Abstract
Green synthesis of nanomaterials has received increasing attention as an eco-friendly technology in materials science. Here, we have used two types of extractions from green tea leaf (i.e., total extraction and tannin extraction) as reducing agents for a rapid, simple and one-step synthesis method of mesoporous silica nanoparticles/iron oxide nanocomposite based on deposition of iron oxide onto mesoporous silica nanoparticles. Mesoporous silica nanoparticles/iron oxide nanocomposite were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, energy-dispersive X-ray, vibrating sample magnetometer, N2 adsorption and high-resolution transmission electron microscopy. Mesoporous silica nanoparticles/iron oxide nanocomposite was used as a solid adsorbent for removal of lindane pesticide from aqueous solutions. The developed system possesses the advantages of silica as core that include large surface area and advantages of iron oxide (shell) that include the capability to interact with chlorinated compounds and ability to release by using external magnetic field. UV-Vis technique was used as a simple and easy method for monitoring the removal of lindane. Effects of pH and temperature on the removal efficiency of the developed mesoporous silica nanoparticles/iron oxide nanocomposite toward lindane pesticide were also investigated. Fourier transform infrared spectroscopy, high-performance liquid chromatography and gas chromatography techniques were used to confirm the high ability of mesoporous silica nanoparticles/iron oxide nanocomposite for sensing and the capture of lindane molecules with high sorption capacity (about 99%) that could develop a new eco-friendly strategy for detection and removal of pesticide and as a promising material for water treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic nanoparticles (NPs) have wide applications in several fields such as medical applications (e.g., target drug delivery, magnetic resonance imaging, cancer hyperthermia treatment) and nano-sorbents in environmental engineering (Johannsen et al. 2007). Several techniques have been used for the synthesis of iron oxides magnetite NPs such as chemical precipitation method (Maity et al. 2010), thermal decomposition of the organic iron precursor in organic solvents (El-Ghandoor et al. 2012), polyol process (Rockenberger et al. 1999), sol–gel method (Cai and Wan 2007), sonochemical synthesis (Dang et al. 2009) and solvothermal synthesis (Zhang et al. 2011). Although most of these methods could produce pure and well-defined NPs, they are relatively expensive and dangerous to the environment (Bhattacharya and Rajinder 2005). Green synthesis methods have many advantages such as the (1) reduction or elimination of hazardous wastes, (2) utilization of sustainable processes, (3) uses of eco-friendly chemicals, solvents or renewable materials (El-Said et al. 2014). Inactivated plant tissue (Padil and Cerník 2013), plant extracts (Shameli et al. 2012), exudates (Lukman et al. 2011) and other parts of living plants (Parsons et al. 2007) were used as environmentally friendly, non-toxic and safe reagents for synthesis of metal NPs (Salam et al. 2012).
Tannin “gallotannic acid or tannic acid” is a mixture of pentadin alloy glucose, pentagalloyl glucose and proanthocyanidins that could be extracted from several plant sources as a colloidal solution in water. Due to its chemical structure (Scheme 1), it could be used as a reducing agent (Cataldo et al. 2013).
Lindane is one of the organochlorine pesticides, which is considered as an environmental hazard that have an estimated 9.3% dermal absorption rate and is absorbed even more efficiently across abraded skin (Yavuz et al. 2007).
Several methods including catalytic dechlorination (Simagina et al. 2003), photolysis (Wang et al. 2007), hydrothermal decomposition (Prabowo et al. 2007), reductive dechlorination (Chang et al. 2008), bacterial degradation (Fouad and Mohamed 2011) and photodegradation process (Fouad et al. 2015) were applied for the decomposition of different types of pesticides “especially organochlorine pesticides” from water.
Due to the high stability of organochlorine pesticides in for many years after their use (Shukla et al. 2006), thus their removal by different degradation processes showed a low efficiency. And in some instances, decomposition of pesticides results in transformation products that are more toxic, so they represent a greater risk to the environment than the parent molecule (Bavcon et al. 2003). Among the different methods for water treatment, the adsorption method by using solid adsorbents has many advantages over the other methods; these advantages include the simple design, ease of using, low cost and ability for recycling of the adsorbents. Much progress has been made in recent years on adsorption application by using different materials. Developing new adsorbent materials with high efficiency and economic feasibility to remove these pollutants is in demand to improve the water quality.
Mesoporous silica nanoparticles (MSNPs) have received increasing attention in several scientific and technical fields due to their unique features including large surface area, capability for interacting with atoms, ions and molecules on their surfaces and along their porous networks (Akhtar et al. 2015; El-Said et al. 2010).
