (Pekey and Arslanbaş 2008). In the outdoor environment, industrial and vehicular emissions are considered as major sources of VOCs, while in the indoor environment, building material emissions, paints, perfumes, and fragrances are typical (Guo et al. 2003). The most common method of sampling these VOCs is passive sampling and it is as precise as active sampling. The present study aims to characterize the indoor air quality of different schools located in different areas of Kuwait. The indoor and outdoor measurements of VOC concentration were carried out and assessed. The indoor-to- outdoor ratio (I/O) was calculated and used as a determinant to assess the IAQ of the schools monitored. The studied VOCs were categorized into four classes depending on their nature.
Materials and methods
The monitoring study was conducted in nine different mechanically ventilated schools. The schools were selected from different provinces of the country located in the prime areas characterized by industries, alarming traffic, and population. Simultaneous measurements of indoor and outdoor VOCs were conducted. The selection of the schools was based on the age-group of the students. The idea was to monitor the VOC concentration at these schools and their health impact on the different educational stages. The classrooms were occupied during the weekdays from 7.00 to 14.00. Samples were also taken from the classrooms during weekends. Canisters were deployed in the schools allowing sampling for a stipulated time frame of either 8 or 24 h. This sampling schedule assured a generalized pattern in the sample collection as well as effective interpretation and precise quantification of the pollutant concentrations. Further, the sampling pattern covered a weekday and weekend, which enabled the comparison of concentrations on those days. The whole air samples were analyzed for 72 VOCs, which were grouped under four separate chemical classes as nine aliphatic hydrocarbons (HCs), eight aromatic hydrocarbons (AHCs) 29 halogenated hydrocarbons (HHCs), and 26 oxygenated hydrocarbons (OHCs). The capabilities and limitations of the sampling and analytical methods were considered while interpreting the ambient air monitoring data. To judge and characterize the present study, a combination of the two EPA approved methods TO14 A and TO15 was used (USEPA 1999a, b).
Sampling sites
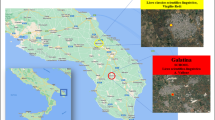
The schools selected for this study and their site features are shown in Table 1. At each location, duplicate samples were collected from classroom indoors of three different schools. Similarly, to get the background concentration, outdoor samples were collected from the roof of the schools at each site. The scheduled sampling was conducted at a particular location, during the months of November and December 2015 when the schools were open. A map showing the site and the sampled schools are shown in Fig. 1.
Sampling and analytical procedures
The sampling protocol used in this study was compliant with the USEPA sampling procedures (USEPA 1999b), and the whole air samples were collected in 6 L evacuated SILONITE™-coated polished stainless steel canisters. Pump-less canisters were employed for sampling. Prior to sampling, the passivated canisters were cleaned using a combination of nitrogen purge followed by evacuation with an optional 100 °C heating, which helped to facilitate the removal of surface-bound contaminants. Canisters were subjected to several such cycles of purge/evacuation and heating in accordance with USEPA TO14A (USEPA 1999a) using Entech instrument model 3100 Smart Lab Canister Cleaning System. The prepared canisters were deployed at the monitoring stations, and samples were collected over the specified averaging period. Entech instrument model CS 1200 high-purity flow regulator precisely controlled the sampling flow rate and filled the canisters at a constant rate without requiring power. The air samples collected in the canisters were analyzed for seventy-two volatile organic compounds using gas chromatography (GC) equipped with flame ionization detector (FID). The air samples from the canister were concentrated to get using a three-stage cryogenic concentrator 7100A from Entech Inc. and were analyzed with Agilent instrument model 7890A GC/FID analyzer. The samples were cryogenically trapped at − 150 °C and desorbed to 180 °C which were then focused at − 150 °C in the focusing trap before transferring for GC/FID analysis. The VOC components eluting from the GC column were further detected by the FID. The chemical ionization of the organic components in the FID flame produced electrical signals that were subsequently interpreted using Agilent Chem. Station software. For the quantitative determination of the samples, the instrument was initially calibrated using a NIST (National Institute of Standards and Technology) traceable external standard of seventy-two components in 50 ppbv concentration (Air Environmental Inc.). The accuracy of the sampling, adequacy of the methodology and analysis were ensured by analyzing duplicates samples from the same location and checking the repeatability.
