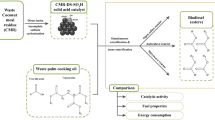
Abstract
The need for renewable environmentally friendly energy resources is growing every day. Biodiesel is one of the most promising alternatives to the conventional non-renewable energy resources. Heterogeneous catalysts proved a high efficiency in the transesterification of oils to produce biodiesel. In this research, activated carbon was tested as a heterogeneous catalyst in the transesterification of two non-edible oils (waste cooking oil and Jatropha oil) with methanol to produce biodiesel. Activated carbon was characterized using X-ray diffraction, scanning electron microscope and Fourier transformed infrared. The effect of different operating parameters, namely operation time (30, 60, 120 and 180 min), alcohol-to-oil molar ratio (4:1, 6:1, 8:1 and 10:1), catalyst loading [0.5, 1, 2, 3 and 5% (w/w)] and rotational speed (100, 200, 300 and 400 rpm), was investigated. Results showed that increasing the operational time, the alcohol-to-oil molar ratio and the catalyst loading increases the conversion to biodiesel but only to some extent; increasing the stirring rate was found to be beneficial to the process. The optimum conditions were found to be 2 h of heating, 6:1 alcohol-to-oil ratio, 1 wt% catalyst loading and 400 rpm stirring. Under optimum conditions, the conversion to biodiesel reached 93.95 and 93.27% for the waste cooking oil and the Jatropha oil, respectively. The properties of the obtained biodiesel (density, viscosity, flash point, pour point and cloud point) were measured giving promising results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing rates of population in today’s world impose a similar increase in the global energy demand. The fuel consumption almost doubled in 32 years; in 1980, it was 6630 million tons of oil equivalents (Mtoe), and in 2012 it became 12,239 Mtoe. The International Energy Agency is expecting the global energy demand to increase by 53% by 2030 (Heikal et al. 2013).
Today’s world’s energy is mainly produced by fossil fuels which account for 81% of the total energy obtained. Relying on this energy source has to be diminished; these fuels are non-renewable (Torres-Rodríguez et al. 2016). Moreover, fossil fuels combustion is the main participant in the increased level of CO2 emission in the atmosphere, leading to global warming; more than 15 billion tons of CO2 are released annually in the earth’s atmosphere (Kamm et al. 2016; Yusuf and Kamarudin 2013), and the gas emissions from fossil fuels are estimated to increase by 39% by 2030. These are the main reasons for the development of renewable clean alternative fuels which are domestically available, environmentally friendly and can be practically produced became a universal goal (Endalew et al. 2011).
Biodiesel seems as one of the best choices among other sources due to its environment-friendly behavior and similar functional properties with diesel (Mofijur et al. 2013). Biodiesel possesses many advantages over the petroleum-based diesel which made it an interesting subject for many diverse researches; it has low sulfur content, it emits 94% less carcinogenic agents than those in diesel emissions (Luque 2010; Luque et al. 2010), it has higher combustion efficiency and cetane number than diesel fuel (Demirbas 1998), it biodegrades faster and more than 90% biodiesel can be biodegraded within 21 days (Mudge and Pereira 1999; Speidel et al. 2000). Biodiesels have high flash points and may be blended with diesel fuels due to their similar properties (Dharma et al. 2016; Torres-Rodríguez et al. 2016). Furthermore, it reduces most exhaust emissions except NOx, such as monoxide, unburned hydrocarbons, and particulate matters (Canakci et al. 2006; Knothe et al. 2006; Lin et al. 2009; Lopez et al. 2009; Zhang et al. 2003). It is easy to handle, transport and store (Luque 2010; Luque et al. 2010). It can be used directly in unaltered commercial diesel engines (Lee et al. 2014).
American Society for Testing and Materials (ASTM) defines biodiesel as a monoalkyl ester of fatty acids or fatty acid (m)ethyl ester derived from renewable feedstocks, such as vegetable oils or animal fats (Yaakob et al. 2013; Yusuf et al. 2011). To produce biodiesel from vegetable oils, several methods can be used: pyrolysis, micro-emulsification, blending and transesterification (Demirbas 2009; Yusuf et al. 2011). Transesterification is the most commonly used, and it involves the catalyzed reaction between an alcohol and a vegetable oil to produce biodiesel and glycerol (Lam and Lee 2011). The biodiesel produced using this method has a lower viscosity which prevents incomplete combustion and poor atomization and consequently prohibits engine deterioration.