Fouad et al. have previously reported on fabrication of silica–gold nanocomposites for removal of methacrifos (methyl (E)-3-dimethoxyphosphinothioyloxy-2-methylprop-2-enoate) pesticides that show high ability for removal of organophosphorus pesticides up to 90% (Fouad et al. 2017). However, there is a high need for the fabrication of cost-effective materials that have ability to capture the pesticides molecules. Nanomaterials have wide applications in several fields including, chemistry and environment, energy, medicine, agriculture, information and communication, heavy industry (El-Said et al. 2011; Chen et al. 2012; Wang and Chen 2016). The nanomaterials may be unsafe for the biological system, since some of these materials could enter in the body due to the small size and their higher specific surface area and be a toxic at the cellular level (Vishwakarma et al. 2010; Jin et al. 2005). Also, they could easily bind with and transport toxic pollutants, which when inhaled can cause a number of pulmonary diseases in mammals. However, iron oxide nanoparticles (Fe3O4 NPs) are one of the biocompatible materials that have been widely used for many biotechnology and medical applications such as in magnetic resonance imaging, cell recognition and drug delivery (Laurent et al. 2008; Jain et al. 2005; Modo and Bulte 2005), in addition to some clinical applications including detection of liver tumors, metastatic lymph nodes, inflammatory and degenerative diseases. Therefore, we have selected Fe3O4 NPs as eco-friendly materials that also enable easy method for extraction from the medium by using external magnetic field.
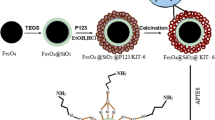
In the present work, we reported a fast, non-toxic, and green synthesis of magnetite MSNPs/Fe3O4 nanocomposites by using extractions from green tea as reducing and capping agents. The aim of this work is using MSNPs/Fe3O4 nanocomposite as a solid adsorbent material for the removal and detection of organochlorine pesticides (lindane). The magnetic feature of the developed MSNPs/Fe3O4 nanocomposites is an additional advantage for magnetic separation of organochlorine pesticides from wastewater based on applying an external magnetic field as shown in Scheme 2. Our results indicated that we have developed a simple and rapid method to remove lindane from water by using green-synthesized MSNPs/Fe3O4 nanocomposite with high efficiency.
This work has been started in May 2016, and it was carried out at the laboratories of Faculty of Science, Assiut University.
Materials and methods
Materials
Tetraethyl orthosilicate (TEOS, ≥99.9%) was obtained from Merck; cetyltrimethylammonium bromide (CTAB, ≥98%) was purchased from Sigma-Aldrich. Ferric chloride and ammonia solution (NH4OH, 32%) were obtained from Alpha Chemika. Standard pesticide such as lindane was purchased from Riedel-de Haën. All other chemicals and solvents were obtained as an analytical grade and used without any purification. All the solutions were prepared with deionized water (18.2 MΩ cm), which was purified with a Purite purification system.
Preparation of MSNPs
MSNPs were prepared by using CTAB surfactant as the structure-directing agent according to the previously reported method (Fouad et al. 2017). In a typical synthesis procedure, 24 mL of deionized water (DIW), 9.6 g of 0.5 M NaOH and 1.0 g of CTAB were added in 100-mL beaker under vigorous stirring at room temperature. 2 mL of TEOS as the silicon source was dropwise added to the mixture and stirred continuously for 8 h. The product material was filtered off, washed three times with DIW and ethanol, and then, dried overnight at 60 °C. The final product was calcined at 550 °C for 5 h to remove all CTAB from the MSNPs.
Preparation of two types of green tea extractions
Two types of extractions were obtained from green tea leaf and used for the synthesis of MSNPs/Fe3O4 nanocomposite as following:
-
(1)
The first type of the green tea leaf extraction (total extraction) was obtained by boiling about 5 g of dried green tea in 50 mL of DIW for 60 min, the color of the aqueous solution was changed from watery to yellow and the mixture was allowed to cool to room temperature. The aqueous extract of green tea was separated by filtration with Whatman filter paper and centrifuged at 1000 rpm for 5 min to remove any heavy biomaterials.
-
(2)
Second type of the green tea leaf extraction (tannin extraction) was prepared by boiling about 5 g of dried green tea in 50 mL of ethanol for 60 min. The mixture was allowed to cool to room temperature and to evaporate ethanol under vacuum evaporation to obtain the tannin.