Results and discussion
The variation in the mean value of the total VOCs measured at different schools located in different areas of the country is shown in Table 2. A notable variation in the indoor and the outdoor concentrations of the VOCs was noticed in the monitored schools, depending on their location. The samples collected from the classroom interiors had the highest average VOC concentration compared to the outdoor samples indicating the activities taking place inside the classroom. However, the average concentration of the studied VOCs at certain schools showed higher values in weekend samples compared to weekday samples. Among the 72 VOC compounds measured certain potentially, health risk compounds were selected to study the indoor air quality. The selection of the compounds was based on the background information available on their toxicity. Moreover, the compounds which are primarily present indoors, or emitted from building materials were also assessed.
At each school, the variation in the component concentration was found to be different, which reflected the characteristics of that site. In all areas, the oxygenated group concentration was higher in the indoor compared to the outdoor samples. The total VOC concentration in the samples collected was higher during the weekend than that of the weekday from Area 1 and Area 3. The main components that showed a significant increase in their concentration from these areas were the aliphatic and the halogenated groups. However, in Area 2, the weekday concentration was higher compared to the weekend concentration of the total VOCs measured. The oxygenated components and the aromatic groups were predominant in the schools sampled from this area. Meanwhile, the aliphatic and the halogenated concentration showed an increase during the weekend, which could possibly be due to the infiltration from outdoor sources. Another reason could be the possibility of low ventilation rate during weekends which was not effective to emit these VOC to the outdoors. The total concentration of the VOCs from the outdoor samples showed similar levels during the weekday as well as the weekend. The compounds that were prevalent at the sites sampled were propane, pentane, and cyclohexane from the aliphatic group and benzene toluene and the xylenes from the aromatic group. Among the halogenated groups, 1,2-dichlorotetrafluoroethane and vinyl chloride were detected, while ketones like acetone, 2-pentanone, 4-methyl-2-pentanone, alcohols like ethanol, and 2-propanol were predominant among the oxygenated components.
From these results, it can be seen that there is an indirect influence of the outdoor spheres to the concentration of VOCs in the indoor spaces. Apparently, these factors are further augmented by the activities involved in the environment leading to an increase in the concentration indoors. For a detailed study of this, an indoor (I)-to-outdoor (O) ratio was calculated for the components to see whether these compounds are in filtered from outdoor emission sources classroom indoors. It was considered that an indoor (I)-to-outdoor (O) ratio of 1.0 ± 0.5 indicates the VOC is mainly emitted from an outdoor source, whereas a VOC with a high ratio, usually > 5.0, is emitted primarily from indoor sources (Al-Khulaifi et al. 2014). From the I/O indicator values presented in Table 3, it can be assumed that there has been a considerable influence of the outdoor emission sources on the mean concentration of the VOC categories measured from the indoor environment. Specifically, the increase in the concentration of the aliphatic, aromatic, and halogenated groups gives an indirect indication of the possibility of seeping of these compounds from outdoor to the indoor atmosphere, which could be further increased by the regular activities taking place indoors. The concentrations of different classes of VOCs monitored indoors against outdoors are plotted and shown in Figs. 2, 3, 4 and 5a, b with the component variation during weekdays and weekends.