Several types of vegetable oils can be used in the production of biodiesel, such as sunflower oil, rapeseed oil, palm oil, coconut oil, peanut oil and soybean oil (Campanelli et al. 2010; Dizge et al. 2009; Issariyakul and Dalai 2014; Tan et al. 2011; Zhang et al. 2003). However, many non-governmental organizations criticized the use of edible oils in the production of biofuels regarding the hunger and starvation millions of people are facing around the world (Ashraful et al. 2014). This gave rise to a new trend of using non-edible oils as feedstock in the biodiesel production such as jojoba oil, linseed oil, tobacco oil, neem oil, Jatropha oil (Ashraful et al. 2014) and also waste cooking oil. This study focuses on the use of Jatropha oil and waste cooking oil as feedstock in the transesterification to produce biodiesel.
Jatropha curcas non-edible oil seems to be a suitable feedstock for the biodiesel production because the Jatropha tree can tolerate different hard conditions and grows in lands that are unfavorable for other plants to grow in (Basir et al. 2015). The seeds contain around 35–40% of the J. curcas oil, while the kernels contain from 50 to 60% of the oil. The percentage of the saturated and unsaturated fatty acids in the oil are approximately 21 and 79%, respectively (Takase et al. 2015). The composition of the Jatropha oil is reported to be 44.5% of oleic acid, 35.4% of linoleic acid, 13% of palmitic acid and 5.8% of stearic acid.
Waste cooking oil is another non-edible feedstock for the biodiesel production through transesterification. The waste cooking oil costs less than the fresh vegetable oil by two or three times which reduces the total production cost and makes it an economic option. The rapidly increasing human population along with augmented food consumption contributed in producing enormous amounts of waste cooking oil in houses and restaurants (Phan and Phan 2008); the disposal of these large amounts through drainage or landfill may cause water and soil pollution and disturbance of aquatic ecosystems (Van Kasteren and Nisworo 2007). This renders the use of waste cooking oil as feedstock for biodiesel production a sustainable choice.
Homogeneous and heterogeneous catalyses are used in the biodiesel production. However, recent researches focused on the heterogeneous catalysts for the benefits they have over the homogeneous ones including the ease of separation, the elimination of crude ester washing step, the environmental safety, the prevention of saponification and hydrolysis reactions in case of using waste cooking oil, the lower energy consumption, the lower catalyst requirements per ton of biodiesel produced and the insensitivity to acidity value (Agarwal et al. 2012; Berchmans and Hirata 2008; de Araújo et al. 2013; Meng et al. 2008; Torres-Rodríguez et al. 2016; Yaakob et al. 2013). Also, nanocatalysts and nano-based catalysts being able to display the benefits of both homogenous and heterogeneous catalysts, namely high efficiency and selectivity, stability, easy recovery and recycling could find their role in biodiesel production (Gardy et al. 2016; Mahto et al. 2016; Sano et al. 2017) as they did in many other applications including water purification (Santos et al. 2015; Yeom and Kim 2016), fuel cells (Jameel et al. 2016), photocatalytic degradation of dyes and organic pollutants (Narayanan and Stephen 2013; Saleh and Gupta 2011, 2012a, b; Saravanan et al. 2011, 2013a, b) and wastewater treatment (Gupta et al. 2011, 2012; Saleh et al. 2011; Saleh and Gupta 2012a, b; Saravanan et al. 2013a, b, 2015).
This study aims to test the use of activated carbon as a heterogeneous catalyst to produce biodiesel through transesterification starting by J. curcas oil and waste cooking oil as feedstock and to evaluate the effect of different operating parameters.
Materials and methods
Materials
Jatropha seeds were supplied by Agriculture Researches Station (Ismailia, Egypt), mechanically pressed to obtain the oil, liquid at room temperature with a clear brown color. The waste cooking oil was collected from domestic home waste cooking vegetable oil. This oil was used for 2–4 times at 120–130 °C. All used chemicals were AR grade. The sulfuric acid was purchased from ADWIC, the methanol and potassium hydroxide from Sigma and Aldrich Company, the activated charcoal from Nouresh’shark Co., phenolphthalein from RANKEM company and diethyl ether from SDFCL company.
Apparatus
The esterification–transesterification reactions were carried out in a bench-scale setup consisting mainly of three-neck round-bottom flask placed in an adjusted temperature water bath. This flask is provided with reflux condenser, magnetic stirrer and funnel for methyl alcohol addition.
Methods
Oil analysis
Titration against 0.1 N KOH was used to determine the free fatty acid percentage of the two oil types used as given in Table 1.
Catalyst characterization
The activated carbon was characterized using Fourier transform infrared spectroscopy, X-ray diffraction and scanning electron microscopy.