Green synthesis of MSNPs/Fe3O4 nanocomposites
Magnetite MSNPs/Fe3O4 nanocomposite was synthesized based on using tea extraction as follows:
Typically, 0.4 g of the prepared MSNPs was dispersed in 50 mL of DIW, and then, 1.11 gm of FeCl3·6H2O was added, and the solution heated at 80 °C under mild stirring for 10 min. Then, 50 mL of tea extractions was added, and immediately the yellowish color of the ferric chloride solution changed into reddish brown color and its pH was strong acidic (about 1). After 5 min, hot ammonia aqueous solution was added to the mixture until it became alkaline (about pH = 8). NH4OH was added with rate of 1 mL/min for allowing the precipitation of magnetite uniformly under mild stirring for about 60 min at 100 °C. The mixture was allowed to cool down to room temperature. And the MSNPs/Fe3O4 nanocomposite was purified by washing three times by using DIW and ethanol. Finally, the MSNPs/Fe3O4 nanocomposites were dried overnight at 80 °C and calcined at different annealing temperatures for 5 h.
Measurements
The chemical, crystal structure and morphologies of MSNPs and MSNPs/Fe3O4 nanocomposites were characterized by HRTEM analysis by using a JEOL-2000 instrument operated at high voltage 120 kV. X-ray diffraction (XRD) analysis of test samples was performed with a Philips (The Netherlands) diffractometer (Model PW 2103, λ = 1.5418 Å, 35 kV and 20 mA) with a source of CuKα radiation (Ni-filtered). The diffraction patterns were recorded in the range of the diffraction angle 2θ from 10° to 90° with a step of 0.06°. Fourier transform infrared (FTIR) spectra of MSNPs and MSNPS/Fe3O4 nanocomposite were measured by using a Nicolet™ iS™10 FTIR spectrometer in the wave number range 400–3800 cm−1. The samples have been admixed with KBr under vacuum and at pressure of 1.88 tons/cm2. Thermogravimetric analysis (TGA) was conducted by using Shimadzu thermogravimetric analyzer model (TGA-60H) in the temperature range of 0–750 °C, with a heating rate of 10 °C/min under air atmosphere. Nitrogen adsorption was recorded for mesoporous silica using a model NOVA 3200 automated gas sorption system (quanta chrome, USA). Magnetic properties of the samples were measured using a vibration sample magnetometer (VSM; susceptibility meter 4700). UV-Vis spectroscopy double beam PC scanning spectrophotometer UV Evolution 300 from Labomed a computer data system is UV Win 5 software v 5.0.5 used for measuring wavelength and absorbance. Spectrophotometer range from 200 to 900 nm using 1-cm matched Stoppard quartz cells was used for monitoring the rate of pesticides removal. High-performance liquid chromatography (HPLC) for the tested samples was promptly analyzed using Agilent 1100 high-performance liquid chromatography system fitted with a diode array detector and an autosampler. Gas chromatograph (GC) using Agilent 6890 equipped with a Ni63 electron capture detector. Raman spectra were recorded with a Bruker Senterra Raman microscope (Bruker Optics Inc., Germany) with 785 nm excitation, 1200 rulings mm−1 holographic grating and a charge-coupled device (CCD) detector. The acquisition time was 3 s with power of 50 mW.
Results and discussion
Chemical and structural features of MSNPs and MSNPs/Fe3O4 nanocomposite
In the present work, MSNPs have been synthesized using TEOS as source of silicon in the presence of CTAB as a cationic surfactant under basic conditions. The chemical, morphological and cryptographical features of the prepared MSNPs and MSNPs/Fe3O4 nanocomposite were investigated, also the optimum conditions for the preparation of MSNPs/Fe3O4 nanocomposite, were investigated by using the SEM, HRTEM, FT-IR, EDX, XRD, nitrogen adsorption and Raman spectroscopic analyses.
Effect of concentration of green tea extracts on the crystallography and structure of Fe3O4 NPs
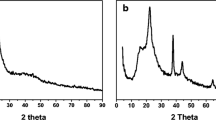
In order to optimize the green synthesis of Fe3O4, we have used different concentrations of the tea extractions to investigate the effect of the amount of tea on the chemical structure of iron oxide NPs by using XRD. Figure 1a–c shows the XRD patterns of the as-prepared iron oxide NP samples by using different amounts of green tea (1, 5 and 8 gm) to reduce 1.11 gm of FeCl3·6H2O; these results demonstrated that using the extraction from 1 gm of green tea results in obtained Fe2O3 NPs as shown in Fig. 1a. By increasing the amount of green tea until 5 gm, Fe3O4 NPs was obtained (Fig. 1b). Furthermore, by increasing the amount of extracted tea over 5 gm results in decreasing the intensity of the XRD peaks that related to the adsorption of green tea extraction onto the surface of Fe3O4 NPs (Fig. 1c). Thus, we have selected the usage of the extraction from 5 gm of green tea as optimum amount of tea under these conditions for the further experiments.
