Abstract
Understanding of the landscape response to agricultural practices mainly in relation to soil trace metals requires particular attention. Consistent with this, the trend and possible pollution of total and DTPA fraction of Mn, Zn, Cu, and Cd in the agricultural soils developed on different landscape positions involving piedmont alluvial plain (PAP), river alluvial plain (RAP), plateau (PL), and lowland (LL) were investigated. The content of the metal in different soil profiles, grouped by landscape positions, varied in the following orders: total and DTPA-Mn as LL > PAP > RAP > PL, total Zn and Cu as PAP > RAP > LL > PL, total Cd as RAP > PAP > PL > LL, DTPA-Zn as RAP > PAP > PL > LL, and DTPA-Cu as RAP > LL > PL > PAP. A wide variation in the total fraction of Mn (89–985 mg kg−1), Zn (24–152 mg kg−1), Cu (8–27 mg kg−1), and Cd (0.6–1.7 mg kg−1) and in the DTPA fraction of Mn (1.2–11 mg kg−1), Zn (0.3–4.4 mg kg−1), Cu (0.3–3 mg kg−1), Cd (0.03–0.09 mg kg−1) observed as a result of the effects of agricultural practices and landscape properties. The values of both total and DTPA-extractable Mn, Zn, and Cu were enriched in the AP horizon probably due to anthropogenic activities particularly successive use of agrochemical compounds and manure during numerous years. Using soil pollution indices [single pollution (PI) and comprehensive pollution (PIN)], the study soils were categorized mainly as low to moderate pollution and Zn was identified as the major element affecting on the yield of these indices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Distribution, pattern, mobility, bioavailability, and toxicity of trace metals in agricultural soil depend mainly on the parent rock composition and the soil formation process (such as precipitation–dissolution and complexation–dissociation) (He et al. 2005; Rezapour et al. 2014). Nevertheless, the natural amount of the metals may be exceeded in some soils by agricultural practices and human operations. In this case, irrigation water, chemical and organic fertilizers, use of agrochemical (e.g., pesticides and fungicides) and organic amendments are considered as the most vital sources of trace metals in farming soils (Rezapour et al. 2011). The input of such metals through wastewaters (untreated and treated wastewaters) is particularly important in arid and semi-arid regions where water supplies are limited and farmers often have no other decision than utilizing wastewater. In India, Masto et al. (2009) found a significant increase in DTPA-extractable Zn, Cu, Fe, Cd, and Ni after 20 years of use of wastewater compared to the adjacent rain-fed reference. In Iran, Rezapour and Samadi (2011) reported a significant build-up in both DTPA-extractable and total metals of Zn, Cu, Cd, Pb after long-term irrigation with wastewaters in the soils form a semi-arid region. Others, particularly chemical fertilizers, fungicides, manure, have variable levels of Cd, Cr, Ni, Pb, and Zn relying upon their sources (Rezapour et al. 2015b). Among chemical fertilizers, phosphatic compounds are known as one of the most ubiquitous sources of soil pollution through the world. On a global mean, the concentration of As, Cd, Cr, Cu, Pb, and Zn was reported as 11, 25, 188, 32, 10, and 239 mg kg−1, respectively, in phosphate rock (He et al. 2005). Total Cd inputs to soils through phosphate fertilizers have been assessed to be around 334 t year−1 in the nations of the European Union (Kabata-Pendias and Mukherjee 2007). Additionally, long-term repeated use of manure and metal-containing pesticides and fungicides can also gradually add the harmful levels of trace metals in soils. For example, the concentration of Cu has been observed to be as high as 10–20 times greater than the background level in some orchard soils of Florida because of repeated application of Cu-containing fungicides/pesticides/herbicides for managing citrus production (He et al. 2005). Similar patterns were found for Zn, Mn, and As in the soils from England and Spanish (Nicholson et al. 2003; Ramos and Lopez-Acevedo 2004).
It has long been known that agricultural activities and environmental conditions strongly influence on the concentration and trend of soil trace metals. However, the impacts of long-term continuous cultivation and landscape properties on distribution, abundance, and toxicity of trace metals, especially in the calcareous soils and semi-arid regions, are not well recognized. When considering trend and risk of soil trace metals, also, the study region is especially important for the following reasons: (1) It is located near the Urmia Lake with rich water bodies; and (2) the region is one of the biodiversity hot spots in the northwest of Iran. Accordingly, the level and trend of some soil trace metals was investigated in a field under continuous long-term cropping with various types of landforms in a calcareous semiarid condition, where intensive farming has a historical record over five decades. The aims of the research were: (1) to determine the DTPA-extractable and total concentration of Mn, Zn, Cu, and Cd in the cultivated soils under different landscape positions, (2) to describe the effects of long-term cultivation and landscape position on the pattern and distribution of the elements and (3) to assess the rate of possible pollution of the examined soils by Mn, Zn, Cu, and Cd using some soil contamination indices.