From Fig. 2a, b, it is evident that the concentration of the aliphatic hydrocarbons in the schools monitored is influenced by the concentration of these compounds outdoors and the concentration of these compounds are more in Area 1 which is characterized as an industrial area. The variation in the concentration of the aromatic hydrocarbons during the weekdays is more influenced by the indoor activities in the region 2 as shown in Fig. 3a, b, while the schools in Areas 1 and 3 are strongly exposed to some indoor sources of these classes of compounds during the weekend. Furthermore, a strong outdoor intrusion of the halogenated group components to the indoor spaces is shown in Fig. 4a, b, while for the oxygenated class of compounds, indoor sources are more relevant than the outdoor sources on the days of sampling,
In Area 1 which was characterized as an industrial area, frequently occurring aliphatic hydrocarbons components were propane, pentane, and cyclohexane. The aromatic species benzene, which is considered to be a hazardous pollutant, was detected only at School 1A. However, the concentration benzene could not be quantified appropriately. Toluene was detected in all the samples, although in low concentrations. Among the halogenated groups that appeared during the sampling were 1,2-dichlorotetrafluoroethane and vinyl chloride, and their concentrations were higher during the weekend compared to the weekday. Critical issues related to certain compounds were evident at some schools from the area they were sampled. In School 1A, the weekend concentration of the measured VOCs was very high compared to the weekday. Certain critically adverse compounds were detected from this school environment. The critical compounds encountered in this school were acetonitrile, cyclohexane, benzene, toluene, m-p-xylene, o-xylene/styrene, 1,3,5-trimethylbenzene, 1,2,3-trimethylbenzene, 1,2-dibromoethane, m-dichlorobenzene, 4-methyl-2-pentanone and ethanol. While the concentration of the ethanol component was negligibly small in the outdoors, a background concentration of vinyl chloride from outdoor emissions was evident from the results.
As for Area 2 which was considered as a heavy traffic congested area, the overall VOC concentration was higher during the weekday compared to the weekend in the indoor and outdoor samples. In the aliphatic group, the compounds that were prevalent on the days of the sampling were propane, pentane, and cyclohexane. The I/O ratio of these components with a value lesser than 1.5 indicates their significant sources are from outdoors which were further elevated due to the indoor activities. From the I/O ratio calculated, the components with high indoor predominance were aromatics as well as oxygenated groups. Benzene was not detected from the outdoor samples. However, xylenes were mostly found in indoor samples collected from which could be related to their indoor origin. The halogenated compounds which were considered to be from outdoor sources were 1,2-dichlorotetrafluoroethane (1,2-DCTFE), and vinyl chloride. While the I/O of 1, 2-DCTFE was below 1, that of vinyl chloride was equal to 1, which indicates their prevalence from outdoor sources. These compounds that have a long persistence in the environment should possibly be considered for their predominance in the outdoor samples. Another group of compounds with important indoor origins was the oxygenated group, which includes compounds like acetaldehyde, nonanal, acetone, 2-pentanone, 4-methyl-2-pentanone, methanol, ethanol, and 2-propanol that are particularly used as solvents. The concentration of these compounds varied significantly in the indoor samples as well as outdoor samples. A higher I/O ratio suggests their presence from the indoor sources. Also, compounds like 2-pentanone, 4-methyl-2-pentanone, and 2-propanol were not detected from the outdoor samples. The concentration of ethanol in the elementary school reached nearly 1 ppm.
