Abstract
Biodiesel appears to be a possible substitute for non-renewable fossil fuels; however, its production requires the presence of a catalyst to accelerate the reaction. Serving the purpose of finding effective, cheap and environmentally safe, heterogeneous catalysts, this research used the fig leaves in three different forms, calcined, activated by KOH, and activated by both K2CO3 and CaCO3. Their efficiency in biodiesel synthesis, from spent cooking oil, was examined and compared with that of activated carbon which has been previously investigated. The properties of different catalyst forms were specified using X-ray diffraction, scanning electron microscope and Fourier transform infrared spectroscopy. Operating parameters studied for the three catalysts were reaction time (from 30 to 180 min), alcohol-to-oil molar ratio (from 4:1 to 10:1), catalyst loading (from 0.5 to 5% by wt.), and stirring speed (from 100 to 400 rpm). The increase in reaction time, molar ratio, and catalyst loading proved to have a favorable effect on % conversion to biodiesel but to a certain degree; increasing the stirring speed augmented the conversion. At optimum conditions (2 h of heating, 6:1 alcohol-to-oil molar ratio, 1% by wt. catalyst loading, and 400 rpm stirring), fig leaves activated by KOH provided the highest conversion to biodiesel (92.73%). The measured properties of the produced biodiesel (density, viscosity, flash point, cloud point, and pour point) yielded encouraging results.

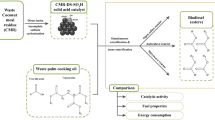
Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reliance of today’s modern societies on traditional fossil fuels as an energy source must be diminished as they are not environmentally friendly: their exhaust gases bring global warming, acid rain, and ozone layer exhaustion (Maeda et al. 2008; Chouhan et al. 2013; Sani et al. 2015; Mansir et al. 2018; Živković and Veljković 2018; Devarajan et al. 2018). These drawbacks triggered the attention to the urgency of developing new renewable, economical, feasible, and sustainable energy resources (Mahmudul et al. 2017; Zareh et al. 2017). Biodiesel was thought to be a good replacement for the fossil fuels, especially in the vehicles, thanks to its physical and chemical properties (Dos Santos et al. 2017; Joshi et al. 2017). Biodiesel has a lower sulfur content than petroleum diesel permitting less sulfur oxide emission. The volume of noncombustible hydrocarbons and carbon monoxide in the exhaust gas can be reduced, when using biodiesel, by approximately 67% and 48%, respectively. Biodiesel utilization lowers the content of particulate matter in air by 47% and hence lowers the black smoke and smog. Biodiesel decreases the cancer risks by nearly 80% as it is less polluting and produces less polycyclic aromatic hydrocarbons, spotted as possible cancer-causing compounds (Knothe et al. 2006; Koh and Ghazi 2011; de Araújo et al. 2013). Utilizing biodiesel and its blends reduce the carbon dioxide emissions (Kavitha et al. 2019). Biodiesel possesses a higher cetane number than petroleum diesel and it has a better lubricating ability protecting engines from wear. Biodiesel has a higher flash point (100–170 °C) than that of petroleum diesel (60–80 °C) making it safer to handle. Biodiesel degradation is faster and easier than that of petroleum diesel (Zabeti et al. 2009; Mofijur et al. 2013). Also, biodiesel can be used in compression-ignition engines instead of any other fuel with minor or without modifications to the engine components (Ullah et al. 2017; Kataria et al. 2019; Ueki et al. 2018). Various methods are available and feasible to produce biodiesel, from raw oils or oil blends, namely micro-emulsions, pyrolysis, esterification, and transesterification (Koh and Ghazi 2011). Currently, transesterification is the most commonly used method to produce biodiesel, where triglycerides (algae oil, animal fats, or vegetable oils) react with alcohol (commonly methanol or ethanol) using a catalyst that boosts the transformation of the triglyceride fatty acids into fatty acid methyl/ethyl esters and glycerol as by-product (Rattanaphra and Harvey 2010). The catalyst is essential in transesterification as the alcohol is weakly soluble in oil, in respect to their polarity difference; hence, the catalyst accelerates the reaction. Owing to its reduced cost and physiochemical properties, methanol is the most utilized alcohol (Singh and Singh 2010; Ullah et al. 2017; Kataria et al. 2019). Vegetable oils are the most favorable feedstock for biodiesel production as they are renewable and environmentally friendly. Either edible or non-edible oils can be used. Edible oils produce around 95% of the total biodiesel production as they are widely produced and provide biodiesel with competing properties (Leung et al. 2010). Nevertheless, they cause competition