Materials and methods
Area description and soil sampling
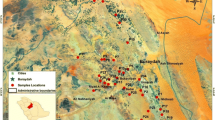
This study was carried out in the Urmia region, the catchments of the Urmia Lake, situated in the south of the Urmia City from 45°07′50″ to 45°17′23″E longitude and 37°20′22″ to 37°30′26″N latitude in West Azerbaijan Province, northwest Iran (Fig. 1). Elevation of the study area ranges between 1290 and 1330 m above sea level, encompassing upper terraces, piedmont, and plain. The geology of the region is mainly formed by the alluvium and recent deposits belonging to the late Quaternary period (10000 BP). The climate of the area is a semi-arid type, characterized by hot–dry summers, wet winters, temperate autumn, and spring with medium occasional precipitation. The mean annual rainfall is 330 mm, and a large portion of it falls from December to April. Mean annual temperatures vary from −6 to 15 °C in winter and between 15 and 31 °C in summer, with an average value of 12 °C. Xeric and mesic are soil moisture and temperature regimes, respectively.
The area is known as one of the most important agroecosystem regions in northwest of Iran, which has been continuously cultivated with wheat, barley in the winter and maize, sugar beet, and horticultural crops in the summer and autumn. This area has a historical record over 50 years, irrigated by rainfall, ground water, and/or seasonal river’s water. Agricultural inputs are especially involved organic and inorganic fertilizers, irrigation, and tillage activities. Chemical and organic fertilizers have been applied at a rate of about 250 kg ha−1 year−1 of urea, 150 kg ha−1 year−1 of superphosphate, and 30–60 t ha−1 year−1 of manure (Malakouti and Gheibi 2000) in all landscape positions. In addition, farmers apply largely various agrochemicals to plant pests and fungi in the area.
A total of 48 surface soils (0–30 cm) and 8 soil profiles were described, classified (Soil Survey Staff 2014), and sampled. Profiles sites were selected based on a reconnaissance survey from different landscape position (Fig. 1): Profiles 1 and 2 developed on the piedmont alluvial plain (PAP), profiles 3 and 7 on the river alluvial plain (RAP), profiles 4 and 5 on the plateau (PL), and profiles 6 and 8 on the lowland (LL). At around each profile, six individual subsamples (0–30 depth) were collected (using a stainless steel spade) within a radius of 10 m of a central point (surface horizon each profile). Subsamples were mixed to achieve a composite soil sample for each sampling site and analyzed in triplicate. Soil samples were air-dried and passed through a 2-mm sieve prior to laboratory analyses. In Table 1, some environmental data are showed for the examined soils.
Physicochemical analyses
The particle-size distribution of the soils was estimated by the hydrometer method (Bouyoucos 1962). Soil pH and EC were measured in a saturated paste and saturated extract, respectively. The values of calcium carbonate equivalent (CCE) and active carbonate calcium (ACC) were estimated by treating the soil samples with 1 N HCl (Nelson and Sommers 1982) and 0.1 N KMnO4 (Drouineau 1942), respectively. Soil organic carbon was determined by a wet digestion method (Nelson and Sommers 1982). The cation exchange capacity (CEC) was determined using sodium acetate (1 M NaOAc) at pH 8.2 (Chapman 1965). Amorphous Fe oxides (Feo) and free Fe oxides (Fed) were determined by ammonium oxalate and citrate-dithionite-bicarbonate (Holmgren 1976), respectively.
The DTPA fraction of Mn, Zn, Cu, and Cd was extracted by shaking a 10 g soil with 20 ml of diethylene-triamine pentaacetic acid (DTPA) extractant (Lindsay and Norvell 1978) followed by centrifugation and filtration. The total fraction of the metals was extracted by heating a 2 g soil in 10 cm3 of HNO3 (1:1) for 15 min at 95 °C following the addition of 2 cm3 deionized water and 3 cm3 of 30% H2O2 (Soon and Abboud 1993). The amounts of Mn, Zn, Cu, and Cd were determined by an atomic absorption spectrophotometer (Shimadzu AA-6300). The accuracy of the procedure was carried out by investigating guaranteed standard reference materials (National Institute of Standards and Technology, USA) replicate analysis of samples and blanks. Descriptive statistics were conducted using SPSS 16 for Windows. The ANOVA was applied to compare the impact of landscape position on trace metal concentration.
Soil pollution indices
Using the international literature, the pollution of soil trace metal can be highlighted by a single trace metal or a combination of several trace metals. In this study, the type of metals responsible for pollution was identified by single-factor pollution index (PI) and the cumulative effect of all elements was described using comprehensive pollution index (PIN) or Nemerow pollution index (Zhang et al. 2013; Bailin et al. 2016). These pollution indices were calculated by following relations:
where C i is the determined concentration of the metal i at each sampling point, and S i is the regional background amount of the metal i; max (PI) = the maximum content of the single-factor pollution index, n = number of studied metals, and i = ith the metals. The values of PI and PIN proposed in some descriptive classes as shown in Table 1. We considered the content of trace metals in the C or CB horizon as background (Zhang et al. 2013).