Unlike other areas sampled, in the residential area marked as Area 3, the mean VOC concentration was escalated during weekend compared to a weekday in the indoor as well as the outdoor samples. Also, the concentration was predominant at Schools 3C and 3A during weekends. The outdoor sample collected from this area showed a similar trend with the weekends being very high compared to the weekday results. It can be assumed that a change in the outdoor activities on the particular day of sampling could have affected the overall readings in that area. However, the result does not show an increase in concentration in the oxygenated group in the roof sample, indicating the source for this group is solely from the indoor activities. Unlike the other areas, the aliphatic and aromatic composition was measured higher in all the samples, either indoor or outdoor. The halogenated group composition was very high in this area with the outdoor sample having a higher concentration compared to the indoor samples. The significant components that were predominant in the indoor atmosphere were aromatic and the oxygenated groups as reflected from their I/O ratio. However, the aliphatic and the halogenated groups have their I/O ratio roughly falling in the range that correlates to the outdoor predominance. Among the aliphatic compounds, propane, pentane, and cyclohexane were found to be higher with the propane concentration reaching almost 200 ppbv in the indoor samples. The aromatic class of compounds was not detected much in the weekday or weekend samples and was low below 7 ppbv. Compounds like benzene and toluene were detected in Schools 3A and 3B, respectively, during the working weekday. Toluene was found in all the samples during the weekend but with a low concentration. The halogenated compounds which showed their higher accumulation in the samples were 1,2-dichlorotetrafluoroethane (1,2-DCTFE), vinyl chloride, dichloromethane (DCM), and chloroform. Their I/O was between 0.5 and 1.5 specifying their occurrence outdoors. Additionally, DCM and chloroform were detected from this site alone. While DCM was detected on both at weekends and weekdays, chloroform was detected only during the weekend. The significant oxygenated compounds were acetaldehyde, butanal, nonanal, acetone, 4-methyl-2-pentanone, methanol, ethanol, 2-propanol, and methyl tertiary butyl ether (MTBE). While for the alcohols like methanol, ethanol, 2-propanol, the I/O ratio suggest an indoor source correlation, the aldehydes, and other compound concentrations varied significantly both indoor and outdoor.
While comparing the three areas under study and from the results discussed previously, it can be generalized that the variation in the concentration of the oxygenated compound was mostly related to indoor sources, whereas the aliphatic and the halogenated compounds were related to their outdoor origins. The change in the concentration of these compounds in the indoor atmospheres is due to the infiltration of the outdoor atmosphere, which was further increased by the indoor day-to-day activities. The aliphatic and halogenated concentrations were higher at Area 1 in the weekday compared to other sites. The trace level of these compounds found in the Area 1 region indicates the saturation of the ambient air with these compounds. The Area 1 region being an industrial area is suspected to have these compounds in high levels, which is also reflected in the results. Refinery emissions should include a certain amount of the light hydrocarbons (Gariazzo et al. 2005) as evident in the results. The halogenated hydrocarbons are widely used as solvents and they are the by-products of many industrial processes. They are persistent in the atmosphere as they are resistant to photochemical breakdown (Tiwari et al. 2010). The aromatic hydrocarbons were detected in very low levels and are mostly related to the indoor sources like the indoor environments by the use of consumer products such as tobacco smoke, deodorizers, air fresheners, liquid process photocopiers, solvents, carpet glue, paints, varnishes, gasoline emissions, and automobile exhaust (NYSDH 2013). The high levels of the oxygenated compounds especially alcohols in the indoor environment might be due to the extensive use of perfumes, room deodorants, fragrances as well as other indoor activities. The oxygenated concentration was higher in the elementary school in Area 2 compared to other locations.
During the weekends, the variation in the concentration of the groups was almost similar to that of weekdays. However, the overall concentration was high in Area 3 region during the weekend. At Area 2, the weekend concentration was very low for the VOCs. However, at Area 1, an unusual trend was seen in the high school where the weekend concentration shot up compared to the other locations sampled at this site. The increase in concentration in Area 3 region during the weekend could be due to some outdoor incidents, which could have happened during the time of sampling. The roof samples also show a similar trend in the concentration at this site. The aliphatic and the halogenated concentrations in Area 1 region were almost the same showing a consistency in the concentration of these compounds at this site. The unusual trend was seen only at the high school in this area. In Area 2, the overall concentration lowered apparently due to low traffic and other activities during the weekend.
Conclusion
The aim of this study was to evaluate the IAQ of the different schools located in different areas. This has yielded the identification and the quantification of the different pollutants in the different stages of formal education levels. The effect of outdoor air pollution on indoor air pollution was evaluated. The influence of the outdoor sources to the indoor exposure has been determined by the I/O ratio. Although the human exposure in the indoor environment is mostly due to the indoor emission source, there are many factors of the area emissions that augmented the indoor personal exposures. Further studies are needed to examine the relationship between the concentration variation of the components with the mechanical ventilation rates that could affect the performance and the comfort level of the students. However, as the results from this study showed a considerable influence of the outdoor emission sources toward the classroom interiors, it is suggested that the mechanical ventilation system should be well maintained with increased ventilation rates in order to avoid any health risks and thus to improve the performance of the occupants.