Structure analysis and phase change of Fe3O4 NPs
To obtain Fe3O4 NPs in pure form, we have studied the effect of annealing temperature on the chemical structures as well as the crystallography of the Fe3O4 NPs. Figure 2a–d shows the XRD patterns of the as-prepared iron oxide NPs and calcined samples at different annealing temperatures (200, 400 and 600 °C). These results reveal several prominent peaks at 2θ values including 32.82°, 35.40°, 44.17°, 49.35°, 53.71° and 63.87°, which well matched with the planes (220), (311), (222), (400), (422) and (511), respectively; these results indicated the formation of Fe3O4 monoclinic structure [reference: POWD:01-089-0951]. In addition, the intensity of all XRD peaks was increased by increasing the annealing temperature that indicates the decomposition of the all organic materials (tea’s extraction) and increase in the crystallinity of Fe3O4 NPs.
Uses of tannin extraction for the fabrication of Fe3O4 NPs and MSNPs/Fe3O4 nanocomposite
Furthermore, we have used tannin extracted instead of total extraction from green tea for synthesis of Fe3O4 NPs. Figure 3a shows the XRD pattern of the as-prepared iron oxide NPs that have been prepared by using tannin, which indicated the formation of Fe3O4 NPs. Thus, tannin is the active ingredient compound in green tea that is responsible for the formation of Fe3O4 NPs. Finally, we have applied the optimum conditions for fabrication of MSNPs/Fe3O4 based on deposition of Fe3O4 onto MSNPs in the presence of tannin as a reducing agent. Figure 3b represents the XRD pattern of MSNPs/Fe3O4 nanocomposite, which demonstrated the appearance of a new peak at 2θ equal 23° accredited to (100) that indicates the successful formation of MSNPs/Fe3O4 nanocomposite.
Chemical and structural features of MSNPs/Fe3O4 nanocomposite
The surface morphologies of MSNPs and MSNPs/Fe3O4 nanocomposite have been studied by using SEM. Figure 4a, b represents the SEM images of MSNPs and MSNPs/Fe3O4 nanocomposite, respectively, which indicated the formation of tiny MSNPs; aggregation of the particles and small grains are present at the surface. In addition, Fig. 4c, d shows the HRTEM images of MSNPs and MSNPs/Fe3O4 nanocomposite, respectively, which indicate the formation of a quasi-spherical in shape MSNPs/Fe3O4 with polydispersed size distribution. The aggregation of small nanocomposite is a result of magnetic features effects during the preparation of MSNPs/Fe3O4 nanocomposite for HRTEM analysis. TGA of the as-prepared MSNPs/Fe3O4 nanocomposite is shown in Fig. 5 that represented the weight loss versus temperature until 750 °C in air, which demonstrated the presence of three weight loss stages. The first stage showed weight loss of about 7.931% in the sample at around 195 °C that likely is due to the elimination of the water molecules absorbed by MSNPs/Fe3O4 nanocomposite. The second stage demonstrated weight loss of about 6.265% in the sample at around 410 °C that could be caused by the decomposition of the tea extractions that absorbed on the nanocomposite surface. The third stage showed the presence of little weight loss of about 2.499% at about 600 °C that could be related to the decomposition of the residual organic materials of the tea extracts, and then, the sample weight was almost constant, which indicates the thermal stability of the sample. Figure 6a shows the FT-IR spectra of MSNPs and MSNPs/Fe3O4 nanocomposite, indicating that the Fe3O4 molecules were deposited onto silica NPs. FT-IR spectrum of MSNPs shows a broad peak at 1077 cm−1, which could be assigned to Si—O—Si bond of silica, and the peak at 960 cm−1 can be attributed to Si—OH bond and broad peak at 3400 cm−1 that corresponding to O–H bond. FTIR spectrum of MSNPs/Fe3O4 nanocomposite (Fig. 6a) demonstrated that functionalized silica particles with iron molecules result in reducing the intensity of Si—O—Si and Si—OH peaks significantly, also the deposition of Fe3O4 molecules was characterized by two strong absorption peaks at around 560 and 440 cm−1, which correspond to the Fe–O stretching vibration band of Fe3O4 (Wu et al. 2004). Raman spectroscopy was also used to confirm the deposition of Fe3O4 into MSNPs; Fig. 6b shows the Raman spectrum of MSNPs, which revealed a peak at 1363 cm−1 corresponding to mesoporous silica (SiO2), while Raman spectrum of MSNPs/Fe3O4 nanocomposite (Fig. 6b) demonstrated the appearance of two new wide peaks at 730 and 1438 cm−1 corresponding to Fe3O4 phases, which indicated the formation of MSNPs/Fe3O4 nanocomposites. Furthermore, EDX spectral analysis of MSNPs/Fe3O4 nanocomposites reveals the presence of carbon, silicon, oxygen and iron in synthesized nanocomposite as shown in Fig. 6c. The appearance of peak of carbon in EDX spectra is due to the presence of organic molecules (tea extracts).