Results and discussion
General soil quality
The soils were classified as Inceptisols [Typic Haploxerepts (profiles of 1 and 2), Typic Endoaquepts (profiles of 3 and 7), Typic Calcixerepts (profiles of 4 and 5), and Typic Halaquepts (profiles of 6 and 8) according to Keys to Soil Taxonomy [(2014) (Table 1)]. These soils are developed mainly on alluvial deposits and were characterized as the slightly alkaline (pH 7.5) to the alkaline category (pH 8.2) and moderately (calcium carbonate, 2–10%) to very high calcareous class (calcium carbonate, 20–30%).
Long-term agricultural practices on the landscape cause some variations in the soil physicochemical attributes because of changes in both natural and anthropogenic processes (Table 2). There was a remarkable change in the particle-size distribution among the different positions of the landscape (e.g., the values of clay ranked as RAP > PL > PAP > LL) mainly due to variation in geomorphologic condition of the landscape (such as slope and topography) and soil type (Rezapour et al. 2010). The silt content varied significantly among landscape positions with the lowest values in the river alluvial plain (39.87 ± 2.39), while the highest (55.25 ± 2.3) was observed in the piedmont alluvial plain.
Soil developed on the lowland position possessed lower slope and topography along with more non-leaching and reducing conditions compared to those in other landscape positions as showed by the presence of redoximorphic features and gleization process. Hence, the values of soil pH (7.95 ± 0.20) and soil EC (1.9 ± 1.4 dS m−1) were significantly higher in the lowland compared to other positions mainly as a result of overland flow and inflow of soluble salts from around the area to the lowland position (Rezapour et al. 2011, 2012). However, the values of soil pH (7.5–8.2) and EC (0.3–2.5 dS m−1) were in an optimal range for the development of numerous plant species in semi-arid environments (Brady and Weil 1999). The content of organic carbon varied from 0.22 to 0.78, 0.34 to 1.74, 0.18 to 1.1, and 0.22 to 1.2 g kg−1 in PAP, RAP, PL, and LL, respectively. In the light of this, it could be considered into very low (0.4–0.6 g kg−1) to moderate (1–1.8 g kg−1) categories in the majority of the soil samples (Brady and Weil 1999). Organic carbon content was the highest in the river alluvial plain where the highest value of the clay fraction recorded. This might be related to the fact that clay can protect organic matter from oxidation and degradation through making clay–humus complexes (Rezapour et al. 2014). The values of soil CEC (cation exchange capacity) classified as medium category [(12–25 cmol kg−1) (Brady and Weil 1999)] and ranked in the order of RAP > PAP > PL > LL. The amount of CEC was the highest in the river alluvial plain (24.43 ± 4.39), linked to the high rate of clay fraction and organic carbon. This suggests that the impacts of clay and organic carbon may be attributed significantly to soil CEC as was evidenced by past works (Rezapour 2014; Rezapour et al. 2015a). The content of soil CCE (calcium carbonate equivalent) and ACC (active carbonate calcium) some varied among landscape positions, with the lowest value in the lowland whereas the highest were recorded in the plateau. In the plateau position, calcareous parent material was tilled, deeply, to cultivate a certain depth of soil by farmers, thereby enhanced the amounts of soil CCE and ACE (Rezapour and Samadi 2011).
The vertical distribution of trace metals
The weighted mean (WM) and the vertical distribution of total and DTPA-extractable forms of Mn, Zn, Cu, and Cd are presented in Fig. 2 and Table 3. As can be seen, impact of cultivation and landscape properties resulted in considerable changes in the distribution of the trace metals.
The content of total Mn was in the range of 89–985 mg kg−1 with a weighted mean from 315 to 555 mg kg−1. These values are within the ranged reported (2–10,000 mg kg−1, an average amount of 1000 mg kg−1) for agricultural soils (Kabata-Pendias and Mukherjee 2007). Total Mn showed association with landscape positions and decreased in the order of the LL (555 mg kg−1) > PAP (521 mg kg−1) > RAP (388 mg kg−1) > PL (316 mg kg−1). Sharma et al. (2005) made similar findings for calcareous alkaline soils in different landforms of the Siwalik Himalaya. The distribution of total Mn also showed an irregular pattern into soil profiles, although its amount was typically greater in AP horizons where interaction between the used agricultural input (e.g., irrigation water, farm manures, biosolids) and its receiving soils was occurring. McBride (2004) reported an increasing trend in the values of total Mn in the soils from Upstate New York after the use of biosolids and composts. The range of DTPA-extractable Mn was 1.2–11 mg kg−1 with a weighted average of 3–6 mg kg−1. It constituted about 0.88–1.2% of the total Mn, which is consistent with past observations (Rezapour et al. 2011). Its profile distribution followed a pattern of higher accumulation in the soil solum (AP and B horizons) where the greater temperature (by tillage activities) and the number of wetting–drying cycles (by irrigation practices) were occurred. Such condition can lead to increase in the weathering of Mn-bearing minerals (Rezapour et al. 2015b), thereby enhanced the amounts of DTPA-extractable Mn.
