Abstract
In a biological treatment of an industrial effluent, the indigenous bacteria have already been used. In this study, the three bacteria strains which are potent in the decolorization of azo dyes were isolated from dairy effluent and used for decolorization of Reactive Black5 and Reactive Red120 azo dyes and decolorization test of each dye was conducted at five concentration levels (10, 50, 100, 150, and 200 mg/l). The pH of which was adjusted to 7 and incubated at 37 °C for 3 days. The strains were identified as Staphylococcus sp. and Micrococcus luteus strains by 16S rRNA gene sequences analysis. The strains were deposited in GenBank with accession numbers of KX180131, KX180132, and KX180133 and submitted to laboratory identifier named Staphylococcus sp. MEH038S, Micrococcus luteus strain SEH038S, and Micrococcus luteus strain FEH038S. Three days into incubation, the lowest efficiency was at a concentration level of 200 mg/l for each dye. Decolorization efficiencies for Staphylococcus sp. MEH038S, Micrococcus luteus strain SEH038S, and Micrococcus luteus strain FEH038S at the concentration level of 200 mg/l for Reactive Red120 were 89.7, 87.1, and 89.3%, and for Reactive Black5 were 90.8, 90.0, and 89.9%, respectively. Based upon this study, dairy effluents can be used as a suitable alternative to the decolorization of textile wastewater. This study demonstrates a report on grounds of elucidation for the use of non-indigenous bacteria in the treatment of industrial wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The primitive man simply extracted the pigments from the natural environment around himself and used them for dyeing clothes and textiles. The use of natural dyes has continued for centuries, until 1856 when the first synthetic dye was accidentally discovered by William Henry Prkyn (Saratale et al. 2011). Today, over 10,000 commercially available synthetic dyes exist and are used extensively in many fields of industries such as textile, leather, paper, food. (Crini 2006; Lade et al. 2015). Over 0.7 million tons of such dyes are produced annually worldwide (Saharan and Ranga 2011; Tripathi and Srivastava 2011; Saroj et al. 2014). Low price, facilitating the production, high stability to the sunshine and temperature, detergents sustainability of synthetic dyes in contrast to natural dyes have caused an increased industrial attraction to the use of such dyes (Lade et al. 2015; Kumari and Naraian 2016; Nouren et al. 2017); 70% of all dyestuffs used in textile are azo dyes (Jirasripongpun et al. 2007; Ogugbue et al. 2012; Tripathi and Srivastava 2011). The azo dyes containing aromatic rings are joined by one or more azo bond (–N = N–) (Gupta 2009; Hussain et al. 2013).
The textile industries consume large quantities of water. Between 60 and 400 L (on average, 200 L) of water is used to produce 1 kg of textile (Ali et al. 2009). As a result, a large volume of colored wastewater is discharged into the environment (Buthelezi et al. 2012; Daâssi et al. 2013; Hema and Suresha 2014). Moreover, some amounts of these dyes are usually directly lost in wastewater in textile industries, depending on the type of dyes and fibers. In the case of reactive dyes, more than 50% of the initial loading dyes are released in the vicinity of regional dyeing facilities into a waste reservoir (Pandey et al. 2007). Less than 1 mg/l of dyes dissolved in water is highly visible and undesirable, while the amounts of dye concentration in the textile effluents are in the range of 10-200 mg/l (Pandey et al. 2007; Lade et al. 2015). It is reported that around 280,000 tons of dyes discharge into nature annually worldwide. Discharging dyes into receiving water is undesirable and causes damage to the water bodies, such as spoiling water appearance or aesthetic problems, obstructing light penetration, reducing the generation of oxygen by photosynthesis process, and creating anoxic conditions that are lethal to aquatic life. (Kurade et al. 2012; Rana and Sharma 2013). Also, azo dyes in soil have different effects on microbial activity and caused changes in the microbial community composition (Imran et al. 2015). Hence, the effluent from textile industry is one of the most problematic wastewater and cannot be discharged directly into the environment without thorough treatment of such water (Miao 2005; Ali et al. 2009).