Fourier transform infrared spectroscopy (FTIR) was recorded to identify the chemical bonds and the functional groups such as oxygen containing groups, carbonyl, hydroxyl and carboxylic groups, and FTIR was carried out using Shimadzu FTIR-8400 S, Japan. About 2–3 mg of sample was mixed with 100 mg of KBr and grinded to uniform particle size and then pressed as KBr pellet using hydraulic press. Measurements were taken in wave number range of 4000–350 cm−1, with 4 cm−1 resolution (Fig. 1).
X-ray diffraction (XRD) patterns were taken to figure out the structure of the catalyst whether crystalline or amorphous and to determine the crystals size, and the patterns were recorded with X-ray diffractometer (XRD Schimadzu-7000, Japan), using CuKα radiation (λ = 1.5418 Å) and step-scan mode (2θ range 5–100°, step time: 0.50 s, step width: 0.1°) (Fig. 2).
Scanning electron microscopy (SEM) analysis was performed using a scanning electron microscope (JEOL JSM 6360LA, Japan) at accelerating voltage 20 kV, to show the surface morphology and the major features of the physical structure of the catalyst at room temperature.
Esterification
Hundred milliliters oil was weighed and fed to the three-neck round-bottom flask in the bench-scale system, and then, alcohol was added and stirred for few minutes. The catalyst (sulfuric acid) was added with continuous stirring. At the end of the reaction, the oil was taken from the reactor in a separating funnel. The oil was washed with 150 ml water to stop the reaction and to separate the alcohol from the oil phase.
Transesterification
Thirty milliliters of the oil layer was taken to the bench-scale system, and then, alcohol and catalyst were added with continuous stirring. At the end of the reaction, the oil was taken from the reactor to separate the catalyst by filtration using filter paper. The oil was poured in a separating funnel. Two layers formed, the upper layer was the biodiesel and the lower layer was glycerol. For the Jatropha oil and waste cooking oil, the effects of operation time, alcohol-to-oil ratio, catalyst loading and rotational speed were investigated.
Purification of biodiesel
After transesterification, the oil layer may contain traces of the catalyst, methanol and residual glycerol. These impurities were removed by washing using hot water and washing the oil with 50 °C distilled water for 2–3 times until the water layer becomes clear.
Gentle washing by using warm water prevents precipitation of saturated fatty acid esters and retards the formation of emulsions and also leads to rapid and complete phase separation.
Drying of the product
After the completion of the purification process, the oil layer may contain some water and methanol. This should be removed before the final use of biodiesel. Methanol reduces the flash point of fuel, and it has corrosive nature to fuel hoses. Water content is responsible for the growth of biological organisms, and it also increases the acid value of fuel. Hence, the oil layer was heated in water bath with shaking speed of 150 rpm, at 100 °C for 15–30 min to remove the water and methanol content present in the product (biodiesel). Finally the dried biodiesel can be stored.
Biodiesel analysis
The biodiesel yield was determined using a gas chromatograph (GC). The used device was HP (Hewlett Packard) 6890 GC (Agilent Technologies/Hewlett Packard Company, USA). The carrier gas was nitrogen, with flow 1 ml/min, using FID (flame ionization detector) at temperature 250 °C. The injector temperature was 220 °C, injection volume 2 µl, splitless mode. The temperature program of the GC was as follows: 2 min isothermal at 150, 150–200 °C with 10 °C/min, 9 min held at 200, 200–250 °C with 5 °C/min. The used column was HP-5 (5% diphenyl, 95% dimethyl polysiloxane), 30 m, 0.32 mm ID and 0.25 µm film thickness at temperature 200–250 °C with 5 °C/min. The GC% was calculated from Eq. (1)
Biodiesel evaluation
To assess the quality of the produced biodiesel and compare it to the universal standards, five of the main properties of biodiesel were measured:
-
1.
Density: The density was measured using a density meter (KEM/DA-640) provided by Kyoto Electronics MFG CO., LTD, the compartment cell of the density meter is cleaned, dried, the sampling is made and once the oscillation frequency becomes stable, the measurement comes to an end and the density is shown.
-
2.
Viscosity: A viscometer bath (KV6) from Stanhope-Seta Co. was used to measure the viscosity (according to the ASTM D445-03 method); in this test, the time is measured for a fixed volume of liquid to flow under gravity through the capillary of a calibrated viscometer under a reproducible driving head and at a closely controlled and known temperature. The kinematic viscosity (determined value) is the product of the measured flow time and the calibration constant of the viscometer. Two acceptable determined values are needed for the calculations (the dynamic viscosity is the product of the kinematic viscosity and the density).
-
3.