The range of total Zn was 24–152 mg kg−1 with a weighted mean from 66.5 to 92.2 mg kg−1 in different landscape positions. Such patterns are comparable to the reported data for the Inceptisols of northwest Iran (Rezapour and Samadi 2011). The profile distributions showed higher accumulation of total Zn in the surface horizons (AP horizon) in the majority of soil profiles, and the intensity of accumulation was more pronounced in the piedmont alluvial plain, followed by the river alluvial plain. The DTPA-Zn varied from 0.3 to 4.4 mg kg−1 (with a weighted average of from 0.88 to 2.2 mg kg−1) and constituted 1.6–2.4 of the total Zn. Generally, its values were greater in the surface horizons than to those in subsurface B and C horizons which could be explained by biomining and turnover by plant residues (Brady and Weil 1999; Rezapour and Samadi 2011). Based on the literature, complexing factors produced by organic compounds promote metal availability in soils (Brady and Weil 1999). Absence of statistically significant correlation between total Zn and DTPA-Zn shows that the amount of DTPA-Zn is not related to total Zn in the soils.
The total Cu amount in different landscape positions varied from 8 to 27 mg kg−1 with a weighted mean between 11 and 20 mg kg−1. More total Cu was observed in the soil of PAP (16–21.5 mg kg−1, WM = 18.3) and RAP (8.5–23 mg kg−1, WM = 18) than to that in other positions, a pattern similar to that for total Zn. There was a weak coefficient of correlation (r = 0.30) between clay and total Cu and Zn (r = 0.29), implying that clay fraction is not the sole determinant of the Cu and Zn distribution in these soils. Manta et al. (2002) and Eze et al. (2010) observed a similar data in the soils of the Italy and Ghana, respectively. In this study, the reason for the weak correlation between Cu and Zn with clay could be found in the mineralogical constituents, where illite, chlorite, and kaolinite together constitute more than 80% of the mineral constituents in the clay fraction of these soils (Rezapour and Samadi 2011, 2012). Generally, the clay minerals such as illite and chlorite that possess low CEC have a lower affinity to absorb trace metals (Brady and Weil 1999). Consequently, the greater amount of the above-mentioned clay minerals and corresponding low surface area and CEC in these soils probably led to the weak influence of the clay fraction on the adsorption and retain of trace metals. Rezapour et al. (2014) found higher and stronger sorption of copper and zinc on swelling clay minerals (e.g., montmorillonite and vermiculite) whereas it was at least on illitic compounds. The concentrations of total Cu as well as total Zn are so lower than to those described for their maximum permissible level (Cu < 100 mg kg−1 and Zn < 300 mg kg−1) in different soils (Kabata-Pendias and Mukherjee 2007).
The content of DTPA-Cu ranged between 0.30 and 3.0 mg kg−1, with a weighted average from 0.38 to 1.9 mg kg−1. It makes around 2–5% of the total Cu. DTPA-Cu was higher in the soils developed on river alluvial plain (WM = 1.82 mg kg−1) followed by lowland, terrace, and piedmont alluvial plain (Fig. 2), a trend similar to that for organic matter (Table 2). Probably, larger amount of organic matter in the soils of RAP compared to those in other positions (Table 2) further lends support to the greater Cu availability through the Cu-strong complex formations in the soils of river alluvial plain (Sharma et al. 2005). Such assessment was evidenced by a significant correlation recorded between organic carbon with both total (r = 0.56, P < 0.01) and DTPA-Cu (r = 0.44, P < 0.05). Adriano (1986) point out that Cu forms the most stable complexes with organic matter. DTPA-Cu showed a positive significant correlation with total Cu (r = 0.61, P < 0.01), suggesting that total Cu may be a factor determinant of available Cu in soils that is compatible with the previous studies (Rezapour et al. 2012).