with edible oil markets raising both the edible oil and the biodiesel prices. As a solution to this problem, focus has been directed to non-edible oils as they cannot be consumed by humans (Kansedo et al. 2009; Leung et al. 2010). The expenses of the feedstock, estimated to contribute to 40–60% of the total biodiesel production cost, can be minimized by using low-cost feedstocks (Sharma et al. 2008); the pre-used cooking oil is a cheap suitable example (Jacobson et al. 2008). It has been recorded that around 15 million tons of spent cooking oil are disposed annually worldwide (Lee et al. 2014) posing an environmental threat. Thus, using this waste in biodiesel production will serve two purposes: decreasing the cost of feedstock and reducing water pollution resulting from the waste discharge (Lou et al. 2008; Dos Santos et al. 2017). The main drawback of non-edible oils is their high content of free fatty acids (FFA) (Shu et al. 2010; T-Kiakalaeih et al. 2013); a small part of this FFA reacts with the basic catalyst in the presence of water and forms soap. The soap formation consumes the catalyst, complicates the separation of glycerol, and reduces enormously the ester yield (Lou et al. 2008). Therefore, the production of biodiesel starting with high FFA content feedstock takes place through two steps: the first step is the esterification of the oil to decrease the FFA content employing an acid catalyst (Marchetti and Errazu 2008) and the second step is the transesterification of the esterified oil using a basic catalyst (Muhammad et al. 2014; Pirouzmand et al. 2018). Currently, homogeneous catalysts are commonly used for biodiesel production in both reactions (Nata et al. 2017). Homogeneous catalysts have their flaws because they are non-reusable and generate excess wastewater during product purification rendering the process harder. They are highly corrosive and require careful handling. As a result, heterogeneous catalysts are being developed to solve these problems as they are easily separable, can be recycled, and facilitate the purification process of the product. Different heterogeneous catalysts have been used in biodiesel production as alkaline earth metal oxides, heteropolyacids, and zeolite (Gupta and Rathod 2018). Numerous efforts are directed, nowadays, to promote new heterogeneous, environmentally friendly catalysts, mainly from agriculture wastes.
Agricultural wastes are undesirable materials produced entirely from agricultural operations resulting from growing of crops, e.g., grapevines, fig leaves, and peanut hulls. Using agricultural wastes, in the preparation of catalysts, serves double purposes. One is for environmental consideration as it converts unwanted agricultural wastes to useful, valuable materials instead of disposing them incorrectly, and the other for economical purposes, as using agricultural byproducts reduces the high preparation expenses of the catalyst.
From this perspective, using fig leaves (Ficus carica) as a heterogeneous catalyst to produce biodiesel from waste cooking oil is discussed in this work. The fig leaves were treated with three different methods: calcination, potassium hydroxide activation, and activation using a mixture of calcium carbonate and potassium carbonate. The catalyst efficiency was assessed and compared to that of activated carbon, investigated in a previous study (Kamel et al. 2017); also, the effect of the numerous operation parameters was determined.
Materials and methods
Materials
Fig leaves (Ficus carica) were collected as agricultural waste from a private farm in King Mariut, Alexandria, Egypt. Domestic waste cooking oil was used as the raw material. Methanol, sulfuric acid, potassium hydroxide, potassium carbonate, and calcium carbonate used in this study were of AR grade and were used without further purification. The sulfuric acid was purchased from ADWIC, the methanol and potassium hydroxide from Sigma and Aldrich Company.
Methods
Catalyst preparation
-
(a)
Calcined fig leaves (CFL)
Fig leaves were thoroughly washed with running tap water three times to remove dirt, soil, and dust, then rinsed with distilled water. The washed fig leaves were dried at 70 °C, crushed, ground, and calcined for 2 h using muffle furnace at 800 °C.
-
(b)
KOH-activated fig leaves (KFL)
1 M KOH solution was mixed with the washed and dried fig leaves (50 g of the dried fig leaves: 50 ml of KOH solution), the mixture was heated at 70 °C till the mixture converted to a paste. The produced KFL paste was heated at 450 °C for 3 h.
-
(c)
Preparation of activated fig leaves with both potassium carbonate and calcium carbonate (MFL)
A mixture of calcium carbonate and potassium carbonate was prepared in a ratio 1:1 by wt. One gram of this mixture was added to 1 g of dried fig leaves then calcined in a muffle furnace at 450 °C for 3 h (Sharma et al. 2012).