Flash point: The flash point was determined by the Normalab half automated cleveland flash point (NCL-120), according to the ASTM D 93-02a method. In this test, a brass cup of specified dimensions, filled to the inside mark with test specimen and fitted with a cover of specified dimensions, is heated and the specimen stirred at specified rates. An ignition source is directed into the test cup at regular intervals with simultaneous interruption of the stirring until a flash is detected.
-
4.
Pour point: It was measured using the compact cloud and pour point cryostat (94100-3) from Stanhope-Seta Co. (according to the ASTM D 97-02 method). After preliminary heating, the sample is cooled at a specified rate and examined at intervals of 3 °C for flow characteristics. The lowest temperature at which movement of the specimen is observed is recorded as the pour point.
-
5.
Cloud point: The Seta compact cloud and pour point cryostat (94100-3) from Stanhope-Seta Co. was used to measure the cloud point, according to the ASTM D2500-02 method, where the specimen is cooled at a specified rate and examined periodically. The temperature at which a cloud is first observed at the bottom of the test jar is recorded as the cloud point.
Results and discussion
Catalyst characterization
Figure 1 shows FTIR spectra of the activated carbon which was recorded in the range from 350 to 4000 cm−1.Characteristic of various groups is observed as follows:
The band at wavenumber 3433.41 cm−1 is attributed to O–H stretching in hydroxyl functional groups. Band at wavenumbers 2347.45 is corresponding to C–H bonds. In addition, band at 2206.6 cm−1 corresponds to C≡C group. The weak and broad band at 1533.46 cm−1 corresponds to the C=C bonds, while the band at 1176.62 corresponds to C–O bond (Saleh 2011; Saleh et al. 2014).
Figure 2 shows the XRD analysis performed by passing X-rays through the material and registering the diffraction (scattering) image of the rays. The XRD pattern shows a broad beak with low angle at 2θ–8 which contributes to the cellulosic material. This low angle indicated the presence of mesoporous structure in the substance. This refers to that the particles are ordered in the preferred orientation to give very thin peaks beside each other. Also, another broad peak at 2θ–25° can be indexed as the C(0 0 2) reflection of the hexagonal graphite structure. The other characteristic diffraction peaks of graphite at 2θ of about 45.6° and 88.38° are associated with C(1 0 0) and C(1 1 0) diffractions of graphite, respectively (Saleh 2011).
Figure 3a–c shows a typical morphology for the activated carbon studied by SEM with three levels of magnification. The figures show that the activated carbon particles are irregular in shape with well-defined particles. The image reveals an irregular amorphous morphology, and some cracks are observed on the external surface as well as some aggregates. Different size pores are also detected, and they provide the high surface area required.
Effect of different operating conditions on the biodiesel yield
At the end of each run, the volumetric biodiesel yield was calculated from Eqs. (2) to (3) and was used to calculate the actual biodiesel yield
Effect of time
Contact time is an important factor that affects the catalyzed transesterification reaction. To study the effect of time on the conversion to biodiesel, the runs duration ranged from 30 to 180 min. The reaction was carried out at a constant temperature of 60 ± 1 °C, stirring speed of 400 rpm, 1% w/w of heterogeneous catalyst loading and 6:1 methanol-to-oil ratio.
Figure 4 shows that the rate of conversion of the fatty acid esters was slow at the beginning of the reaction, which may be attributed to the mixing and dispersion of alcohol into the oil and the slow mass transfer, and then, after about 60 min, the rate is obviously increased and reaches a maximum after around 120 min, when enough time was available for the adsorbent and adsorbate to be in contact. The biodiesel yield in case of Jatropha oil attained a maximum of 93.27%, while in case of waste cooking oil it attained 93.95%.
With further increase in the reaction time the yield decreases, this is probably caused by the reversed reaction of the transesterification, leading to esters loss and more soap formation by fatty acids. These observations are matching with findings from previous studies (Eevera et al. 2009; Freedman et al. 1984; Hawash et al. 2011; Leung et al. 2010; Ma et al. 1998).