During the last few decades, several physicochemical techniques were used for the treatment of colored textile effluents. Despite these methods to have a good efficiency in decolorization, such problems like large amounts of toxic sludge, formation of hazardous by-products may arise. Furthermore, in a method like adsorption, the dyes only transfer from one phase to another and it leaves the problem unsolved (Aksu and Dönmez 2003; Crini 2006; Saratale et al. 2011; Vijayanand and Hemapriya 2013; Govindwar et al. 2014; Saroj et al. 2014). Despite other methods, biodecolorization is considered as an environmental friendly and viable alternative that can lead to complete mineralization of xenobiotic compounds at low cost and produces less toxic compounds or sludge with less water consumption (Saratale et al. 2011; Hema and Suresha 2014; Pathak et al. 2014).
Recently, numerous microorganisms, including bacteria, fungi, yeasts, and algae, were investigated for decolorization of azo dyes (Liao et al. 2013; Rana and Sharma 2013). Previous studies had used the indigenous microorganisms in the treatment of wastewater (Ali et al. 2009; Jain et al. 2012; Forss et al. 2017; He et al. 2017). Dairy effluent is one of the resources, which possesses various types of microorganisms such as bacteria and yeasts. The main purpose of this study is the use of bacteria strains isolated from dairy effluent to decolorization of two commonly used azo dyes in the textile industry. In addition, this work investigates the (I) screen and phenotypic identification of azo dye decolorizing bacteria strains from dairy wastewater, (II) determine the potentiality of the isolated strains in the decolorization of Reactive Black5 (RR5) and Reactive Red120 (RR120) azo dyes, and (III) genetic identification of strains based on the 16S rRNA gene. This research was conducted in Shahrekord, the province of Chaharmahal and Bakhtiari in Iran in 2016.
Materials and methods
Dyes, growth mediums, and chemicals
Textile azo dyes used in this study consist of the RB5 and RR120 that were purchased from Sigma–Aldrich (USA). All growth mediums including nutrient broth, nutrient agar, and Luria broth were purchased from Merck, Germany. Ethidium bromide, Taq DNA Polymerase, DNA ladder 100 bp, dNTP, preparation solutions, wash buffer, and 10X PCR buffer were purchased from CinnaGen, Iran. Primers were purchased from Faza Biotech, Iran.
Sample collection and primary isolation
The dairy effluent samples were collected from Zagros Pak Pooyan Co. (Shahrekord, Iran). Three samples of dairy effluent were collected in sterile clean plastic containers every 8 h and transported to the laboratory in a coolbox. In the laboratory, the samples were mixed and a composite sample was prepared.
In the primary isolation of bacteria, the dilutions of dairy effluent samples were prepared through successive dilution techniques to 10−5 times using sterile distilled water. Diluted sample was spread on nutrient agar and incubated for 24 h at 37 °C. After 24 h of incubation, growing colonies were picked up. Purification of bacteria was performed using four-quadrant streaking on the nutrient agar and gram staining. Cultivation and gram staining of purification of bacteria were repeated several times. Initially, isolated colonies were assessed in terms of the ability of growth in the environment contaminated with dyes. For this purpose, single colonies were streaked on nutrient agar medium containing different dye concentrations (10, 50, 100, 150, and 200 mg/l).
Secondary isolation and screening of high-efficiency strains in decolorization
To select the most potent bacterial strains in the decolorization, the pretest was carried out on primarily separated colonies. For this purpose, nutrient broth medium containing 200 mg/l of each dye (RB5 and RR120) was used and 20 ml Pyrex tubes were selected as bioreactors. In each of the tubes, the bacterial strains were inoculated and incubated at 37 °C for three days and decolorization was compared with a control sample through direct observation.
Appropriate wavelength alternative for decolorization assay
To select the maximum absorption wavelength for each dye, nutrient broth mediums supplemented with a concentration of 10 mg/l of dyes were used. They were autoclaved to match decolorization assay and neutralize the effect of temperature on dyes. Then, the absorption was measured with a spectrophotometer (DR6000, Hach, Germany) at different wavelengths. In the end, the maximum absorption wavelength was selected for the decolorization assay.