References
Agostinho J, Alvim F, Catarina F, Sousa S (2012) Indoor air pollution on nurseries and primary schools: impact on childhood asthma—study protocol. Biol Sci Environ Sci Pollut Manag 12:1
Al-Khulaifi NM, Al-Mudhaf HF, Alenezi R, Abu-Shady ASI, Selim MI (2014) Seasonal and temporal variations in volatile organic compounds in indoor and outdoor air in Al-Jahra City, Kuwait. J Environ Prot 5:310–326
de Gennaro G, Farella G, Marzocca A, Mazzone A, Tutino M (2013) Indoor and outdoor monitoring of volatile organic compounds in school buildings: indicators based on health risk assessment to single out critical issues. Int J Environ Res Public Health 10:6273–6291
Gariazzo C, Pelliccioni A, Filippo P, Sallustiand F, Cecinato A (2005) Monitoring and analysis of volatile organic compounds around an oil refinery. Water Air Soil Pollut 167:17–38
Geiss O, Giannopoulos B, Larsen B, Moreno J, Tirendi S (2011) The AIRMEX study—VOC measurements in public buildings and schools/kindergartens in eleven European cities: statistical analysis of the data. Atmos Environ 45:3676–3684
Godwin C, Batterman S (2007) Indoor air quality in Michigan schools. Indoor Air 17:109–121
Guo H, Lee SC, Li WM, Cao JJ (2003) Source characterization of BTEX in indoor microenvironments in Hong Kong. Atmos Environ 37:73–82
Lee A, Rumchev K, Spickett J, Stick S, Zhang G (2006) Indoor environmental quality in a ‘low allergen’ school and three standard primary schools in Western Australia. Biol Sci Environ Sci Pollut Manag 16:74–80
NYSDH (2013) Volatile organic compounds (VOCs) in commonly used products. New York State Department of Health. https://www.health.ny.gov/environmental/indoors/voc.html. Accessed 16 Feb 2014
Ohura T, Amagai T, Senga Y, Fusaya M (2006) Organic air pollutants inside and outside residences in Shimizu, Japan: levels, sources, and risks. Sci Total Environ 366:485–499
Pekey H, Arslanbaş D (2008) The relationship between indoor, outdoor and personal VOC concentrations in homes, offices, and schools in the metropolitan region of Kocaeli, Turkey. Water Air Soil Pollut 191:113–129
Sexton K, Adgate JL, Ramachandran G, Pratt GC, Mongin SJ, Stock TH et al (2004) Comparison of personal. Indoor, and personal exposures to hazardous air pollutants in three urban communities. Environ Sci Technol 38:423–430
Stafford T (2015) Indoor air quality and academic performance. J Environ Econ Manag 70:34–50
Tiwari V, Hanai Y, Masunaga S (2010) Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Qual Atmos Health 3:65–75
USEPA (1999a) Compendium of methods for the determination of toxic organic compounds in ambient air: method TO-14A. United States Environmental Protection Agency, Cincinnati
USEPA (1999b) Compendium of methods for the determination of toxic organic compounds in ambient air: method TO-15. United States Environmental Protection Agency, Cincinnati
Acknowledgements
The authors would like to thank Kuwait Institute for Scientific Research (KISR) for providing the facility to conduct this study. This study was under service project EM001 M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Mohamed Fathy Yassin.
Rights and permissions
About this article
Cite this article
Yassin, M.F., Pillai, A.M. Monitoring of volatile organic compounds in different schools: a determinant of the indoor air quality. Int. J. Environ. Sci. Technol. 16, 2733–2744 (2019). https://doi.org/10.1007/s13762-018-1838-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1838-0