Oil analysis
Titration with 0.1 N KOH solution was used to specify the free fatty acid percentage of WCO. The average water content of the used oil was specified as 0.17% by wt. applying the Karl Fischer titration. Table 1 shows the WCO composition.
Catalyst characterization
Scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR) were used to characterize the catalysts as in our previous study (Kamel et al. 2017).
Biodiesel synthesis
-
(a)
Esterification
One hundred milliliters of oil were weighed and fed to the three-neck round-bottom flask in the bench-scale system and heated at 50 °C. Then, alcohol was added (6:1 alcohol-to-oil molar ratio) with stirring for few minutes. The catalyst (sulfuric acid, 1% by wt. catalyst loading) was added, with continuous stirring, for 2 h, at 400 rpm. At the end of the reaction, the oil was transferred from the reactor to a separating funnel. The oil was washed using 150 ml distilled water to stop the reaction and to separate the alcohol from the oil phase. The acid value of the esterified oil was then measured two times.
-
(b)
Transesterification
Thirty milliliters of the esterified oil was mixed with the alcohol and the catalyst with continuous stirring. At the end of the reaction, the oil was collected, filtered, and poured in a separating funnel. Two layers formed: the top layer was the biodiesel and the bottom layer was glycerol.
Different operating parameters studied, for the three catalysts, were reaction time (from 30 to 180 min), alcohol-to-oil molar ratio (from 4:1 to 10:1), catalyst loading (from 0.5 to 5% (w/w)), and stirring speed (from 100 to 400 rpm). Each experiment was repeated three times for result verification.
Biodiesel analysis
After transesterification, the oil contained residues of the catalyst, methanol, and glycerol. To remove these impurities, the oil was washed with 50 ml distilled hot water for 2–3 times. Then, the oil layer was heated in a water bath, at 100 °C for about 20 min to remove methanol and water from the product (biodiesel).
The FAME (fatty acid methyl esters) content of the biodiesel layer was quantified using gas chromatography (GC).
Two equations ((1) and (2)) were employed to compute the % conversion to biodiesel to investigate the effect of different variables.
Biodiesel property characterization
To appraise the quality of the produced biodiesel and compare it to the universal standards, five of the main properties of biodiesel were measured:
- 1.
Density: The density was measured using a density meter (KEM/DA-640) provided by Kyoto Electronics MFG CO., LTD.
- 2.
Viscosity: A viscometer bath (KV6) from Stanhope-Seta Co. was used to measure the viscosity (according to the ASTM D 445-03 method).
- 3.
Flash point: The flash point was determined by the Norma lab half automated Cleveland flash point (NCL-120), following the ASTM D 93-02a method.
- 4.
Pour point: It was measured using the compact cloud and pour point cryostat (94100-3) from Stanhope-Seta Co. (according to the ASTM D 97-02 method).
- 5.
Cloud point: The Seta compact cloud and pour point cryostat (94100-3) from Stanhope-Seta Co. was used to measure the cloud point following the ASTM D2500-02 method (Kamel et al. 2017).
Results and discussion
Catalyst characterization
Scanning electron microscopy
Figure 1 displays the morphology of the untreated and the treated fig leaves qualitatively using the SEM technique which produces images by scanning the surface of the sample with a focused beam of electrons giving information about the surface morphology. It is observed that raw fig leaves, RFL (Fig. 1a), have a longitudinal, fibrous, more or less regular and non-porous surface. After calcination, the CFL (Fig. 1b) surface roughness is highly increased; hollow cavities, some cracks, and some aggregates can be noted on the external surface. The leaves treated with the potassium hydroxide, KFL (Fig. 1c), show a highly amorphous morphology with deep holes and pores increasing largely the surface area. Finally, the mixed activated fig leaves, MFL (Fig. 1d), show the presence of a great number of mesopores and a very porous outer surface. From the observed morphology it is obvious that the different treatments of the fig leaves produced better irregular porous structures yielding a higher surface area.
