Abstract
Mesoporous silica nanoparticles–gold nanoparticles (MSNPs/AuNPs) nanocomposite was prepared using mono-dispersed mesoporous silica as a template for gold nanoparticles without any functional group on the silica surface. The growth of gold layer over the surface of mesoporous silica nanoparticles (MSNPs) was demonstrated via scanning electron microscopies (SEM), Fourier transform infrared (FTIR) spectra, high-resolution transmission electron microscopy (HRTEM), and ultraviolet-visible (UV) adsorption spectrum. The presence of gold on the mesoporous nanoparticles gave an absorption band at 520 nm, also X-ray diffraction (XRD) indicated the crystal structure of gold NPs which has a cubic shape. Raman spectra proofed the high ability of MSNPs/AuNPs nanocomposite for removing methacrifos from water which was also confirmed by using high-performance liquid chromatography (HPLC) at room temperature where the removal rate exceeded 98 %. MSNPs/AuNPs nanocomposite has high capacity as an adsorbent which could be considered as a new eco-friendly strategy for pesticide removal and appears to be the new promising material in water treatment application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development of new metal nanoparticles has become a demanding task in different fields of research owing to their controlled and unique properties, which are very different from the behavior of the respective size material [1–4]. Gold nanoparticles (AuNPs) have attracted great attention because of their bio-sensing electronic, catalytic, photo-chemical pesticide removal, bio-medical, and surface-enhanced Raman scattering (SERS) properties [5]. Combining the properties of AuNPs with mesoporous silica nanoparticles (MSNPs) materials leads to the formation of advanced nanocomposite with superior adsorption capacity, since the MSNPs have high Brunner–Emmett–Teller (BET) specific surface area [6]. They are also considered as organophilic solids due to their ability to absorb significant quantities of organic molecules in gas phase, such as n-hexane, benzene, toluene, as well as various alcohols [7, 8]. MSNPs/AuNPs nanocomposite has rapid development in bio-medical fields such as the drug releasing, the cancer treating, biological sensor, imaging, and also in removal pesticide.
Pesticides are considered as an environmental pollution. Organophosphorous pesticide is one of the classes which are actually more widely used in the world [9], which affect the nervous system of insects and humans [10]. These chemical agents inhibit the activity of the enzyme cholinesterase (ChE), responsible for the nervous impulse in organisms [11]. Thus, it is important to prevent the increase of these compounds into the environment. Several methods are applied for removing organophosphorous pesticides from water including membrane technology (microfiltration, ultra filtration, nanofiltration and reverse osmosis), solid phase extraction, ozone and chemical oxidation, Fenton, photocatalysis, and adsorption on various substances such as activated carbon [12–16], but in some instances, the need of high energy, high costs of regeneration and the need to renew the adsorbent and decomposition of pesticides result in transformation products that are more toxic and are the disadvantages of these methods since they represent a greater risk to the environment than the parent molecule [17, 18]. Among the different techniques for the removal of organophosphorous pesticides, the adsorption process by using solid adsorbents has many advantages over the other methods due to its simple design, ease of using, low cost, and ability for recycling. Our focus is to develop adsorbent materials with high efficiency and economic feasibility to remove these pollutants to be able to improve the water quality.
In this study, we report a new method for the production of silica–gold nanoparticles for the removal of methacrifos (methyl (E)-3-dimethoxyphosphinothioyloxy-2-methylprop-2-enoate) pesticides selected as a model organophosphorous pollutant. AuNPs have rapid detection of organophosphorous pesticides up to 0.5 ppm. MSNPs/AuNPs nanocomposite has high capacity as an adsorbent which resulted in rapid detection of methacrifos up to 0.5 ppm which presents a new bio-compatible and eco-friendly strategy for pesticide removal and appears to be a new promising material in water treatment application and fast, non-toxic, and single-step method. MSNPs/AuNPs nanocomposite has high ability for the removal of organophosphorous pesticides up to 90 % which was indicated using high-performance liquid chromatography (HPLC) chromatogram.
Materials
TEOS (Tetra ortho silicates (≥99.9 % Sigma-Aldrich)), CTAB cetyltrimethylammonium bromide (≥98 % Sigma-Aldrich), HAuCl4 gold chloride (≥99.9 % Sigma-Aldrich), and ascorbic acid used were from Sigma-Aldrich; NH4OH ammonia solution 32 % was from Sigma-Aldrich, ethanol absolute used was from Adwic, and standard pesticides such as methacrifos used were from Sigma-Aldrich.