Preparing the inoculums concentrations
To prepare the inoculation density, one loop of isolated bacteria was added in 100 ml of Luria broth medium and incubated for 24 h at 37 °C and was shaken (150 rpm). Then, the density of inoculation was adjusted to McFarland 0.5 turbidity standard (0.1 at 625 nm).
Decolorization assay
Decolorization test was carried out at concentrations of 10, 50, 100, 150, and 200 mg/l for each dye, and then, 200 ml nutrient broth medium containing different dye concentrations was poured in 250 ml Erlenmeyer flasks. The pH of mediums was adjusted to 7 and autoclaved. Aliquots of 10% (v/v) of inoculums were added to each flask. The flasks were closed with sterilized cotton and aluminum foil, incubated at 37 °C, and shaken at 150 rpm. Every 24 h interval, aliquots of 45 ml were regularly withdrawn from each flask in sterile conditions and poured in 50 ml Falcon tubes, and cells were pelleted by centrifugation for 15 min at 4500 rpm. The obtained clear supernatant was transferred to the Cuvette and tested with a spectrophotometer (DR6000, Hach, Germany) at maximum absorption wavelength. Percentage of decolorization was calculated using Eq. (1):
where C0 is the initial and C1 is the executed decolorized dye concentration. All the experiments were performed in triplicates, and the mean values were considered.
Assessing the biological resistance of strains, against RB5 and RR120 azo dyes
In order to determine the ability and tolerance of isolated strains, resiliency test was done in different dye concentrations. To do that, different dye concentrations were added to nutrient agar medium and autoclaved. One loop of each colony was streaked in each plate and incubated for 48 h at 37 °C. The test was continued to obtain a minimum inhibitory dye concentration.
Differential identification of strains
The initial identification of most potent bacterial strains in decolorization was conducted based on morphological characters on nutrient agar media and differential identification including gram staining, biochemical activity (motility, indole, catalase, fermentation of glucose, and mannitol) and physiological properties were implemented using the protocol of the Bergey’s Manual of Determinative Bacteriology (Buchaman and Gibbons 1974).
The final identification of bacteria using 16S rRNA Gene Sequencing
The genomic DNA was extracted from the pure culture pellets via a Cinnagen DNA extraction kit according to the manufacturer protocol. The 16S rRNA gene was amplified by PCR using the bacteria universal primers 27F (5′-CAGCGGTACCAGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-CTCTCTGCAGTACGGCTACCTTGTTACGACTT-3′), and 1500 bp products were amplified. PCR steps including an initial denaturation were performed for 5 min at 94 °C followed by 32 cycles of 1 min at 94 °C, 1 min at 58 °C, and 2 min at 72 °C. The amplicons were purified using Cinnagen several reactive gel extraction kit and sequenced. The phylogenetic tree was constructed by the neighbor-joining method using Kimura-2-parameter distances in Mega 6 package. The support of monophyletic groups was assessed by the bootstrap method with 1000 replicates. The stock was prepared for an extended time preservation of strains.
Results and discussion
Isolation and screening of azo dye decolorizing bacterial strains
At primary screening, ten bacterial isolates were obtained from cultured dairy effluents on nutrient agar media. Eight of these colonies showed good growth on medium supplemented with a dye concentration of 200 mg/l. Figure 1 shows the samples of isolated single colonies and the colonies grew on nutrient agar medium containing dyes.
The results of the decolorization pretest showed that among the eight selected isolates, only three of which had a desirable decolorization efficiency within three days of incubation. The three most potent colonies in decolorization were coded from 1 to 3. The visual comparison of decolorization by colonies is shown in Fig. 2.
The maximum absorption wavelength for the RB5 and RR120 dyes
The results showed that the maximum absorption wavelength for the RR120 and RB5 dyes were occurring at 538 nm and 597 nm, respectively.