Effect of methanol-to-oil molar ratio
Figure 5 depicts the effect of methanol-to-oil molar ratio. Four molar ratios were used: 4:1, 6:1, 8:1 and 10:1; The reaction was carried out at a constant temperature of 60 ± 1 °C, stirring speed of 400 rpm and 1% w/w of catalyst for 120 min. The yield, as shown, increases steadily until arriving at a maximum value at a ratio of 6:1. Further increasing in the molar ratio reverses the yield behavior and causes continuous yield decrease after reaching the maximum value. This can be explained as follows: The transesterification reaction goes through a sequence of intermediate reversible reactions to be completed; the triglyceride is first converted to diglyceride, then to monoglyceride and finally to glycerol and methyl esters. To achieve reaction completion the stoichiometric ratio of methanol to oil is 3:1, practically a higher ratio is used to shift the reaction equilibrium toward the forward direction and increases the production of methyl esters and glycerol (Qian et al. 2010). However, using too much alcohol has two unfavorable effects: (1) It increases the polarity of the reaction medium, increasing in turn the solubility of glycerol in the ester phase and shifting the reaction equilibrium toward the reversed direction and hence decreasing the yield of methyl ester (Issariyakul and Dalai 2014), and (2) it can flood the active sites of the catalyst impeding the formation of glycerol and methyl ester. Similar results concerning the behavior of the molar ratio increase were deduced in previous studies (Phan and Phan 2008; Shu et al. 2010). It was found that, in general, the alcohol-to-oil ratio depends on the catalyst used; in case of acid catalyst, the molar ratio ranged from 30:1 to 150:1, while with alkali catalysts lower ratios were needed ranging from 6:1 to 15:1 (Qian et al. 2010).
Effect of catalyst loading
The amount of catalyst added is an important factor influencing the conversion to biodiesel. To study the effect of the catalyst loading, five runs, for each type of oil, were performed with catalyst amount ranging from 0.5 to 5% (w/w) based on the oil weight, with methanol-to-oil molar ratio of 6:1, at 60 ± 1 °C, stirring velocity of 400 rpm and each run lasted for 120 min.
Figure 6 shows that biodiesel yield increased with increasing the catalyst amount from 0.5 to 1% (w/w) to achieve a maximum of 93.27% in case of Jatropha oil and 93.95% in case of waste cooking oil. This is can be attributed to the fact that an increase in adsorbent dosage increases the number of active sites available for adsorption (Al-Saadi et al. 2013). However, with catalyst loading range from 1 to 3% (w/w), the yield decreases sharply and then remains almost constant from 3 to 5% (w/w). Previous studies concerning heterogeneous catalysis concluded that the limiting step in the transesterification of vegetable oils is the liquid–solid mass transfer or the surface reaction (Dossin et al. 2006; Gryglewicz 1999). With increasing the catalyst concentration, adsorption of some produced biodiesel becomes noticeable, and hence, the amount of biodiesel obtained will be less. Also, adding excess catalyst can result in the deactivation of activated molecules by collision with ground state molecules (Gupta et al. 2012).
Effect of stirring velocity
To investigate the effect of stirring velocity four velocities were used, namely 100, 200, 300 and 400 rpm with the molar ratio of 6:1, at 60 ± 1 °C, 1% (w/w) of catalyst and each run lasted for 120 min. Figure 7 shows that as the stirring velocity increases, the biodiesel yield increases. This effect of stirring may be explained in light of steps of catalytic reactions, catalytic reactions involve seven steps: (1) mass transfer (diffusion) of the reactants from the bulk fluid to the external surface of the catalyst, (2) diffusion of the reactants from the pore mouth through the catalyst pores to the internal catalytic surface, (3) adsorption of reactants onto the catalyst surface, (4) reaction on the surface of the catalyst, (5) desorption of the products from interior of pellets to pore mouth, (6) diffusion of products from the interior of pellets to the pore mouth, (7) mass transfer of products from external pellets surface to bulk fluid. The overall rate of reaction is equal to the rate of the slowest step in the mechanism. Previous studies concluded that in case of heterogeneous reactions the rate limiting step is the either the liquid–solid mass transfer or the surface reaction (Dossin et al. 2006; Gryglewicz 1999), and stirring helps decreasing the thickness of the diffusion layer leading to an increase in the diffusion of adsorbate into the surface of the adsorbent ensuring a better mass transfer and a higher biodiesel yield (Fogler 2010; Al-Saadi et al. 2013).
Biodiesel evaluation
Table 2 shows the measured values of the produced biodiesel properties and compares them with the average standards worldwide. The properties of the product lie within the average range of the standard values.
It would be instructive to compare the outcome of the present experimental work with previous similar studies; the biodiesel yield obtained from several related works is presented in Table 3 including the operation conditions in each case.
Comparing the yield obtained in the different cases illustrates that the present work has one of the highest yield in the both cases of Jatropha oil and waste cooking oil. Though some studies obtained higher yields, considering the operation conditions used the present study could reach the optimization between the high yield and the mild operation conditions.
The promising results of the obtained biodiesel using the activated carbon as the heterogeneous catalyst for the transesterification give rise to an economic process since the activated carbon could be synthesized from the agricultural wastes of the biodiesel production (Buasri et al. 2013; Foo and Hameed 2009) which makes the process sustainable.