Experimental
Preparation of MSNPs
The MSNPs were prepared by using CTAB surfactant as the structure-directing agent. Twenty-four milliliters of deionized water (DIW), 9.6 g of 0.5 M NaOH, and 1.0 g of CTAB were added in 100-ml beaker under vigorous stirring at room temperature. Two milliliters of TEOS as the silicon source was dropwise added and stirred continuously for 8 h. The product material was filtered off, washed for three times with DIW and ethanol, and then dried overnight at 60 °C. In order to remove CTAB, the final products were calcined at 550 °C for 5 h [19].
Synthesis of Mesoporous Silica-AuNPs nanocomposite
0.5 g of the prepared mesoporous silica materials was mixed with 50 ml of deionized and stirred vigorously at room temperature for 1 h. Ten microliters of gold chloride was added until white MSNPs particles showed a golden color during stirring; 50 ml of (1 mmol) ascorbic acid was added for reducing gold on the surface of MSNPs particles where the color of the solution changed into wine red. After stirring for 2 h, the solution was filtrated and washed with deionized water for three times. The mixture was dried overnight at 200 °C for 3 h.
Instrumentation
The crystal structure, and morphologies of silica NPs and MSNPs/AuNPs nanocomposite were characterized by high-resolution transmission electron microscopy (HRTEM) analysis using JEOL 2000 operated at high voltage 120 kV.
X-ray diffraction (XRD) analysis of test samples was performed with a Philips (The Netherlands) diffractometer (Model PW 2103, λ = 1.5418 A°, 35 kV, and 20 mA) with a source of Cu Kα radiation (Ni filtered). The diffraction patterns were recorded in the range of the diffraction angle 2θ from 10° to 90° with a step of 0.06°. Fourier transform infrared (FTIR) spectra of MSNPS/Fe3O4 were measured using a Nicolet™ iS™10 FTIR spectrometer in the wave number range 400–4000 cm−1. The samples have been mixed with KBr under a vacuum and at pressure of 1.88 t/cm2. Nitrogen adsorption was recorded for mesoporous silica using a model NOVA 3200 automated gas sorption system (Quantachrome, USA). Ultraviolet-visible (UV-VIS) spectroscopy double beam PC scanning spectrophotometer UV Evolution 300 from Labomed a computer data system is the UV Win 5 software v 5.0.5 used for measuring the wavelength and absorbance. Spectrophotometer range 200–900 nm using 1-cm matched Stoppard quartz cells was used for following the pesticide removal. HPLC samples were analyzed using Agilent 1100 HPLC system fitted with a diode array detector and an autosampler. Raman spectra were recorded with a Bruker Senterra Raman microscope (Bruker Optics Inc., Germany) with 785-nm excitation, 1200 rulings per millimeter holographic grating, and a charge-coupled device (CCD) detector. The acquisition time was 3 s with the power of 50 mW.
Results and Discussion
Characterizations of MSNPs/AuNPs Nanocomposite
The structural properties and morphology of MSNPs/AuNPs nanocomposite were characterized using different tools:
-
N2 adsorption of MSNPs/AuNPs nanocomposite
The synthesis of MSNPs/AuNPs nanocomposite is based on deposition of Au onto MSNPs. Loading of Au molecules onto the surface of mesoporous silica results in decreasing the BET surface area to become 412 m2/g as showed in Table 1.
-
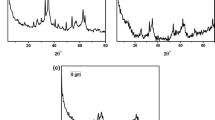
X-ray diffraction
The powder XRD patterns (Fig. 1) were used to study the structural properties of the synthesized MSNPs/AuNPs nanocomposite, as shown in Fig. 1a; one intense diffraction peak at 2θ = 22 is found, which corresponds to 100 indicating the formation of ordered mesostructure. The XRD pattern of gold nanoparticles showed in Fig. 1b which demonstrated characteristic reflections of face-centered cubic (fcc) gold planes. The diffraction features appearing 2θ = 38.20°, 44.41°, 64.54°, and 77.48° corresponds to the (111), (200), (220), and (311), respectively. Therefore, the XRD result indicates gold nanoparticles with a cubic shape.
The XRD pattern suggests the formation of crystalline AuNPs with fcc structure. Broadening of XRD peaks clearly indicates that the samples are nanocrystalline in nature.