The range in the total Cd content was 0.6–1.7 mg kg−1 with a weighted mean from 078 to 1.5 mg kg−1. Total Cd also reflected association with landscape positions and decreased in the order of the RAP (WM = 1.18 mg kg−1) > PAP (WM = 1.0 mg kg−1) > PL (WM = 0.9 mg kg−1) > LL (WM = 0.83 mg kg−1). In the 50% of the examined soils (profiles of 1, 3, 4, and 8), its profile distribution followed a pattern of higher accumulation in the subsoil than topsoil, an opposite trend to that for total Mn, Zn, and Cu (Table 3). Such pattern seems to be as a result of the mobilization of Cd from surface horizons to subsurface horizons under the influence of the strong irrigation and intensive cropping system over a long time (Kabata-Pendias and Mukherjee 2007). Rezapour et al. (2011) and Rezapour and Samadi (2011) also reported similar observations for some calcareous alkaline soils in northwest of Iran. The vertical distribution of DTPA-Cd varied from 0.03 to 0.09 mg kg−1 with a weighted average between 0.045 and 0.068 mg kg−1. It makes around 3–8% of the total Cd which is higher than the shares of DTPA-extractable forms of Mn, Zn, and Cu to their respective total fractions. The profile distribution of DTPA-Cd did not show any regular pattern with depth in the majority of the soil profiles; however, its amount was greater in B horizons in the 50% of the studied soil profiles, a similar trend to that for total Cd. The content of DTPA-extractable Cd showed a negative correlation with the clay fraction which could be due to the predominance of illite and chlorite minerals in clay fractions of the soils (Rezapour and Samadi 2011) and their high base (Ca, Mg, Na, and K) saturation, which does not allow much preferential exchange sites for Cd2+ adsorption (Brady and Weil 1999; Sharma et al. 2005). The values both DTPA-Cd and total were within the typical range of non-polluted soils [(DTPA-Cd < 0.5 mg kg−1) Malakouti and Gheibi 2000; total Cd < 5 mg kg−1) Kabata-Pendias and Mukherjee 2007].
In general, the total amount of trace metals is remarkably greater in topsoil than in the subsoil, with few exceptions, because of their strong affinities with the soil solid phase mainly associated: (a) with organic matter and (b) with alumino-silicate minerals and iron oxides as the major products of bedrock weathering in the surface soil (Rezapour et al. 2014; Esmaeili et al. 2014). The content of a metal in surface soil divided by the content of that metal in the corresponding subsurface soil (RTE) (Rezapour and Samadi 2011) is in the following ranges: Mn from 0.88 to 1.36, Zn from 0.77 to 2.09, Cu from 0.68 to 1.2, and Cd from 0.54 to 1.09 in the different landscape positions. Irrespective of landscape position, the values of RTE also were in the sequence of Mn > Zn > Cu > Cd. This sequence shows that Cd was the most mobile element and Mn, in contrast, was the element most strongly retained by the soils. Esmaeili et al. (2014) and Rezapour et al. (2014) found similar data.
In spite of the fact that soil pH is considered as the most effective soil attribute controlling the behavior and pattern of soil trace metals (Brady and Weil 1999), there was a nonsignificant correlation between the level of soil pH to: total Mn (r = −0.05), DTPA-Mn (r = −0.18), total Zn (r = −0.007), DTPA-Zn (r = −0.26), total Cu (r = −0.35), DTPA-Cu (r = −0.36), total Cd (r = −0.23), and DTPA-Cd (r = −0.2). Such trend could be related to the narrow range of soil pH contents (7.5–8.2) and the soil condition in the study area (neutral-alkaline condition) which agrees with the findings of Rezapour and Samadi (2012) and Esmaeili et al. (2014) from the calcareous soils of Iran. Other researchers (Eze et al. 2010; Bailin et al. 2016) also obtained similar results from different soils of the world. On the other hand, the trace metals mainly Zn, Cu, Cd showed a nonsignificant correlation with other soil characteristics, [e.g., soil CEC, EC, CCE, sand, silt (data not shown)], probably as a result of their contribution from various anthropogenic sources (Zhou et al. 2014).
The trend and pattern of soil trace metals in the surface horizons
Considering the fact that anthropogenic processes (e.g., irrigation and tillage practices, the application of fertilizers and agrochemicals) and root activities are more exposed in the topsoil (AP horizon), the variability of the examined trace metals was statistically compared in the different position of landscape (Table 4). Irrespective of the landscape position, the data on surface distribution of the values of total fraction of different trace metals revealed the dominance of Mn, followed by Zn, Cu, and Cd, a pattern different to the order established for the available fraction (Mn > Cu > Zn > Cd).
Total Zn, Cu, and Cd showed association with landscape position and decreased in the order of the PAP > RAP > LL > PL. A considerable variation in the average content of total Mn (468–506 mg kg−1), Zn (67–104 mg kg−1), Cu (17.5–21 mg kg−1), and Cd (0.98–1.2 mg kg−1) was observed among the different position of landscape in the topsoil, although the differences were not significant statistically. Considering the fact that parent material is almost the same in the region, the differences may be due to geomorphic and physicochemical processes as was well documented by Rezapour et al. (2011, 2012). However, the origin of anthropogenic process cannot be ruled out.