Magnetic measurements
The magnetic behavior of the prepared MSNPs/Fe3O4 nanocomposite was performed by using VSM at room temperature. Figure 6d shows the hysteresis loop obtained from the magnetization (M) versus field (H) data for MSNPs/Fe3O4. The magnetic hysteresis loop (M–H curve) of MSNPs/Fe3O4 nanocomposite demonstrated a typical behavior of ferromagnetic materials at room temperature. The value of their saturation magnetization (M s) was 40 emu/g. The excellent magnetic feature was necessary for magnetic separation of organochlorine pesticides from wastewater by applying an external magnetic field on MSNPs/Fe3O4 nanocomposite (Lu et al. 2004). The magnetic susceptibility of the materials was demonstrated in a simple laboratory setup with a handheld magnet. MSNPs/Fe3O4 nanocomposite could be completely separated from the aqueous solution within 5 min.
Nitrogen sorption isotherm
The morphology and surface features of the prepared MSNPs and MSNPs/Fe3O4 nanocomposite were characterized using the nitrogen sorption isotherm (Nunes et al. 2006). Analysis of the nitrogen adsorption/desorption branches of MSNPs showed a BET surface area of 818 m2/g and a total pore volume of 0.64 cm3/g. The pore size, calculated from the desorption isotherm using the BJH theory, was found to be 33 Å in diameter with a uniform distribution. MSNPs/Fe3O4 nanocomposite has been fabricated based on deposition of Fe3O4 onto MSNPs by using total green tea extraction and tannin extraction. Our results demonstrated that loading of Fe3O4 particles onto the surface of MSNPs results in decrease in the BET surface area to become 93.12 m2/g as shown in Table 1.
Detection of lindane using the MSNPs/Fe3O4 nanocomposite
The high stability of the organochlorine pesticides and their long resistance time in the environment is an environmental challenge; therefore development of simple, easy of use, cost-effective with high capability for recycling adsorption process by using solid adsorbents is urgently needed. The absorption of lindane by using MSNPs/Fe3O4 nanocomposite as solid adsorbent was confirmed by FT-IR technique. As shown in Fig. 6a, pure lindane showed its characteristic peaks, especially the functionality of C–Cl was observed at 1385 cm−1. Upon the binding of MSNPs/Fe3O4 nanocomposite, the increase and shift of FTIR peaks were observed, which confirmed the adsorption of lindane into MSNPs/Fe3O4 nanocomposite.
Removal of lindane using the MSNPs/Fe3O4 nanocomposite
Purification of water from the pesticides traces represents an environmental challenge due to their high stability and long resistance time in the environment. Here, we have applied MSNPs, Fe3O4 and MSNPs/Fe3O4 nanocomposite as a solid sorbent for removal of lindane at low concentration. In order to optimize the conditions of the removal process and the removal efficiency of MSNPs/Fe3O4 nanocomposite, we have investigated the effects of temperature and pH of the medium. Figure 7a shows the UV-Vis spectra of lindane before and after being treated with MSNPs/Fe3O4 nanocomposite for different treatment periods up to 7 h, which illustrated that the UV-Vis spectrum of standard solution of lindane showed an absorbance peak at wavelength of about 273 nm and the intensity of this absorption peak was decreased with the increase in the contact time. Figure 7b shows the time profile of lindane removal with MSNPs/Fe3O4 nanocomposite, which indicated that the uptake percentage of lindane was increased with the increase in the contact time until reaching the saturation phase after 5 h. Also, it confirmed that the lindane adsorption is a rapid process with almost 60% lindane being removed within approximately 5 h, which indicates the high affinity between the lindane pesticide and the MSNPs/Fe3O4 nanocomposite. Furthermore, we have studied the behavior of lindane pesticide in solutions of different pH values within the range from 3 to 12. Figure 7c shows the UV-Vis spectra of lindane in different pH solutions, which showed an absorption peak for lindane in neutral solution centered at about 270 nm, and this peak was shifted to longer wavelengths with the increase in the pH of alkaline solution (pH at 9 or 12) to 286 nm. The shifting of the absorption peak of lindane in alkaline medium dechlorination of lindane indicates that lindane pesticide is unstable in alkaline medium. Thus, we have performed all the next experiments in the neutral medium. Figure 8a shows the absorbance spectra of different concentrations of lindane within range from 1 to 40 ppm, which demonstrated that the intensity of the absorbance peak at wavelength of about 273 nm was increased as the concentration of lindane