-
Fourier transform infrared (FTIR) spectra
FTIR spectrum of MSNPs and MSNPs/AuNPs nanocomposite shown in Fig. 2 indicates that the Au molecules were deposited onto silica NPs, from Fig. 2(A). The broad peak at 1081.85 cm−1 can be assigned to Si–O–Si bond, and the peak at 976.05 cm−1 can be attributed to Si–OH bond Also, broad peak at 3269.12 cm−1 can be assigned to NH bond. In the spectrum of the MSNPs/AuNPs nanocomposite, the intensity of Si–O–Si and Si––OH peaks has been reduced significantly. This indicates the presence of AuNPs in silica particle as shown in Fig. 2(B).
-
UV-VIS spectroscopy
AuNPs were found to be attached to mesoporous silica. It could be observed that the originally white particles of silica turned into wine red color. Optical absorption was recorded using UV-VIS spectroscopy. Optical absorption of MSNPs/AuNPs nanocomposite was found at 520 nm as shown in Fig. 3.
-
Scanning electron microscope (SEM) and energy diffusive X-ray (EDX) of MSNPs/AuNPs nanocomposites
EDX spectrum recorded a strong signal for the silica and gold atoms as shown in Fig. 4a. The elemental analysis measurements show that the MSNPs/AuNPs nanocomposite composed of metallic Au and Si and some other contaminants is also present. SEM images of MSNPs/AuNPs refer to the aggregation of the gold nanoparticles, and small grains are present at the surface of the silica as shown in Fig. 4b.
-
High-resolution transmission electron microscopy (HRTEM)
HRTEM images of AuNPs/MSNPs nanocomposite (Fig. 5) showed a cubic shape of gold nanoparticles, and it is clearly seen that gold nanoparticles have been attached to the surface of silica.
Removal Organophosphorous Pesticides Using MSNPs/AuNPs Nanocomposite
Continues monitoring of the pesticides represents an environmental challenge due to the stability and long resistance time in the environment, therefore, developing simple, ease of using, and low cost with high ability for recycling adsorption process by using solid adsorbents.
Our first attempt is to study the ability of gold nanoparticles to detect and remove methacrifos. AuNPs were mixed with various concentrations of pesticide (40–0.05 ppm). A change in the color of the mixture of AuNPs and methacrifos was observed. Changing the color was highly dependent on methacrifos concentration. The high concentration (40 ppm) of methacrifos changed its color dramatically from red to blue as seen in Fig. 6c, which could be attributed to the surface plasmon phenomena [19, 20]. Surface plasmon has the ability to confine (trap) light at a metal/dielectric interface, which either localizes or propagates depending upon the dimensionality of the nanostructured material [21]. The extent of color change of low concentration (0.05 ppm) was much less compared to high concentration. As the pesticide concentration increased, the absorbance at 522 nm decreased as shown in Fig. 6a, b. For larger particles, the light cannot polarize the nanoparticles homogeneously, and as a result, the optical absorption spectra are directly related to the size of the nanoparticles which is known as the extrinsic size effect [22]. The adsorption of methacrifos by using MSNPs/AuNPs nanocomposite as a solid adsorbent was confirmed by the Raman spectra. As shown in Fig. 7, parent MSNPs and AuNPs exhibit Raman peaks at 1360 and 1578 cm−1, respectively; MSNPs/AuNPs nanocomposite was treated with 10 ppm methacrifos solution, and a new peak at 248 cm−1 is noticed that may be assigned to AuNPs-S interaction, possibly in the form of an AuNPs-S complex [23, 24].
HPLC technique was used to confirm the removal of methacrifos by using MSNPs/AuNPs nanocomposite. HPLC technique showed that methacrifos has one sharp peak at retention time 9.5 min; the results shown in Fig. 8 illustrate that by increasing the amount of nanocomposite (20–60 mg) that are added to methacrifos, there is a marked decrease in the peak area and the integration area percentage, and the decrease in the peak area and height confirms the complete removal of the methacrifos solution due to its binding to gold nanoparticles as presented in Fig. 8f.
The sorbet concentration of methacrifos into sorbents was calculated by the difference in the detector response absorbance before (A i) and after (A t) removal.
Equation (1) indicated that the removal efficiency of the developed solid adsorbent against methacrifos pesticide exceeds 98 %.
Conclusion
Our study presents new method for the preparation of MSNPs/AuNPs nanocomposite which has high capacity as an adsorbent which could explore a new bio-compatible and eco-friendly strategy for pesticide removal and appears to be a new promising material in water treatment application. Raman spectra and HPLC at room temperature (RT) proofed the ability of MSNPs/AuNPs nanocomposite for removing methacrifos where the removal rate exceeded 98 %.