X-ray diffraction
The structure of CFL, KFL, and MFL, using XRD analysis, is illustrated in Fig. 2. A significant spectrum is obvious indicating that the material contains crystalline cellulosic features. The main peak at 2θ≈12 indicates highly organized crystalline cellulose, while the secondary peak at 2θ≈22 is a measure of a polysaccharide structure with low organization and assigned to broad peak with low angle. This low angle proves the presence of a mesoporous structure, with a preferred orientation, giving very thin peaks next to each other. Another peak at 2θ ≈ 30 shows more organized & crystallized quartz. Also, a peak appearing at 2θ ≈ 44 is due to the presence of quartz as well. From the XRD configuration in Fig. 2B, it is obvious that the KFL has an amorphous structure which agrees with many researches dealing with agricultural wastes being activated using KOH (Abdul Khalil et al. 2013; Cai et al. 2015; Chouhan and Sarma 2011).
Fourier transform infrared spectroscopy
The characterization of the modified fig leaf (Ficus carica) functional groups, performed by the FTIR spectra in the range of 100 to 4600 cm−1, is shown in Fig. 3. With the three catalysts, a band appears in the wavelength range 1026–1087 cm−1 corresponding to the C–O bond. The next observed band in all catalysts is in the range 1431–1456 cm−1 indicating C=C existence. Figure 3A shows a band at 1668 cm−1 and Fig. 3B shows a band at 1624 cm−1 representing the stretching aromatic carboxyl group of conjugated carbonyl (mainly ketones and esters) of lignin (C=O). The C≡C is manifested in all of the catalysts by a band at 2347–2349 cm−1. Also, Fig. 3B shows a band at 2985 cm−1 that contributes to the symmetrical and non-symmetrical C–H stretching. Finally, a band showing off at ≈ 3400 cm−1 is assigned to the symmetrical and non-symmetrical elongated vibration of O–H absorbed water molecules; this band is very broad for the CFL; this is ascribed to water evaporation as a result of calcination.
Effect of different operation parameters on % conversion to biodiesel
The influence of different parameters is tested with the three prepared catalysts and compared with the results obtained using activated carbon as a heterogeneous catalyst discussed in a previous work as it showed a high % conversion to biodiesel.
Effect of time
Reaction time is among the most influential parameters in the triglyceride conversion as it has a notable role in determining the economics of the transesterification process (Reddy et al. 2014). To explore the effect of time, running time ranged from 30 to 180 min while keeping all other parameters constant. Figure 4 depicts that the transesterification started with a slow rate as the alcohol dispersed slowly to react with triglyceride (Koh and Ghazi 2011). As time elapsed, the % conversion to biodiesel reached a peak value after around 120 min. Further extension in reaction duration resulted in a reverse effect ascribed to the backward reaction causing ester losses (Draphco et al. 2008). Similar behavior, with different optimum time, was reported by different researchers. (Chongkhong et al. 2007; Qian et al. 2010; Zhang et al. 2010; Viriya-empikul et al. 2012; Vi Tran et al. 2016; Nata et al. 2017; Ullah et al. 2017; Kataria et al. 2019; Murugesan 2018). From Fig. 4, it is clear that KFL gives the highest % conversion for all tested time periods, followed by MFL and CFL; this can be attributed to the fact that KFL shows a high amorphous morphology with deep holes and pores.
Effect of methanol-to-oil molar ratio
Another vital parameter playing a distinguished role in the transesterification reaction is the alcohol:oil molar ratio; an optimum ratio has to be obtained to cut the transesterification expenses. Four ratios have been tested, namely 4:1, 6:1, 8:1 and 10:1; while conducting the reaction for 120 min, the temperature was held constant at 60 ± 1 °C, the catalyst loading at 1% by wt., and the stirring speed at 400 rpm. Figure 5 reveals that the % conversion augmented by increasing the ratio until attaining a peak corresponding to the ratio 6:1 and then it started to decline with further ratio increase. This behavior can be explained by the following facts: (i) transesterification reactions are reversible (Draphco et al. 2008). Stoichiometrically, the reaction necessitates three moles of methanol and 1 mol of oil producing 3 mol of FAME and 1 mol of glycerol. Hence, the quantity of alcohol used must be in excess to force the reaction towards the formation of FAME and to enhance the % conversion. (ii) The excess alcohol sweeps the product molecules away from the catalyst surface and consequently serves in the regeneration of the catalyst active sites (Kataria et al. 2019). Nevertheless, an excessive rise in alcohol amount will cause an increase in the solubility of glycerol in the methyl ester layer which will harden the glycerol separation, so the reaction will be driven backwards lowering the conversion to FAME. Several previous studies reported 6:1 also as the optimum methanol-to-oil ratio (Ma and Hanna 1999; Leung and Guo 2006; Payawan Jr et al. 2010; Ramachandran et al. 2011). However, numerous other researchers reported higher optimum ratios (Ullah et al. 2017; Kataria et al. 2019; Mansir et al. 2018); it was found that, generally, the type of catalyst used affects the optimum methanol:oil ratio; with acid catalysts, the ratio ranged from 30:1 to 150:1 while using alkali catalysts lowered the required ratio to be in the range of 6:1 to 15:1 (Qian et al. 2010). The obtained results regarding the level of activity of the three catalysts tested can be attributed to the previously mentioned reasons in “Effect of time.”