increased. Figure 8b shows the UV-Vis spectra of 10 ml of lindane pesticide solution (40 ppm) after treatment with a wide range of concentrations of MSNPs; lindane solution showed an absorbance peak at 273 nm, which demonstrated that the intensity of this peak was slightly decreased with the increase in the amount of the solid adsorbent and reached the saturation at 40 mg of MSNPs after stirring for 6 h at room temperature. This weak removal efficiency of MSNPs is due to the difficulty binding between silica surface and the lindane pesticides. Then, we have used the same doses from Fe3O4 NPs for treating the lindane solution as shown in Fig. 8c, which indicated that the intensity of the absorption peak of lindane was decreased, and this decreasing was higher than that observed by using the same amount of MSNPs. Finally, we have used the same doses from Fe3O4/MSNPs nanocomposite for treating the lindane solution that showed the strongest decrease in the absorption in comparison with MSNPs or Fe3O4 at the same dose (Fig. 8d). Figure 8e shows the relationship between the removal efficiency and the amount of adsorbent, which indicated the removal percentage of lindane by using the same range of amounts of MSNPs, Fe3O4 or Fe3O4/MSNPs nanocomposite, which demonstrated that for the treatment of the lindane solution with MSNPs, the maximum removal percentage reached up to 7.5% by using 40 mg of the solid adsorbent; moreover, there is no further effect by increasing the amount of adsorbent over 40 mg. But in case of the usage of Fe3O4 for the treatment, the maximum removal percentage reached up to 30% by using 60 mg. On the other hand, usage of Fe3O4/MSNPs nanocomposite as solid adsorbent for the treatment of lindane solution shows the highest removal percentage that reached 65% by using 70 mg of the nanocomposite, due to the large surface area and the high capability of Fe3O4. So, functionalization of mesoporous silica with Fe3O4 is an important issue for the removal of lindane solution, since the mechanism of removal depends on the interaction between the chlorine atoms of lindane and the Fe3O4 NPs. In addition, the excellent magnetic feature was necessary for magnetic separation of lindane pesticides from wastewater by applying an external magnetic field on MSNPs/Fe3O4 nanocomposite to release by using external magnetic field. Furthermore, HPLC technique was used to confirm removal of lindane by using MSNPs/Fe3O4 nanocomposite. HPLC technique showed that lindane has one sharp peak at retention time of 3.2 min; the decrease in the area of this band intensity and the area under the peak reflects the removal of the lindane. These results shown in Fig. 9a, b demonstrated that by the increase in the addition of MSNPs/Fe3O4 nanocomposite amount to lindane solution, a marked decrease in the peak area and the integration area percentage was observed. From Fig. 9c, it was found that using of 60 mg of MSNPs/Fe3O4 nanocomposite as solid adsorbent results in decreasing the absorbance unit (mAU) from 49 to 14. The effect of sorbent concentrations within range from 0.02 to 0.06 gm on the remove capacity of lindane was investigated by using MSNPs/Fe3O4 nanocomposite. Our results indicated that the amount of adsorbent is an important parameter which affects the ability of a sorbent for a sorbet. Finally, to verify the UV-Vis data for the removal of lindane by using MSNPs/Fe3O4 nanocomposite, we have used GC technique to monitor the lindane removal by using 8 different concentrations of MSNPs/Fe3O4 nanocomposite over a wide range from 10 to 130 mg. Figure 10 shows the GC chromatograms of lindane solution before and after the treatment with different doses of MSNPs/Fe3O4 nanocomposite (10, 30, 50, 70, 90, 110 and 130 mg), which demonstrated that the GC chromatogram of lindane standard solution has one sharp peak at retention time of 2.5 min (Fig. 10a); the band intensity and the area under the peak of lindane solution were decreased as the amount of the solid adsorbent (MSNPs/Fe3O4 nanocomposite) increased (Fig. 10b–h), which reflects the removal of the lindane. Then, we calculated the mole percent of lindane based on the areas under the curves by using Eq. 1, and these data are listed in Table 2, which illustrates that the removal of lindane was increased by increasing the amount of the solid sorbent within range from 10 to 130 mg, and it reached its maximum removal efficiency at 110 mg of MSNPs/Fe3O4 nanocomposite, which is in good agreement with the UV-Vis results. From Eq. (1), GC data indicated that the removal efficiency of the developed solid adsorbent against lindane pesticide was about 99% at 90 mg of MSNPs/Fe3O4 nanocomposite.