DTPA-Mn, Zn, Cu, and Cd also showed an association with landscape position in the topsoil and decreased in the order of the RAP > LL > Pl > PAP for Mn and Cu, LL > PAP > RAP > PL for Zn, and LL > PL > RAP > APP for Cd. Wherever the contents of clay and organic carbon was accumulated (river alluvial plain), more the amounts of DTPA-Mn and Cu were present; however, it was not the case for DTPA-Zn and Cd (Tables 2, 4). This is perceptible, due to clay and OC are known to retain Mn and Cu in their adsorption and complexes (Sharma et al. 2005). Previous works have also showed that clay and organic carbon amounts considerably control the pattern of Mn and Cu in soils (Rezapour and Samadi 2011; Rezapour et al. 2014). On the other hand, DTPA-Cd and Zn were higher in the topsoil of lowland position where the highest amounts of Fed and Feo were present (Table 2), suggesting that Cd and Zn may be adsorbed and maintenance by iron oxides. A positive significant correlation recorded between DTPA-Cd and Fed (r = 0.54, P < 0.01); DTPA-Cd and Feo (r = 0.49, P < 0.05); DTPA-Zn and Fed (r = 0.61, P < 0.01); DTPA-Cd and Feo (r = 0.56, P < 0.05). These values showed that iron oxides contribute significantly to soil DTPA-Cd and Zn and the role of Fed was more than Feo. These data are comparable with previous literature that iron oxides have a great affinity for the adsorption of some trace metals in soils (Kabata-Pendias and Mukherjee 2007).
Assessment of soil pollution indices
The amounts of the single-factor pollution index (PI) and Nemerow pollution index (PIN) were computed by the total fraction of Mn, Zn, Cu, and Cd in the cultivated soils (AP horizons) developed on the different landscape positions to indicate the relative degree of soil contamination (Tables 5, 6; Figs. 3, 4). The contents of PI ranged as 0.92–1.95, 0.8–4.69, 0.76–2.76, and 0.7–2.68 for Mn, Zn, Cu, and Cd, respectively, indicating the classes of “cleans to high pollution”. Based on regional background values, more than 60% of the soil samples have class 2 and 3 PI for Zn, Cu, and Cd, indicating “low to moderate contamination”. Conversely, just 8% of the total soils were categorized as “high pollution” by Zn. The measured PIN contents were in the sequence of Zn (3.64) > Cu (2.3) > Cd (2.12) > Mn (1.65), an observation similar to that for the values of PI. This implies that the calculated PIN falls in class 5 and 3 for Zn and Mn showing slight (Mn) and high (Zn) pollution. For copper and cadmium, determined PIN fell toward class 4 demonstrating the examined soils are moderately polluted by Cu and Cd.
For the soils developed on the different landscape positions, the mean PI of Mn and Cd decreased in the order of PL > LL > PAP > RAP whereas the order was as PL > LL > RAP > PAP and LL > RAP > PL > PAP for Cu and Zn, respectively (Table 6). This implies that the soils developed on plateau position have a higher affinity to the load rate of Mn, Cd, and Cu compared to other positions.
The data on pattern of PIN content in the soils developed on the different landscape positions (Figs. 4, 5) revealed dominance of the mean PIN of Mn and Cu in PL, followed by RAP, LL, and PAP. The mean PIN of Zn was highest in the soils of the lowland, moderate in the soils of the river alluvial plain and plateau, and lowest in the soils of the piedmont alluvial plain, a dissimilar pattern to that for the mean PIN of Cd. Figure 5 reflects that the PIN of the four trace metals was significantly higher in the lowland position (min = 1.2, max = 3.7, mean = 2), followed by the plateau (min = 1.6, max = 2.7, mean = 1.7), the river alluvial plain (min = 0.92, max = 2.4 mean = 1.5), and the piedmont alluvial plain (min = 1.1, max = 1.7, mean = 1.2), indicating the classes of “slight to moderate pollution”. In this regard, Zn was the highest contributor to the PIN value followed closely by Cu (Fig. 4). The contributing factor to the accumulation of Zn and Cu can be related to application of excessive agrochemical compounds and organic fertilizers on long term. In the light of this, farmers apply largely various agrochemicals (e.g., Bordeaux mixture, Bordeaux fix, Mancozeb, Zineb) to plant pests in the area. In the composition and formulation of such compounds, elements such as Zn and Cu were applied predominantly which could lead to their accumulation in the soils (Rezapour et al. 2015b). Such investigations are in line with other research outcomes. For instance, some agricultural soils in South Australia and Florida contain more than 200 mg kg−1 Cu because of fungicide sprays (He et al. 2005). In England and Wales, it was estimated a total input of 21 and 8 t years−1 of Zn and Cu to be added from agrochemical compounds to agricultural soils (Nicholson et al. 2003).