Conclusion
The performance of activated carbon as heterogeneous catalyst for the production of biodiesel was studied. Two types of oil were used, the Jatropha oil and the waste cooking oil. The effect of different operating parameters was investigated; the maximum biodiesel yield was obtained after 120 min at an alcohol-to-oil ratio of 6:1, catalyst loading of 1% (w/w) and stirring velocity of 400 rpm. The quality of the produced biodiesel is satisfactory which gives a chance to a sustainable feasible biodiesel production process.
References
Agarwal M, Chauhan G, Chaurasia SP, Singh K (2012) Study of catalytic behavior of KOH as homogeneous and heterogeneous catalyst for biodiesel production. J Taiwan Inst Chem Eng 43:89–94
Al-Saadi AA, Saleh TA, Gupta VK (2013) Spectroscopic and computational evaluation of cadmium adsorption using activated carbon produced from rubber tires. J Mol Liq 188:136–142
Ashraful AM, Masjuki HH, Kalam MA, Rizwanul Fattah IM, Imtenan S, Shahir SA, Mobarak HM (2014) Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: a review. Energy Convers Manag 80:202–228
Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef SA (2012) Comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev 16:2070–2093
Basir FA, Datta S, Roy PK (2015) Studies on biodiesel production from Jatropha curcas oil using chemical and biochemical methods—a mathematical approach. Fuel 158:503–511
Berchmans HJ, Hirata S (2008) Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour Technol 99:1716–1721
Buasri A, Chaiyut N, Loryuenyong V, Phakdeepataraphan E, Watpathomsub S, Kunakemakorn V (2013) Synthesis of activated carbon using agricultural wastes from biodiesel production. Int J Chem Nucl Mater Metall Eng 7(1):98–102
Cai Z-Z, Wang Y, Teng Y-L, Chong K-M, Wang J-W, Zhang J-W, Yang D-P (2015) A two-step biodiesel production process from waste cooking oil via recycling crude glycerol esterification catalyzed by alkali catalyst. Fuel Process Technol 137:186–193
Campanelli P, Banchero M, Manna L (2010) Synthesis of biodiesel from edible, non-edible and waste cooking oils via supercritical methyl acetate transesterification. Fuel 89:3675–3682
Canakci M, Erdil A, Arcaklioglu E (2006) Performance and exhaust emissions of a biodiesel engine. Appl Energy 83:594–605
de Araújo CDM, de Andrade CC, de Silva ES, Dupas FA (2013) Biodiesel production from used cooking oil: a review. Renew Sustain Energy Rev 27:445–452
Demirbas A (1998) Fuel properties and calculation of higher heating values of vegetable oils. Fuel 77:1117–1120
Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energy Convers Manag 50:14–34
Dharma S, Masjuki HH, Ong HC, Sebayang AH, Silitonga AS, Kusumo F, Mahlia TMI (2016) Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers Manag 115:178–190
Dizge N, Aydiner C, Imer DY, Bayramoglu M, Tanriseven A, Keskinler B (2009) Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour Technol 100:1983–1991
Dossin TF, Reyniers M-F, Marin GB (2006) Kinetics of heterogeneously MgO-catalyzed transesterification. Appl Catal B Environ 62:35–45
Eevera T, Rajendran K, Saradha S (2009) Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew Energy 34:762–765
Endalew AK, Kiros Y, Zanzi R (2011) Heterogeneous catalysis for biodiesel production from Jatropha curcas oil (JCO). Energy 36:2693–2700
Fogler HS (2010) Essentials of chemical reaction engineering. Prentice Hall, New Jersey
Foo KY, Hameed BH (2009) Utilization of biodiesel waste as a renewable resource for activated carbon: application to environmental problems. Renew Sustain Energy Rev 13:2495–2504
Freedman B, Pryde EH, Mounts TL (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc 161:1638–1643
Gardy J, Hassanpour A, Lai X, Ahmed MH (2016) Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl Catal A Gen 527:81–95
Gryglewicz S (1999) Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour Technol 70:249–253
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32:12–17
Hawash S, El Diwani G, Abdel Kader E (2011) Optimization of biodiesel production from jatropha oil by heterogeneous base catalysed transesterification. Int J Eng Sci Technol 3(6):5242–5251
Heikal EK, Khalil SA, Abdou IK (2013) Jatropha bio-diesel production technologies. IJBBB 3:288–291
Issariyakul T, Dalai AK (2014) Biodiesel from vegetable oils. Renew Sustain Energy Rev 31:446–471