a UV spectra of different concentrations of lindane solution over range from 1 to 40 ppm, b UV spectra of removal lindane by using different amounts of MSNPs, c UV spectra of removal lindane by using different amounts of Fe3O4, d UV spectra of removal lindane by using different amounts of Fe3O4/MSNPs nanocomposite and e influence of amount three different solid adsorbents on removal of lindane
Conclusion
Here, we have reported a rapid, single step and completely green synthetic method for Fe3O4 particles. The MSNPs/Fe3O4 nanocomposite has been characterized by SEM, XRD, EDX, FTIR and HRTEM. Particle size analysis of MSNPs/Fe3O4 nanocomposite was 30 nm. MSNPs/Fe3O4 nanocomposite was used in the treatment of water from lindane pesticide as a model of OCP. Removal percentage of this type of pesticides exceeds 99% by using 90 mg of MSNPs/Fe3O4 nanocomposite. This nanocomposite possesses high capability for lindane capture, which could explore a new biocompatible and eco-friendly strategy for pesticide removal, and appears to be the new promising material in water treatment application.
References
Akhtar N, El-Safty SA, Khairy M, El-Said WA (2015) Fabrication of a highly selective nonenzymatic amperometric sensor for hydrogen peroxide based on nickel foam/cytochrome c modified electrode. Sens Actuators B Chem 207:158–166
Bavcon M, Trebse P, Zupancic-Kralj L (2003) Investigations of the determination and transformations of diazinon and malathion under environmental conditions using gas chromatography coupled with a flame ionisation detector. Chemosphere 50:595–601
Bhattacharya D, Rajinder G (2005) Nanotechnology and potential of microorganisms. Crit Rev Biotechnol 25:199–204
Cai W, Wan J (2007) Facile synthesis of superparamagnetic magnetite nanoparticles in liquid polyols. J Colloid Interface Sci 305:366–370
Cataldo F, Ursini O, Angelini G (2013) A green synthesis of colloidal silver nanoparticles and their reaction with ozone. Eur Chem Bull 2:700–705
Chang YC, Kikuchi S, Kawauchi N, Sato T, Takamizawa K (2008) Complete dechlorination of tetrachloroethylene by use of an anaerobic clostridium bifermentans dph-1 and zero-valent iron. Environ Technol 29:381–391
Chen Y, Wang D, Zhu X, Zheng X, Feng L (2012) Long-term Effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environ Sci Technol 46:12452–12458
Dang F, Enomoto N, Hpjo J, Enpuku K (2009) Sonochemical synthesis of monodispersed magnetite nanoparticles by using an ethanol–water mixed solvent. Ultrason Sonochem 16:649–654
El-Ghandoor H, Zidan HM, Khalil MMH, Ismail MIM (2012) Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int J Electrochem Sci 7:5734–5745
El-Said WA, Yea C-H, Jung M, Kim H-C, Choi JW (2010) Analysis of effect of nanoporous alumina substrate coated with polypyrrole nanowire on cell morphology based on AFM topography. Ultramicroscopy 110(6):676–681
El-Said WA, Kim T-H, Yea C-H, Kim HC, Choi JW (2011) Fabrication of gold nanoparticle modified ITO substrate to detect β-amyloid using surface-enhanced raman scattering. J Nanosci Nanotechnol 11:768–772
El-Said WA, Cho HY, Yea CH, Choi JW (2014) Synthesis of metal nanoparticles inside living human cells based on the intracellular formation process. Adv Mater 26(6):910–918
Fouad DM, Mohamed MB (2011) Studies on the photo-catalytic activity of semiconductor nanostructures and their gold core–shell on the photodegradation of malathion. Nanotechnology 22:455705–455713
Fouad DM, El-Said WA, Mohamed MB (2015) Spectroscopic characterisation of magnetic Fe3O4@Au core shell nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc 140:392–397
Fouad DM, El-Said WA, Ali MH, El-Gahami MA (2017) Silica-gold nanocomposite for removal of organophosphorous pesticides. Plasmonics 12(3):869–875
Jain TK, Morales MA, Sahoo SK, LesliePelecky DL, Labhasetwar V (2005) Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm 2(3):194–205