Animal manure and compost, applied widely in the investigated soils, has also been suggested to contribute to Zn as well as Cu levels in agricultural soils. Consistent of this, a contribution about 37–40% of total Zn inputs has been reported from livestock manure in farming soils of England and Wales (Nicholson et al. 2003). Ramos and Lopea-Acevedo (2004) found that the use of cow manure significantly enhanced amounts of the total Zn and DTPA-extractable Zn in basic soils from Spain regions. In addition, McBride (2004) reported high Zn and Cu concentrations in manure due to the Zn and Cu-enriched feed additives used to promote animal growth. However, the association of Zn and Cu with Cd as highlighted before (He et al. 2005) suggests that the application of phosphate fertilizers is the important source for Zn and Cu in the agricultural land. Based on international literature (Adriano 1986; Kabata-Pendias and Mukherjee 2007), phosphate rock, on average, contains 25, 32, and 240 mg kg−1 of Cd, Cu, and Zn, respectively. Reports from China (Du et al. 2015) showed that the accumulation of considerable amounts of Cd (6 mg kg−1) and Cu (756 mg kg−1) in this country, particularly because of the wide use rate of phosphate fertilizers (about 7.48 × 1010 t in a year) to the soil.
As a result, uncontrolled application rates of pesticides–fungicides, animal wastes, and manure as well as phosphate fertilizers over a long time were known as major sources for the input of Zn, Cu, and Cd to the studied soils. Indeed, the input, release, and pattern of the soils trace metals were as functions of several sources in the area. Contributions by the trace metals to multiple sources were evidenced by the absence of significant correlations between soil attributes and trace metals which is in agreement with the dates of Du et al. 2015 (2008) and Rezapour et al. (2015b). The results, also, show that Zn and Cu positively correlate at P > 0.01 (r = 0.59), implying a similarity in their occurrences or an anthropogenic source that control their trend.
Conclusion
Impact of long-term cultivation and landscape properties on the amount, trend, and pattern of selected trace metals has been examined in this work. Soils developed on the different landscape positions involving PAP, RAP, PL, and LL reflected a heterogeneous response to the level and pattern of both total and DTPA-extractable fractions of Mn, Zn, Cu, and Cd. The weighted mean of the metals in the different soil profiles, grouped by different landscape positions, varied in the following orders: total and DTPA-Mn as LL > PAP > RAP > PL, total Zn and Cu as PAP > RAP > LL > PL, total Cd as RAP > PAP > PL > LL, DTPA-Zn as PAP > RAP > PL > LL, and DTPA-Cu as RAP > LL > PL > PAP. Such patterns suggest a strong association of the trace metals to the landscape positions. In all soil profiles, developed from different landscape positions, total and available Mn, Zn and, Cu were enriched in the surface horizons (AP horizon), where agricultural activities are highlighted, presumably as a result of their strong association with the anthropogenic activities and organic matter. Although the content of both available and total fractions of Mn, Zn, Cu, and Cd was below their maximum permissible limits, there was an enhancing trend in the content of the metal compared to their regional background values, particularly because of the uncontrolled use rates of different agrochemicals compounds (fertilizers, manure, and pesticides–herbicides–fungicides) during numerous years. The pollution indices (PI and PIN) highlighted low to moderate pollution levels for total Mn, Zn,Cu, and Cd in the majority of the studied soils, and zinc was identified as the major element affecting on the PI and PIN yield. The comprehensive pollution index was significantly higher in soils from the lowland position (mean value = 2) followed by the plateau (mean value = 1.7), the river alluvial plain (mean value = 1.5), and the piedmont alluvial plain (mean value = 1.2), fall into the categories of slight to moderate pollution. In Iran, high rates of chemical fertilizers, chemical compounds, and organic manures have been used by farmers for agricultural production. Therefore, the quality of the applied agrochemical should be monitored periodically (a) to minimizing trace metal pollution and (b) to ensuring soil–water–crop heath. To achieve these facts, great attention should be paid to the logical application of organic and inorganic fertilizers, manures, pesticides–fungicides, and balanced fertilization in the agricultural landscape.