Effect of initial dye concentration
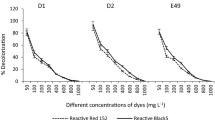
Decolorization efficiency was greatly affected by the initial dye concentration (Singh et al. 2014). As seen in Table 1, decolorization efficiency of RB5 and RR120 by three isolated strain was decreased with an increase in dye concentration. After 24 h of incubation, maximum decolorization efficiency for RR120 was 96.1% at the concentration of 10 mg/l. Also, the minimum decolorization efficiency of RR120 was 79.7% at the concentration of 200 mg/l. Maximum decolorization efficiency for RB5 dye was 85.0% at the concentration of 10 mg/l, and minimum decolorization efficiency of RB5 was 75.5%, at the concentration of 200 mg/l. There is a report in which decolorization was decreased due to increase in initial dye concentration (Jadhav et al. 2008).
Effect of incubation time
It was observed, depending on the initial dye concentration, most decolorization efficiency was occurred within 24 h of incubation. In the first 24 h of incubation, decolorization efficiencies for RR120 and RB5 were 88.9 to 96.1% and 79.7 to 85.0%, respectively. In accordance with Table 1 and Fig. 3, the rise of incubation time only leads from 0.5 to 11% increase in decolorization efficiency. So, dye concentration at high levels required more time to achieve similar efficiencies. Similar results have previously been reported (Kale et al. 2010; Das and Mishra 2016) (Table 2).
Phenotypical and molecular identification of strains
The results of phenotypic characterization and identification of azo dye decolorization strains showed that bacteria code 1 was gram-positive, nonmotile catalase-positive, mannitol fermentation-negative, convex, and white colony according to the Bergey’s Manual of Determinative Bacteriology. Moreover, the bacteria codes 2 and 3 were gram-positive, catalase-positive, mannitol fermentation-negative, convex yellow pigments, and glucose fermentation-negative. Based on the biochemical tests, the bacteria code 1 is Staphylococcus sp. and bacteria codes 2 and 3 are Micrococcus luteus. Also the 16srRNA sequence showed that the bacteria code 1 was the closest phylogenetic neighbors to Staphylococcus sp. IMB003 at an accurate identity of 96%.(Figure 4.), bacteria code 2 was the nearest phylogenetic neighbors to Micrococcus luteus TY21SsR at an accurate identity of 100%, and bacteria code 3 was the nearest phylogenetic neighbors to Micrococcus sp. H5 with an accurate identity of 97% (Fig. 5.). The sequences of the 16srRNA gene of the strains were deposited in GenBank using accession numbers of KX180131, KX180132, and KX180133 for code 1, code 2, and code 3, respectively. Also, the strains were submitted to GenBank using laboratory identifier called Staphylococcus sp. MEH038S, Micrococcus luteus strain SEH038S, and Micrococcus luteus strain FEH038S, in order to which they processed.
In this study, after three days of incubation via Micrococcus luteus SEH038S and Micrococcus luteus FEH038S, the decolorization efficiencies for 200 mg/l concentration of RB5 were 90 and 89.9%, respectively. Nearly similar efficiencies for the same concentration of RR120 were 87.1 and 89.3%, respectively. The removal of RR120 via Micrococcus luteus strains has not yet been reported.
In a study, Hassan et al. applied the Micrococcus luteus, to remove the yellow FN2R, navy WB, orange W3R, red FNR, and blue FNR dyes. Seven days into incubation, the removal efficiencies for yellow FN2R and navy WB were 60% for both, and for orange W3R, red FNR, and blue FNR were between 85 to 90% (Hassan et al. 2013). The two studies showed that the incubation time in the Hassan’s study was 2.3 times higher than that of ours in comparison. In another study done by Singh et al., the removal efficiency of direct orange 16 at 5 mg/l concentration was 96% via Micrococcus luteus strain SSN2 (Singh et al. 2015). Although the removal efficiency in Singh study is very high, the highest removed concentration in our study is over 40 times higher than that done by Singh.