Effect of catalyst loading
The third controlling operating parameter in the transesterification reaction is the quantity of the catalyst used. Once again, the optimum amount of catalyst should be specified to cut down the process expenses. To investigate the effect of the catalyst loading, different amounts were used ranging from 0.5 to 5% by wt. based on the oil weight, with a methanol-to-oil molar ratio of 6:1, at 60 ± 1 °C, and stirring speed of 400 rpm and each run lasted for 120 min. The % conversion increased with increasing the amount of the catalyst, due to the increase in the number of active sites available (Al-Saadi et al. 2013), until reaching a peak at 1% by wt. and then decreased sharply as illustrated in Fig. 6. Several factors may cause this drop: (i) the surplus of catalysts increases the resistance to the mass transfer making the inter-phase contact during the reaction harder and hence diminishing the % conversion (Ullah et al. 2017; Kataria et al. 2019); (ii) the surplus of catalyst may react with the remaining traces of free fatty acids present in the esterified oil decreasing the % conversion (Gupta and Rathod 2018), (iii) raising the catalyst concentration causes the adsorption of a notable portion of the produced biodiesel and therefore the final yield will diminish; (iv) increased catalyst amount may lead to deactivation of activated molecules by collision with ground-state molecules (Gupta et al. 2012); (v) finally, increased catalyst load causes separation issues in the downstream (Joshi et al. 2017).
The obtained results regarding the level of activity of the three catalysts tested can be attributed to the previously mentioned reasons in “Effect of time.”
Effect of stirring speed
Reactants’ mixing is vital for the accomplishment of the reaction and the amelioration of the conversion. The stirring allows the collision between the particles, shortens the reaction duration and increases the conversion (Ullah et al. 2017). To specify the optimum stirring speed, four speeds were used, namely 100, 200, 300 and 400 rpm with the other parameters kept fixed (2 h of heating, 6:1 alcohol-to-oil ratio, and 1% by wt. catalyst loading) as displayed in Fig. 7. It was found that the % conversion increased as the stirring speed increased all the way in the range of 100–400 rpm. This result is ascribed probably to the role of stirring in reducing the thickness of the diffusion layer, allowing a better diffusion of the adsorbate into the surface of the adsorbent providing a better mass transfer and an enhanced % conversion (Fogler 2010; Gupta et al. 2012; Al-Saadi et al. 2013). Once again, the KFL had the maximum conversion which is owed to its amorphous structure.
Comparison between different catalysts
Table 2 shows the maximum % conversion to biodiesel obtained at optimum conditions (2 h of heating, 6:1 alcohol-to-oil molar ratio, 1% by wt. catalyst loading, and 400 rpm stirring) with the various catalysts used. With the different operation parameters tested, the behavior of the catalysts was the same; the activated carbon (AC) has the highest value, followed by the KFL, then the MFL, and, finally, the CFL. The amorphous structure of the KFL, shown by the XRD, may be considered as the main reason for his satisfactory performance. This finding agrees with many researches dealing with agricultural wastes being activated using KOH (Bohlouli and Mahdavian 2019; Ishak and Kamari 2019).
The values obtained are pretty close; meanwhile, the difference in prices between the activated carbon and the prepared agricultural waste is considerable which makes the KFL an appealing effective heterogeneous catalyst for biodiesel synthesis.
Also, the outcome of the present research was compared with similar researches using different catalysts; Table 3 displays the % conversion to biodiesel resulting from previous related studies and the corresponding operation conditions.
Regarding the operation conditions and the nature of the various used catalysts, the KFL is a promising catalyst that can be employed to obtain biodiesel using transesterification at mild conditions.