Jacobson K, Gopinath R, Meher LC, Dalai AK (2008) Solid acid catalyzed biodiesel production from waste cooking oil. Appl Catal B Environ 85:86–91
Jain S, Sharma MP (2010) Prospects of biodiesel from Jatropha in India: a review. Renew Sustain Energy Rev 14:763–771
Jameel U, Zhu M, Tikkanen W, Chen X, Tong Z (2016) Recent fuel cell progress in nano gold hybrid materials for oxygen reduction reaction in alkaline media. Mater Res Bull 84:185–211
Kafuku G, Lee K, Mbarawa M (2010) The use of sulfated tin oxide as solid superacid catalyst for heterogeneous transesterification of Jatropha curcas oil. Chem Pap 64(6):734–740
Kalam MA, Masjuki HH, Jayed MH, Liaquat AM (2011) Emission and performance characteristics of an indirect ignition diesel engine fuelled with waste cooking oil. Energy 36:397–402
Kamm B, Gruber PR, Kamm M (2016) Biorefineries-industrial processes and products. Ullmann’s Encyclopedia of Industrial, Chemistry, pp 1–38
Kartika IA, Yani M, Ariono D, Evon Ph, Rigal L (2013) Biodiesel production from jatropha seeds: solvent extraction and in situ transesterification in a single step. Fuel 106:111–117
Knothe G, Sharp CA, Ryan TW (2006) Exhaust emissions of biodiesel, petrodiesel, neat methyl esters, and alkanes in a new technology engine. Energy Fuel 20:403–408
Koh MY, Ghazi M, Idaty T (2011) A review of biodiesel production from Jatropha curcas L. oil. Renew Sustain Energy Rev 15:2240–2251
Lam MK, Lee KT (2011) Mixed methanol–ethanol technology to produce greener biodiesel from waste cooking oil: a breakthrough for SO4 2−/SnO2–SiO2 catalyst. Fuel Process Technol 92:1639–1645
Lee AF, Bennett JA, Manayil JC, Wilson K (2014) Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem Soc Rev 43:7887–7916
Leung DYC, Wu X, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energ 87:1083–1095
Lin L, Ying D, Chaitep S, Vittayapadung S (2009) Biodiesel production from crude rice bran oil and properties as fuel. Appl Energy 86:681–688
Lopez JM, Gomez A, Aparicio F, Javier Sanchez F (2009) Comparison of GHG emissions from diesel, biodiesel and natural gas refuse trucks of the City of Madrid. Appl Energy 86:610–615
Luque R (2010) Algal biofuels: the eternal promise? Energy Environ Sci 3:254–257
Luque R, Lovett CJ, Datta B, Clancy J, Campelo JM, Romero AA (2010) Biodiesel: a feasible petrol fuel replacement. Energy Environ Sci 3:1706–1721
Ma F, Clements LD, Hanna MA (1998) The effects of catalyst, free fatty acids, and water on transesterification of beef tallow. Trans Am Soc Agric Eng 41:1261–1264
Mahto TK, Jain R, Chandra S, Roy D, Mahto V, Sahu SK (2016) Single step synthesis of sulfonic group bearing graphene oxide: a promising carbo-nano material for biodiesel production. J Environ Chem Eng 4(3):2933–2940
Meng X, Chen G, Wang Y (2008) Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process Technol 89:851–857
Mofijur M, Masjuki HH, Kalam MA, Atabani AE, Shahabuddin M, Palash SM, Hazrat MA (2013) Effect of biodiesel from various feedstocks on combustion characteristics, engine durability and materials compatibility: a review. Renew Sustain Energy Rev 28:441–455
Mudge SM, Pereira G (1999) Stimulating the biodegradation of crude oil with biodiesel preliminary results. Spill Sci Technol Bull 5:353–355
Narayanan V, Stephen A (2013) ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light. Mater Sci Eng C 33:2235–2244
Phan AN, Phan TM (2008) Biodiesel production from waste cooking oils. Fuel 87:3490–3496
Qian J, Shi H, Yun Z (2010) Preparation of biodiesel from Jatropha curcas L. oil produced by two-phase solvent extraction. Bioresour Technol 101:7025–7031
Saleh TA (2011) The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl Surf Sci 257:7746–7751
Saleh TA, Gupta VK (2011) Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J Colloid Interface Sci 362:337–344
Saleh TA, Gupta VK (2012a) Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ Sci Pollut Res 19:1224–1228
Saleh TA, Gupta VK (2012b) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. Colloid Interface Sci 371:101–106
Saleh TA, Agarwal S, Gupta VK (2011) Synthesis of MWCNT/MnO2 and their application for simultaneous oxidation of arsenite and sorption of arsenate. Appl Catal B Environ 106:46–53
Saleh TA, Al-Saadi AA, Gupta VK (2014) Carbonaceous adsorbent prepared from waste tires: experimental and computational evaluations of organic dye methyl orange. J Mol Liq 191:85–91