In this study, the removal efficiencies for the RB5 and RR120 concentrations of 200 mg/l via Staphylococcus sp. MEH038S, within three days of incubation, were 90.8 and 89.7%, respectively. Yan used Staphylococcus cohnii to remove 100 mg/l of acid red B concentration. Yan reported that the removal efficiency after 24 h of incubation was 90 percent (Yan et al. 2012). In another study, Chaieb et al. applied Staphylococcus lentus to remove Congo red, Evans blue, and Eriochrome Black T at 100 mg/l dye concentration (Chaieb et al. 2016). The dyes removal efficiency has been reported to be 100%. In the above studies, dye removal efficiencies were high; however, the dye concentration in our study was twice higher than that used in Chaieb et al. and Yan’s. According to Table 1, it is found that the decolorization efficiency, especially in the case of RR120, occurs mainly during the first 24 h. More research needed to determine the decolorization mechanisms by obtained strains and to identify the cause of major decolorization in the first 24 h. The decolorization pattern varies; it depends on the type of color, the type and strain of microorganism, the mechanism of decolorization, the enzymatic activity, and the toxicity of the intermediate products. In some cases, it has been reported that the enzymatic activity of the organisms was high in the first 24 h and depleted in the following days. As an example, Uhnakova’s study explained that laccase utilized oxygen molecules to oxidize various types of xenobiotic compounds without the need for redox mediator and secondary metabolites (Uhnáková et al. 2011). This theory was supported by Hadibarata who explained that laccase reached the maximum activity during 24 h of incubation (Hadibarata et al. 2012). In other cases, the kinetics of the enzymes and intermediate products can play an inhibition role in decolorization. In a study done by Hu, the toxicity of metabolic products of azo dyes was analyzed, resulted in the kinetics of azoreductase on azo dyes, emphatically suggesting an inhibition model (Hu 2001).
Finally, by comparing our study and previous studies, it is clear that Micrococcus luteus strains and Staphylococcus sp. have high efficiencies in azo dyes removal; however, no studies have yet reported the 90% biological RB5 and RR120 decolorization efficiency at 200 mg/l concentration with applicability of non-indigenous bacteria strains in textile industrial effluents.
Conclusion
Bacterial strains of. Staphylococcus sp. MEH038S, Micrococcus luteus SEH038S, and Micrococcus luteus FEH038S which are capable of decolorizing RR120 and RB5 were isolated from dairy effluents. Discovered strains were identified with 16S rRNA and some biochemical tests. This study was conducted at 10, 50, 100, 150, and 200 mg/l azo dye concentrations for three consecutive days of incubation. These strains were biodecolorized at 200 mg/l concentration of RR120 from 87.1 to 89.7% and that RB5 from 89.9 to 90.8%. Our results suggest that the mixed application of dairy and textile wastewaters in a bioreactor system is congenial in decolorizing of textile effluents. Based upon these findings, future attempts will be performed to isolate more microorganisms from dairy effluents which are capable of azo dye decolorization.
References
Aksu Z, Dönmez G (2003) A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere 50:1075–1083
Ali N, Hameed A, Ahmed S (2009) Physicochemical characterization and bioremediation perspective of textile effluent, dyes and metals by indigenous bacteria. J Hazard Mater 164:322–328
Anwar F et al (2014) Characterization of reactive red-120 decolorizing bacterial strain Acinetobacter junii FA10 capable of simultaneous removal of azo dyes and hexavalent chromium Water. Air, & Soil Pollution 225:2017
Buchaman R, Gibbons N (1974) Bergey’s manual of determinative bacteriology Williams and Wilkins. Baltimore 197:654–701