Biodiesel fuel properties
The quality of the fuel is the key to long-term successful use. Biodiesel quality is reflected by its chemical and physical properties. ASTM International adopted various standards for measuring the biodiesel different properties since 2002. Five of the most significant properties of biodiesel have been measured in the current work using the ASTM standards, compared with the average standards worldwide; and they have been found to be in accordance with the average standards as shown in Table 4.
The measured properties were (i) density (mass/volume): the density of the fuel has an influential effect on its behavior in the engine (penetration, atomization, vaporization, and combustion); (ii) viscosity: the viscosity of a fluid measures its resistance to deformation and determines the degree of resistance to fluid flow; (iii) flash point: it is an important criterion for biodiesel safe storage as it is defined as the least temperature at which the fuel can form an ignitable mixture with air, (iv) cloud point: it is the temperature, during cooling, at which the fuel starts to solidify and the first crystal forms; and (v) pour point: the pour point determines the range of operating temperatures as it represents the temperature at which the biodiesel solidifies and ceases to flow. (Mahlia et al. 2019; Sharma and Duraisamy 2019)
Conclusion
The current study concentrates on the utilization of heterogeneous catalysts from agricultural wastes in producing biodiesel. Three heterogeneous catalysts were prepared from fig leaves (Ficus carica) by three different methods: calcination, activation using KOH, and activation using a mixture of CaCO3 and K2CO3. Their performance, in producing biodiesel by transesterification starting with waste cooking oil feedstock, was tested and compared with that of activated carbon. Different variables were investigated showing that the optimum conditions were 2 h of heating, 6:1 alcohol-to-oil molar ratio, 1% by wt. catalyst loading, and 400 rpm stirring. The fig leaves which were activated with KOH yielded the best results at the above conditions. The present study was compared with another heterogeneous catalyst study (activated carbon); the results were observed to be so close. The obtained biodiesel had outstanding properties, making the fig leaves activated by KOH a suitable catalyst that can be employed for producing biodiesel at mild operating conditions.
Abbreviations
- FFA:
-
Free fatty acids
- CFL :
-
Calcined fig leaves
- FAME:
-
Fatty acid methyl esters
- KFL:
-
Fig leaves activated by KOH
- MFL:
-
Fig leaves activated by a mixture of K2CO3 and CaCO3
- RFL:
-
Raw fig leaves
- WCO:
-
Waste cooking oil
References
Abdul Khalil HPS, Jawaid M, Firoozian P, Rashid U, Islam A, Akil HM (2013) Activated carbon from various agricultural wastes by chemical activation with KOH: preparation and characterization. J Biochem Mol Biol 7:1–7
Al-Saadi AA, Saleh TA, Gupta VK (2013) Spectroscopic and computational evaluation of cadmium adsorption using activated carbon produced from rubber tires. J Mol Liq 188:136–142
Bohlouli A, Mahdavian L (2019) Catalysts used in biodiesel production: a review. Biofuels. https://doi.org/10.1080/17597269.2018.1558836
Cai ZZ, Wang Y, Teng YL, Chong KM, Wang JW, Zhang JW, Yang DP (2015) A two-step biodiesel production process from waste cooking oil via recycling crude glycerol esterification catalyzed by alkali catalyst. Fuel Process Technol 137:186–193
Chongkhong S, Tongurai C, Chetpattananondh P, Bunyakan C (2007) Biodiesel production by esterification of palm fatty acid distillate. Biomass Bioenergy 31:563–568
Chouhan APS, Sarma AK (2011) Modern heterogeneous catalysts for biodiesel production: a comprehensive review. Renew Sust Energ Rev 15:4378–4399
De Araújo CDM, De Andrade CC, Ese S, Dupas FA (2013) Biodiesel production from used cooking oil: A review. Renew Sust Energ Rev 27:445–452
Devarajan Y, Mahalingam A, Munuswamy DB, Nagappan B (2018) Emission and combustion profile study of unmodified research engine propelled with neat biofuels. Environ Sci Pollut Res Int 25(20):19643–19656
Dos Santos LK, Hatanaka RR, De Oliveira JE, Flumignan DL (2017) Experimental factorial design on hydroesterification of waste cooking oil by subcritical conditions for biodiesel production. Renew Energy 114:574–580