Sano N, Yamada K, Tsunauchi S, Tamon H (2017) A novel solid base catalyst for transesterification of triglycerides toward biodiesel production: carbon nanohorn dispersed with calcium ferrite. Chem Eng J 307:135–142
Santos IB, Acchar W, Goncalves JN, Segadães AN (2015) Bactericidal potential of titania and silver nano powders deposited in porous ceramic substrates for low-power water purification reactors. Mater Today 2(1):242–245
Saravanan R, Shankar H, Prakash T, Narayanan V, Stephen A (2011) ZnO/CdO composite nanorods for photocatalytic degradation of methylene blue under visible light. Mater Chem Phys 125:277–280
Saravanan R, Karthikeyan S, Gupta VK, Sekaran G, Narayanan V, Stephen A (2013a) Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater Sci Eng C 33:91–98
Saravanan R, Karthikeyan N, Gupta VK, Thirumal E, Thangadurai P, Narayanan V, Stephen A (2013b) ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light. Mater Sci Eng C 33:2235–2244
Saravanan R, Gracia F, Khan MM, Poornima V, Gupta VK, Narayanan V, Stephen A (2015) ZnO/CdO nanocomposites for textile effluent degradation and electrochemical detection. J Mol Liq 209:374–380
Shu Q, Gao J, Nawaz Z, Liao Y, Wang D, Wang J (2010) Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl Energy 87:2589–2596
Speidel HK, Lightner RL, Ahmed I (2000) Biodegradability of new engineered fuels compared to conventional petroleum fuels and alternative fuels in current use. Appl Biochem Biotechnol 84–86:879–897
Srilatha K, Issariyakul T, Lingaiah N, Prasad PSS, Kozinski J, Dalai AK (2010) Efficient esterification and transesterification of used cooking oil using 12-tungstophosphoric acid (TPA)/Nb2O5 catalyst. Energy Fuels 24:4748–4755
Takase M, Zhao T, Zhang M, Chen Y, Liu H, Yang L, Xiangyang X (2015) An expatiate review of neem, Jatropha, rubber and karanja as multipurpose non-edible biodiesel resources and comparison of their fuel, engine and emission properties. Renew Sustain Energy Rev 43:495–520
Talebian-Kiakalaieh A, Amin NAS, Zarei A, Jaliliannosrati H (2013) Biodiesel production from high free fatty acid waste cooking oil by solid acid catalyst. In: Proceedings of the 6th international conference on process systems engineering (PSE ASIA) 25–27 June 2013, Kuala Lumpur
Tan KT, Lee KT, Mohamed AR (2011) Potential of waste palm cooking oil for catalyst-free biodiesel production. Energy 36:2085–2088
Torres-Rodríguez DA, Romero-Ibarra IC, Ibarra IA, Pfeiffer H (2016) Biodiesel production from soybean and Jatropha oils using cesium impregnated sodium zirconate as a heterogeneous base catalyst. Renew Energy 93:323–331
Van Kasteren JMN, Nisworo AP (2007) A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour Conserv Recycl 50:442–458
Yaakob Z, Mohammad M, Alherbawi M, Alam Z, Sopian K (2013) Overview of the production of biodiesel from waste cooking oil. Renew Sustain Energy Rev 18:184–193
Yeom C, Kim Y (2016) Purification of oily seawater/wastewater using superhydrophobic nano-silica coated mesh and sponge. J Ind Eng Chem 40:47–53
Yusuf NNAN, Kamarudin SK (2013) Techno-economic analysis of biodiesel production Jatropha curcas via a supercritical methanol process. Energy Convers Manag 75:710–717
Yusuf NNAN, Kamarudin SK, Yaakob Z (2011) Overview on the current trends in biodiesel production. Energy Convers Manag 52:2741–2751
Zanette AF, Barella RA, Pergher SBC, Treichel H, Oliveira D, Mazutti MA, Silva EA, Oliveira JV (2011) Screening, optimization and kinetics of Jatropha curcas oil transesterification with heterogeneous catalysts. Renew Energy 36:726–731
Zhang Y, Dube MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour Technol 90:229–240
Acknowledgements
The authors would like to express their gratitude to the Agriculture Researches Station (Ismailia, Egypt) which provided the Jatropha seeds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: V.K. Gupta.
Rights and permissions
About this article
Cite this article
Kamel, D.A., Farag, H.A., Amin, N.K. et al. Biodiesel synthesis from non-edible oils by transesterification using the activated carbon as heterogeneous catalyst. Int. J. Environ. Sci. Technol. 14, 785–794 (2017). https://doi.org/10.1007/s13762-016-1184-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1184-z