References
Costi R, Saunders A E, Banin U (2010) Colloidal hybrid nanostructures: a new type of functional materials. Angew Chem Int Ed Engl 49:4878
Fouad DM, Said WA, Mohamed MB (2015) Spectroscopic characterization of magnetic Fe3O4@Au core shell nanoparticles. Spectrochim Acta A 140:392–397
Fouad DM, Mohamed MB (2011) Studies on the photo-catalytic activity of semiconductor nanostructures and their gold core–shell on the photodegradation of malathion. Nanotechnology 22:455–705
El-Said WA, Fouad DM, El-Safty SA (2016) Ultrasensitive label-free detection of cardiac biomarker myoglobinbased on surface-enhanced Raman spectroscopy. Sensors Actuators B 228:401–409
Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ (2008) Biological applications of gold nanoparticle. Chem Soc Rev 9:37
Ng EP, Mintova S (2008) Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Micropor Mesopor Mater 114:1–26
Zhao XS, GQ L, Hu X (2001) Organophilicity of MCM-41 adsorbents studied by adsorption and temperature-programmed desorption. Colloids Surf A Physicochem Eng Asp 179:261–269
Mangrulkar PA, Kamble SP, Meshram J, Rayalu SS (2008) Adsorption of phenol and o-chlorophenol by mesoporous MCM-41. J Hazard Mater 160:414–421
Colosio C, Tiramani M, Brambilla G, Colombi A, Moretto A (2009) Neurobehavioural effects of pesticides with special focus on organophosphorus compounds: which is the real size of the problem. Neurotoxicology 30:1155–1161
Jokanovic M, Prostran M (2009) Pyridinium oximes as cholinesterase reactivators. structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Curr Med Chem 16:2177–2188
Yair S, Ofer B, Arik E, Shai S, Yossi R, Tzvika D, Amir K (2008) Organophosphate degrading microorganisms and enzymes as biocatalysts in environmental and personal decontamination. Applied Critical Review in Biotechnology 28:265–275
Nyazi K, Baçaoui A, Yaacoubi A, Darmstadt H, Adnot A, Roy C (2005) Influence of carbon black surface chemistry on the adsorption of model herbicides from aqueous solution. Carbon 43:2215–2234
Aksu Z, Kabasakal E (2004) Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep Puri Technol 35:223–240
Chen SS, Taylor JS, Mulford LA, Norris CD (2004) Influences of molecular weight, molecular size, flux, and recovery for aromatic pesticide removal by Nanofiltration membranes. Desalination 160:103–111
Gupta VK, Ali I, Suhas, Saini VK (2006) Removal of 2,4-D and carbofuran pesticides using fertilizer and steel industry wastes. J Colloid Interf Sci 299:556–563
Chingombe P, Saha B, Wakeman RJ (2006) Effect of surface modification of an engineered activated carbon on the sorption of 2,4-dichlorophenoxy acetic acid and benazolin from water. J Colloid, Interf Sci 297:434–442
Bavcon M, Trebse P, Zupancic-Kralj L (2003) Investigations of the determination and transformations of diazinon and malathion under environmental conditions using gas chromatography coupled with a flame ionisation detector. Chemosphere 50:595
Pozo O, Pitarch E, Sancho JV, Hernandez FJ (2001) Determination of herbicide 4-chloro-2-methylphenoxyacetic acid and its main metabolite, 4-chloro-2-methylphenol in water and soil by liquid chromatography-electrospray tandem mass spectrometry. Chromatogr A 75:923
El-Safty SA, Mekawy M, Yamaguchi A, Shahat A, Ogawa K, Teramae N (2010) Organic-inorganic meso porous silica nanostrands for ultrafine filtration of spherical nanopartiles. Chem Commun 46:3917–3919
Fouad DM, Mohamed MB (2013) Malathion-induced surface coupling with gold nanoparticles. Plasmonics 8:937–941
El-Sayed MA (2001) Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res 34:257–264
Kreibig U, Vollmer M (1995) Optical properties of metal clusters. Springer, New York
Liu B, Han G, Zhang Z, Liu R, Jiang C, Wang S, Han MY (2012) Shell thickness-dependent Raman enhancement for rapid identification and detection of pesticide residues at fruit peels. Anal Chem 84:255–261
Sanchez Cortes S, Domingo C, Garcra-Ramos JV, Aznarez JA (2001) Surface-enhanced vibrational study (SEIR and SERS) of dithiocarbamate pesticides on gold films. Langmuir 17:1157–1162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouad, D.M., El-Said, W.A., Ali, M.H. et al. Silica–Gold Nanocomposite for Removal of Organophosphorous Pesticides. Plasmonics 12, 869–875 (2017). https://doi.org/10.1007/s11468-016-0338-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-016-0338-7