Draphco CM, Nhuan NP, Walker TH (2008) Biofuels engineering process technology. McGraw-Hill Companies Inc., New York
Fogler HS (2010) Essentials of chemical reaction engineering. Prentice Hall, New Jersey
Gupta AR, Rathod VK (2018) Calcium diglyceroxide catalyzed biodiesel production from waste cooking oil in the presence of microwave: Optimization and kinetic studies. Renew Energy 121:757–767
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Photo-catalytic degradation of toxic dye amaranth on TiO2/ UV in aqueous suspensions. Mater Sci Eng C 32:12–17
Ishak S, Kamari A (2019) A review of optimum conditions of transesterification process for biodiesel production from various feedstocks. Environ Sci Pollut Res 26:2481–2502
Jacobson K, Gopinath R, Meher LC, Dalai AK (2008) Solid acid catalyzed biodiesel production from waste cooking oil. Appl Catal B Environ 85:86–91
Joshi S, Gogate PR, Moreira PF Jr, Giudici R (2017) Intensification of biodiesel production from soybean oil and waste cooking oil in the presence of heterogeneous catalyst using high speed homogenizer. Ultrason Sonochem 39:645–653
Kamel DA, Farag HA, Amin NK, Fouad YO (2017) Biodiesel synthesis from non-edible oils by transesterification using the activated carbon as heterogeneous catalyst. Int J Environ Sci Technol 14:785–794
Kansedo J, Lee KT, Bhatia S (2009) Cerbera-odollam (sea mango) oil as a promising non-edible feedstock for biodiesel production. Fuel 88:1148–1150
Kataria J, Mohapatra SK, Kundu K (2019) Biodiesel production from waste cooking oil using heterogeneous catalysts and its operational characteristics on variable compression ratio CI engine. J Energy Inst 92(2):275–287
Kavitha KR, Beemkumar N, Rajasekar R (2019) Experimental investigation of diesel engine performance fueled with the blends of Jatropha curcas, ethanol, and diesel. Environ Sci Pollut Res Int 26(9):8633–8639
Talebian-Kiakalaieh A, Amin NAS, Zarei A, Jaliliannosrati H (2013) Biodiesel production from high free fatty acid waste cooking oil by solid acid catalyst, 6th international conference on process systems engineering (PSE ASIA), Kuala Lumpur, 25-27 June.
Knothe G, Sharp CA, Ryan TW (2006) Exhaust emissions of biodiesel, petro diesel, neat methyl esters, and alkanes in a new technology engine. Energy Fuel 20:403–408
Koh MY, Ghazi TIM (2011) A review of biodiesel production from Jatropha curcas L. oil. Renew Sust Energ Rev 15:2240–2251
Lee AF, Bennett JA, Manayil JC, Wilson K (2014) Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem Soc Rev 43(22):7887–7916
Leung D, Guo Y (2006) Transesterification of neat and used frying oil: optimization for biodiesel production. Fuel Process Technol 87(10):883–890
Leung DYC, Wu X, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energy 87:1083–1095
Lou WY, Zong MH, Duan ZQ (2008) Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour Technol 99:8752–8758
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70(1):1–15
Maeda H, Hagiwara S, Nabetani H, Sagara Y, Soerawidjaya TH, Tambunan AH (2008) Biodiesel fuels from palm oil via the non-catalytic transesterification in a bubble column reactor at atmospheric pressure: a kinetic study. Renew Energy 33:1629–1636
Mahlia TMI, Ismail N, Hossain N, Silitonga AS, Shamsuddin AH (2019) Palm oil and its wastes as bioenergy sources: a comprehensive review. Environ Sci Pollut Res 26:14849–14866
Mahmudul HM, Mamat FY, Adam AA, Ishak WFW, Alenezi R (2017) Production, characterization and performance of biodiesel as an alternative fuel in diesel engines: a review. Renew Sust Energ Rev 72:497–509
Mansir N, Teo SH, Rashid U, Saiman MI, Tan YP, Alsultan GA, Taufiq-Yap YH (2018) Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil: A review. Renew Sust Energ Rev 82(3):3645–3655
Marchetti JM, Errazu AF (2008) Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass Bioenergy 32:892–895
Mofijur M, Masjuki HH, Kalam MA, Atabani AE, Shahabuddin M, Palash SM, Hazrat MA (2013) Effect of biodiesel from various feedstocks on combustion characteristics, engine durability and materials compatibility: A review. Renew Sust Energ Rev 28:441–455