Buthelezi SP, Olaniran AO, Pillay B (2012) Textile dye removal from wastewater effluents using bioflocculants produced by indigenous bacterial isolates. Molecules 17:14260–14274
Chaieb K, Hagar M, Radwan NR (2016) Biodegradation and decolorization of azo dyes by adherent Staphylococcus lentus strain. Appl Biol Chem 59:405–413
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Biores Technol 97:1061–1085
Daâssi D, Mechichi T, Nasri M, Rodriguez-Couto S (2013) Decolorization of the metal textile dye Lanaset Grey G by immobilized white-rot fungi. J Environ Manag 129:324–332
Das A, Mishra S (2016) Decolorization of different textile azo dyes using an isolated bacterium Enterococcus durans GM13. Int J Curr Microbiol Appl Sci 5:676–686
Forss J, Lindh MV, Pinhassi J, Welander U (2017) Microbial biotreatment of actual textile wastewater in a continuous sequential rice husk biofilter and the microbial community involved. PLoS ONE 12:e0170562
Govindwar SP, Kurade MB, Tamboli DP, Kabra AN, Kim PJ, Waghmode TR (2014) Decolorization and degradation of xenobiotic azo dye Reactive Yellow-84A and textile effluent by Galactomyces geotrichum. Chemosphere 109:234–238
Gupta V (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90:2313–2342
Hadibarata T, Yusoff ARM, Aris A, Hidayat T, Kristanti RA (2012) Decolorization of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water Air Soil Pollut 223:1045–1054
Hassan M, Alam M, Anwar M (2013) Biodegradation of textile azo dyes by bacteria isolated from dyeing industry effluent. Int Res J Biol Sci 2:27–31
He M, Tan L, Ning S, Song L, Shi S (2017) Performance of the biological aerated filter bioaugmented by a yeast Magnusiomyces ingens LH-F1 for treatment of Acid Red B and microbial community dynamics. World J Microbiol Biotechnol 33:39
Hema N, Suresha S (2014) Bioremediation of textile dye effluent by Shewanella putrefaciens. Int J Pharm Bio Sci 4:109–116
Hu T-L (2001) Kinetics of azoreductase and assessment of toxicity of metabolic products from azo dyes by Pseudomonas luteola. Water Sci Technol 43:261–269
Hussain S, Maqbool Z, Ali S, Yasmeen T, Imran M, Mahmood F, Abbas F (2013) Biodecolorization of reactive black-5 by a metal and salt tolerant bacterial strain Pseudomonas sp. RA20 isolated from Paharang drain effluents in Pakistan. Ecotoxicol Environ Saf 98:331–338
Imran M, Arshad M, Asghar HN, Asghar M, Crowley DE (2014) Potential of Shewanella sp. strain IFN4 to decolorize azo dyes under optimal conditions. Int J Agric Biol 16
Imran M, Shaharoona B, Crowley DE, Khalid A, Hussain S, Arshad M (2015) The stability of textile azo dyes in soil and their impact on microbial phospholipid fatty acid profiles. Ecotoxicol Environ Saf 120:163–168
Jadhav SU, Jadhav MU, Kagalkar AN, Govindwar SP (2008) Decolorization of Brilliant Blue G dye mediated by degradation of the microbial consortium of Galactomyces geotrichum and Bacillus sp. J Chin Inst Chem Eng 39:563–570
Jain K, Shah V, Chapla D, Madamwar D (2012) Decolorization and degradation of azo dye–Reactive Violet 5R by an acclimatized indigenous bacterial mixed cultures-SB4 isolated from anthropogenic dye contaminated soil. J Hazard Mater 213:378–386
Jirasripongpun K, Nasanit R, Niruntasook J, Chotikasatian B (2007) Decolorization and degradation of CI Reactive Red 195 by Enterobacter sp. Int J Sci Technol 12:6–11
Kale R, Sabale M, Thorat P (2010) Decolorization and degradation of textile azo dye golden yellow HE 2 R by adapted bacteria nature. Environ Pollut Technol 9:351–354
Kumari S, Naraian R (2016) Decolorization of synthetic brilliant green carpet industry dye through fungal co-culture technology. J Environ Manag 180:172–179
Kurade MB, Waghmode TR, Kagalkar AN, Govindwar SP (2012) Decolorization of textile industry effluent containing disperse dye Scarlet RR by a newly developed bacterial-yeast consortium BL-GG. Chem Eng J 184:33–41