References
Adriano DC (1986) Trace elements in the terrestrial environment. Springer, New York
Bailin L, Xinwang M, Shiwei A, Saiyong Z, Wenya Z, Yingmei Z (2016) Spatial distribution and source identification of heavy metals in soils under different land uses in a sewage irrigation region, northwest China. J Soils Sediments 5:1547–1556
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analysis of soils. Agron J 54:464–465
Brady NC, Weil RR (1999) The nature and properties of soils. Prentice-Hall, Englewood Cliffs
Chapman HD (1965) Cation exchange capacity. In: Black CA (ed) Methods of soil analysis, Part 2. American Society of Agronomy, Madison, pp 891–900
Drouineau G (1942) Dosage rapide du calcaire du sol, nouvelles donnees sur la separation et la nature des fractions calcaires. Annual Agron 12:441–450
Du P, Xie Y, Wang SH, Zhao H, Zhuo Zhang Z, Wu B, Li F (2015) Potential sources of and ecological risks from heavy metals in agricultural soils, Daye City, China. Environ Sci Pollut Res 22:3498–3507
Esmaeili A, Moore F, Keshavarzi B, Jaafarzadeh N, Kermani M (2014) A geochemical survey of heavy metals in agricultural and background soils of the Isfahan industrial zone. Iran. Catena 121:88–98
Eze PN, Udigwe TK, Stietiya MH (2010) Distribution and potential source evaluation of heavy metals in prominent soils of Accra Plains, Ghana. Geoderma 156:357–362
He ZLL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elements Med Biol 19:125–140
Holmgren GG (1976) A rapid citrate-dithionate extractable iron procedure. Soil Sci Soc Am Proc 31:210–211
Kabata-Pendias A, Mukherjee AB (2007) Trace elements in soils and plants. Springer, Berlin
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Malakouti MJ, Gheibi MN (2000) Determination of critical levels of nutrients in soils, plant, and fruit for the quality and yield improvements of Iran’s strategic crops. Applied Agricultural Science Publishers, Tehran (In Persian)
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieria M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 30:229–243
Masto RE, Chhonkar PK, Singh D, Patra AK (2009) Changes in soil quality indicators under long-term sewage irrigation in a sub-tropical environment. Environ Geol 56:1237–1243
McBride MB (2004) Molybdenum, sulfur, and other trace elements in farm soils and forages after sewage sludge application. Commun Soil Sci Plant Anal 35:517–535
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL (ed) Methods of soil analysis, part 2. American Society of Agronomy, Madison, pp 539–580
Nicholson FA, Smith SR, Alloway BJ, Carlton- Smith C, Chambers BJ (2003) An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci Total Environ 311:205–219
Ramos MC, Lopez-Acevedo M (2004) Zinc levels in vineyard soils from the Alt Penedes-Anoia region (NE Spain) after compost application. Adv Environ 8:687–696
Rezapour S (2014) Response of some soil attributes to different land use types in calcareous soils with mediterranean type climate in north-west of Iran. Environ Earth Sci 71:2199–2210
Rezapour S, Jafarzadeh AA, Samadi A, Oustan S (2010) Distribution of iron oxides forms on a transect of calcareous soils, north-west of Iran. Arch Agron Soil Sci 56:165–182
Rezapour S, Samadi A (2011) Soil quality response to long-term wastewater irrigation in Inceptisols from a semi-arid environment. Nutr Cycle Agroecosyst 91:269–280
Rezapour S, Samadi A (2012) Assessment of inceptisols soil quality following long-term cropping in a calcareous environment. Environ Monit Assess 184:1311–1323
Rezapour S, Samadi A, Khodaverdiloo H (2011) An investigation of the soil property Changes and heavy metal accumulation in relation to long-term wastewater irrigation in the semi-arid region of Iran. Soil Sediment Contamin 20:841–856
Rezapour S, Samadi A, Khodaverdiloo H (2012) Impact of long-term wastewater irrigation on variability of soil attributes along a landscape in semi-arid region of Iran. Environ Earth Sci 67:1713–1723
Rezapour S, Golmohammad H, Ramezanpour H (2014) Impact of parent rock and topography aspect on the distribution of soil trace metals in natural ecosystems. Int J Environ Sci Technol 11:2075–2086
Rezapour S, Najari S, Ghaemian N (2015a) The impacts of long-term intensive agriculture on the Vertisol properties in a calcareous region. Environ Monit Assess. doi:10.1007/s10661-015-4453-2
Rezapour S, Kouhinezhad P, Samadi P, Rezapour M (2015b) Level, pattern, and risk assessment of the selected soil trace metals in the calcareous-cultivated Vertisols. Chem Ecol 8:692–706
Sharma BD, Mukhopadhyay SS, Arora H (2005) Total and DTPA-extractable micronutrients in relation to pedogenesis in some alfisols of Punjab, India. Soil Sci 170:559–572
Soil Survey Staff (2014) Keys to Soil Taxonomy. U. S. Government Printing Office, United States Department of Agriculture, Natural Resources Conservation Service, Washington, D.C.
Soon YR, Abboud S (1993) Cadmium, chromium, and nickel. In: Carter MR (ed) Soil sampling and methods of soil analysis. Lewis Publishers, Boca Raton, pp 101–108
Zhang CL, Li ZY, Yang WW (2013) Assessment of metals pollution on agricultural soil surrounding a lead-zinc mining area in the Karst region of Guangxi, China. Bull Environ Contam Toxicol 6:736–741
Zhou L, Yang B, Xue N, Li F, Seip HM, Cong X, Yan Y, Liu B, Han B, Li H (2014) Ecological risk and potential sources of heavy metals in agricultural soils from Huanghuai Plain, China. Environ Sci Pollut Res 21:1360–1369
Acknowledgements
The researchers wish to thank the Urmia University for the financial support of this research (Grant No. 10/780).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Rezapour, S., Moazzeni, H. Assessment of the selected trace metals in relation to long-term agricultural practices and landscape properties. Int. J. Environ. Sci. Technol. 13, 2939–2950 (2016). https://doi.org/10.1007/s13762-016-1146-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1146-5