Muhammad Y, Mohd W, Wan A, Aziz ARA (2014) Activity of solid acid catalysts for biodiesel production: a critical review. Appl Catal A Gen 470:140–161
Murugesan AA (2018) Prediction capabilities of mathematical models in producing a renewable fuel from waste cooking oil for sustainable energy and clean environment. Fuel 216:322–329
Nata IF, Putra MD, Irawan C, Lee CK (2017) Catalytic performance of sulfonated carbon-based solid acid catalyst on esterification of waste cooking oil for biodiesel production. J Environ Chem Eng 5:2171–2175
Payawan LM Jr, Damasco JA, Sy Piecco KWE (2010) Transesterification of oil extract from locally cultivated Jatropha curcas using a heterogeneous base catalyst and determination of its properties as a viable biodiesel. Philipp J Sci 139:105–116
Pirouzmand M, Anakhatoon MM, Ghasemi Z (2018) One-step biodiesel production from waste cooking oils over metal incorporated MCM-41; positive effect of template. Fuel 216:296–300
Qian J, Shi H, Yun Z (2010) Preparation of biodiesel from Jatropha curcas L. oil produced by two-phase solvent extraction. Bioresour Technol 101:7025–7031
Ramachandran K, Sivakumar P, Suganya T, Renganathan S (2011) Production of biodiesel from mixed waste vegetable oil using an aluminium hydrogen sulphate as a heterogeneous acid catalyst. Bioresour Technol 102:7289–7293
Rattanaphra D, Harvey A (2010) Simultaneous conversion of triglyceride/free fatty acid mixtures into biodiesel using sulfated zirconia. Top Catal 53:773–782
Reddy HK, Muppaneni T, Patil PD, Ponnusamy S, Cooke P, Schaub T (2014) Direct conversion of wet algae to crude biodiesel under supercritical ethanol conditions. Fuel 115:720–726
Sani MY, Alaba PA, Raji-yahya AO, Aziz ARA, Daud WMAW (2015) Acidity and catalytic performance of Yb-doped SO2 for biodiesel production. J Taiwan Inst Chem Eng 59:195–204
Sharma V, Duraisamy G (2019) Production and characterization of bio-mix fuel produced from the mixture of raw oil feedstock, and its effects on performance and emission analysis in DICI diesel engine. Environ Sci Pollut Res 26:16742–16761
Sharma YC, Singh B, Upadhyay SN (2008) Advancements in development and characterization of biodiesel: a review. Fuel 87(12):2355–2373
Sharma M, Khan AA, Puri SK, Tuli DK (2012) Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenergy 41:94–106
Shu Q, Gao J, Nawaz Z, Liao Y, Wang D, Wang J (2010) Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl Energy 87:2589–2596
Singh SP, Singh DD (2010) Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: a review. Renew Sust Energ Rev 14:200–216
Ueki Y, Saiki S, Hoshina H, Seko N (2018) Biodiesel fuel production from waste cooking oil using radiation-grafted fibrous catalysts. Radiat Phys Chem 143:41–46
Ullah Z, Bustam MA, Man Z, Khan AS, Muhammad N, Sarwono A (2017) Preparation and kinetics study of biodiesel production from waste cooking oil using new functionalized ionic liquids as catalysts. Renew Energy 114:755–765
Vi Tran TT, Kaiprommarat S, Kongparakul S, Reubroycharoen P, Guan G, Nguyen MH, Samart C (2016) Green biodiesel production from waste cooking oil using an environmentally benign acid catalyst. Waste Manag 52:367–374
Viriya-empikul N, Krasae P, Nualpaeng W, Yoosuk B, Faungnawakij K (2012) Biodiesel production over Ca-based solid catalysts derived from industrial wastes. Fuel 92:239–244
Zabeti M, Daud WMAW, Aroua MK (2009) Activity of solid catalysts for biodiesel production: A review. Fuel Process Technol 90:770–777
Zareh P, Zare AA, Ghobadian B (2017) Comparative assessment of performance and emission characteristics of castor, coconut and waste cooking based biodiesel as fuel in a diesel engine. Energy 139:883–894
Zhang J, Chen S, Yang R, Yan Y (2010) Biodiesel production from vegetable oil using heterogeneous acid and alkali catalyst. Fuel 89:2939–2944
Živković S, Veljković M (2018) Environmental impacts of production and use of biodiesel. Environ Sci Pollut Res 25(1):191–199
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamel, D.A., Farag, H.A., Amin, N.K. et al. Utilization of Ficus carica leaves as a heterogeneous catalyst for production of biodiesel from waste cooking oil. Environ Sci Pollut Res 26, 32804–32814 (2019). https://doi.org/10.1007/s11356-019-06424-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06424-z