Lade H, Kadam A, Paul D, Govindwar S (2015) Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J 14:158
Liao C-S, Hung C-H, Chao S-L (2013) Decolorization of azo dye reactive black B by Bacillus cereus strain HJ-1. Chemosphere 90:2109–2114
Maqbool Z et al (2016) Use of RSM modeling for optimizing decolorization of simulated textile wastewater by Pseudomonas aeruginosa strain ZM130 capable of simultaneous removal of reactive dyes and hexavalent chromium. Environ Sci Pollut Res 23:11224–11239
Miao Y (2005) Biological remediation of dyes in textile effluent: a review on current treatment technologies. Bioresour Technol 58:217–227
Nouren S et al (2017) By-product identification and phytotoxicity of biodegraded Direct Yellow 4 dye. Chemosphere 169:474–484
Ogugbue CJ, Morad N, Sawidis T, Oranusi NA (2012) Decolorization and partial mineralization of a polyazo dye by Bacillus firmus immobilized within tubular polymeric gel 3. Biotechnology 2:67–78
Oturkar CC, Nemade HN, Mulik PM, Patole MS, Hawaldar RR, Gawai KR (2011) Mechanistic investigation of decolorization and degradation of Reactive Red 120 by Bacillus lentus BI377. Biores Technol 102:758–764
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegradation 59:73–84
Pathak H, Soni D, Chauhan K (2014) Evaluation of in vitro efficacy for decolorization and degradation of commercial azo dye RB-B by Morganella sp. HK-1 isolated from dye contaminated industrial landfill. Chemosphere 105:126–132
Rana S, Sharma R (2013) Microbial degradation of synthetic textile dyes: a cost effective and eco-friendly approach. Afr J Microbiol Res 7:2983–2989
Saharan BS, Ranga P (2011) Optimisation of cultural conditions for decolourization of textiles Azo dyes by Bacillus subtilis SPR42 under submerged fermentation. Int J Adv Biotech Res 2:148–153
Saratale RG, Saratale GD, Chang J-S, Govindwar S (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157
Saroj S, Kumar K, Pareek N, Prasad R, Singh R (2014) Biodegradation of azo dyes Acid Red 183, Direct Blue 15 and Direct Red 75 by the isolate Penicillium oxalicum SAR-3. Chemosphere 107:240–248
Singh RP, Singh PK, Singh RL (2014) Bacterial decolorization of textile azo dye acid orange by Staphylococcus hominis RMLRT03. Toxicol Int 21:160
Singh S, Chatterji S, Nandini P, Prasad A, Rao K (2015) Biodegradation of azo dye Direct Orange 16 by Micrococcus luteus strain SSN2. Int J Environ Sci Technol 12:2161–2168
Tripathi A, Srivastava S (2011) Ecofriendly treatment of azo dyes: biodecolorization using bacterial strains. Int J Biosci Biochem Bioinform 1:37
Uhnáková B et al (2011) Biodegradation of tetrabromobisphenol A by oxidases in basidiomycetous fungi and estrogenic activity of the biotransformation products. Biores Technol 102:9409–9415
Vijayanand S, Hemapriya J (2013) Bacterial bioremediation of textile azo dyes: a review. Ind J Appl Res 3:480–482
Yan B, Du C, Xu M, Liao W (2012) Decolorization of azo dyes by a salt-tolerant Staphylococcus cohnii strain isolated from textile wastewater. Front Environ Sci Eng 6:806–814
Acknowledgements
The authors gratefully acknowledge the assistance of honorable experts at Water and Wastewater Microbiology Laboratory in the Faculty of Health, honorable experts in Cellular and Molecular Research Center of the Shahrekord University of Medical Sciences and Vice Chancellor for Research and Technology. This study was financially supported by the Shahrekord University of Medical Sciences, IR. Iran [Grant Number 122431].
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Tanmoy Karak.
Rights and permissions
About this article
Cite this article
Sadeghi, M., Forouzandeh, S., Nourmoradi, H. et al. /+//Biodecolorization of Reactive Black5 and Reactive Red120 azo dyes using bacterial strains isolated from dairy effluents. Int. J. Environ. Sci. Technol. 16, 3615–3624 (2019). https://doi.org/10.1007/s13762-018-1750-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1750-7