Jin Y, Wu M, Zhao X, (eds) (2005) Toxicity of nanomaterials to living cells. In: Technical proceedings of the 2005 NSTI nanotechnology conference and trade show
Johannsen M, Gneveckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R, Jung K, Jordan A, Wust P, Loening SA (2007) Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperth 23:315–323
Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physico-chemical characterizations and biological applications. Chem Rev 108(6):2064–2110
Lu AH, Li WC, Kiefer A, Schmidt W, Bill E, Fink G, Schuth F (2004) Fabrication of magnetically separable mesostructured silica with an open pore system. J Am Chem Soc 126:8616–8617
Lukman AI, Gong B, Marjo CE, Roessner U, Harris AT (2011) Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. Colloid Interface Sci 353:433–444
Maity D, Chandrasekharan P, Yang CT, Chuang KH, Shuter B, Xue JM, Ding J, Feng SS (2010) Facile synthesis of water-stable magnetite nanoparticles for clinical MRI and magnetic hyperthermia applications. Nanomedicine 5:1571–1584
Modo MMJ, Bulte JWM (2005) Molecular and cellular MR imaging. CRC Press, London
Nunes JS, de Vasconcelos CL, Cabral FAO, de Araújo JH, Pereira MR, Fonseca JLC (2006) Synthesis and characterization of poly (ethyl methacrylate-co-methacrylic acid) magnetic particles via miniemulsion polymerization. Polymer 47:7646–7652
Padil VV, Cerník M (2013) Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomed 8:889–898
Parsons JG, Peralta-Videa JR, Gardea-Torresdey JL (2007) Use of plants in biotechnology: synthesis of metal nanoparticles by inactivated plant tissues, plant extracts, and living plants. Dev Environ Sci 5:463–485
Prabowo B, Veriansyah B, Kim JD (2007) Supercritical water oxidation for the destruction of toxic organic wastewaters: a review. J Environ Sci 19:513–522
Rockenberger J, Scher EC, Alivisatos AP (1999) A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal oxides. J Am Chem Soc 121:11595–11596
Salam HA, Rajiv P, Kamaraj M, Jagadeeswaran P, Gunalan S, Sivaraj R (2012) Plants: green route for nanoparticle synthesis. Int J Biol Sci 1:85–90
Shameli K, Ahmad MB, Zamanian A, Sangpour P, Shabanzadeh P, Abdollahi Y, Zargar M (2012) Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int J Nanomed 7:5603–5610
Shukla G, Kumar A, Bhanti M, Joseph PE (2006) Organochlorine pesticide contamination of ground water in the city of Hyderabad. Environ Int 32:244–247
Simagina V, Likholobov V, Bergeret G, Gimenez MT, Renouprez A (2003) Catalytic hydrodechlorination of hexachlorobenzene on carbon supported Pd-Ni bimetallic catalysts. Appl Catal B 40:293–304
Vishwakarma V, Samal SS, Manoharan N (2010) Safety and risk associated with nanoparticles-a review. J Miner Mater Charact Eng 9(05):455–459
Wang D, Chen Y (2016) Critical review of the influences of nanoparticles on biological wastewater treatment and sludge digestion. Crit Rev Biotechnol 36:816–828
Wang Y, Ma W, Chen C, Hu X, Zhao J, Yu JC (2007) Fe 3+/Fe 2+ cycling promoted by Ta 3 N 5 under visible irradiation in Fenton degradation of organic pollutants. Appl Catal B 75:256–263
Wu P, Zhu J, Xu Z (2004) Template-assisted synthesis of mesoporous magnetic nanocomposite particles. Adv Funct Mater 14:345–351
Yavuz Y, Yurumez Y, Kucuker H, Ela Y, Yuksel S (2007) Two cases of acute endosulfan toxicity. Clin Toxicol (Phila) 45:530–532
Zhang W, Shen F, Hong R (2011) Solvothermal synthesis of magnetic Fe3O4 microparticles via self-assembly of Fe3O4 nanoparticles. Particuology 9:179–186
Acknowledgements
This work was supported by Assiut University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Binbin Huang
Rights and permissions
About this article
Cite this article
El-Said, W.A., Fouad, D.M., Ali, M.H. et al. Green synthesis of magnetic mesoporous silica nanocomposite and its adsorptive performance against organochlorine pesticides. Int. J. Environ. Sci. Technol. 15, 1731–1744 (2018). https://doi.org/10.1007/s13762-017-1530-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1530-9