Abstract
Thirty-nine paired soil samples belonging to Inceptisols, irrigated with wastewater and well water, were examined to monitor the combination of clay mineralogy, physicochemical properties, and trace metals (Zn, Cu, Cd, and Pb) following long-term wastewater irrigation. Results of XRD analysis indicated that the quantity of clay minerals did not change by long-term wastewater irrigation although some modifications in smectite were occurred in Fluvaquentic Endoaquepts compared to control (well water-irrigated soil). Irrigation with wastewater was resulted in an increase in clay content from 3 to 17%, EC from 90 to 160%, organic carbon from 13 to 44%, and CEC from 10 to 13%. In this context, improvement in total N was as 80–110%, available P as 270–330%, and available K as 5–50% as compared to the control soil. Irrigation with wastewater was led to a significant enrichment in both EDTA-extractable and total metals of Zn, Cu, Cd, Pb, mainly as result of the combination of their addition through wastewater along with interaction between the used wastewater and its receiving soils. In the light of this, wastewater irrigation system showed the relative enrichment (RE) for total-Zn, Cu, Cd, and Pb as 3.6–6.8, 2.8–4.9, 4.4–5.7, and 1.8–2.3, respectively. Fine particle-size fraction (<0.002 mm), organic matter, and calcium carbonates were appeared as the main agents in retaining the total trace metals as reflected in significant positive correlation among the physicochemical properties and the examined metals. Degree of contamination (Cd) for the four analyzed metals was in the range of 3–5. Based on Cd values, the wastewater-irrigated soils were classified as moderate degree of contamination (2 ≤ Cd < 4) to high degree of contamination (4 ≤ Cd < 8). Fluvaquentic Endoaquepts highlighted to be a major contributor of the load and contamination rate of trace metals regarding to geoaccumulation index, contamination factor, and Degree of contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Historically, using wastewater on land for irrigation purposes has become a common practice in water-scarce environments, where farmers often have no other choice than using wastewater (Pedrero et al. 2010; Qadir et al. 2010). In the light of this, 70 million m3year−1 of wastewater is used on agricultural lands in Iran (Radcliffe 2004) as a developing country. As urban populations in developing countries increase, and residents seek better living standards, larger amounts of fresh water are diverted to domestic, commercial, and industrial sectors, which generate greater volumes of wastewater (Qadir et al. 2007). In view of fact that wastewater is discharged with little or no treatment in natural water bodies (Qadir et al. 2010), the soil contain wastewater irrigation system can reflect both negative and positive aspect. Examples of characteristics that modify soil quality positively are those that maintained or even increased the level of soil fertility properties. Such properties include the addition of organic matter, mineralisable N, phosphorus, potassium, essential trace elements, microbial biomass, enzyme activities (Arienzoa et al. 2009). Keeley and Quin (1979) reported a significant increase in soil organic carbon (4.45 vs. 3.88%), potassium (1.4 vs. 0.4 cmolc kg−1), and base saturation (87 vs. 66%) after 80 years of application of wastewater compared to the non-irrigated control. Similarly, Masto et al. (2009) manifested that use of sewage for irrigation (after 20 years) improved the organic carbon to 0.51–0.86% and build-up of available N (397 kg ha−1), available P (128 kg ha−1), available K (524 kg ha−1), and available S (65.5 kg ha−1). In contrast, examples of properties that change soil quality are mainly derived through increase of soil salinity, undesirable pH values, and excessive leaching of nutrients and heavy metals (Arienzoa et al. 2009).
In recent years, most of studies concerning the introduction of effluent-associated contaminants to soils were focused on heavy metals accumulation. Indeed, among wastewater threats, trace heavy metals accumulation in the soil have highlighted as an issue of global attention. In this context, most concern has been influenced to zinc (Zn), copper (Cu), cadmium (Cd), and lead (Pb) which are commonly occur in considerable values in wastewater and other pollutants. Contamination of soils with heavy metal appear when an element or a substance is present in greater than natural (background) concentrations as a results of human activity and has a net detrimental effect on the environment and its components (Kabata-Pendias and Mukherjee 2007). Soil is the main sink for adsorb and retain heavy metals in wastewater irrigation system. Through this paradigm, when the capacity of soil to retain toxic metal is declined following continuous loading of wastewater or changes in soil properties, soil can release the metals into environment for plant uptake (Mapanda et al. 2005). By the interpretations, long-term using wastewater irrigation can enrich heavy metal to phytotoxic levels and result in reduced plant growth and/or enhanced metal concentration in plants, leading to enter in food chain as pathways: Soil → Plant → Animal → Human.
With regarding to the scarce of historical soil data before irrigation, the most commonly used methods to determine the impact of wastewater irrigation on soil properties and contaminants accumulation is to compare soil parameters and pollutants levels between wastewater-irrigated and reference soil (Xu et al. 2010). Accordingly, we studied a region irrigated with treated wastewater and non-treated from north-west of Iran, where consume the large volume of fresh water and generate huge amounts of wastewater promote farmers in using wastewater as attractive and available source of irrigation. The objective of present study was: (1) to assess some soil quality indices following long-term wastewater irrigation, (2) to determine the DTPA-extractable and total concentration of Zn, Cu, Cd, and Pb, and (3) to investigation some trace metals enrichment factors and the degree of contamination of the studied soil.
Materials and methods
Description of study site
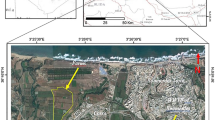
The investigated site was situated on Urmia area (45°05′ to 45°08′ E; 37°32′ to 37°38′ N) in The Western-Azarbaijan province, north-west Iran. A semi-arid climate exists in the region with soil moisture and temperature regimes xeric and mesic, respectively. The area is containing agricultural fields that have been continuously cropped with fruit tree species [such as apple (Pyrus malus), apricot (Prunus armeniaca), peach (Prunus persica)], and horticultural crops [such as cabbage (Brassica oleracea), beans, and lettuce (Lactuca sativa)] and has received wastewater for at least 40 years as flooding irrigation. The main sources of wastewater include municipal wastewater, household, and industrial effluents, which are used directly by local farmers. A total of 78 soils belonging to three soil types were described (Soil Survey Staff 2003), classified (Soil Survey Staff 2010), and sampled from the wastewater-irrigated zone (as two-third (2/3) wastewater and one-third (1/3) groundwater) and the adjacent zone (as reference) irrigated by well water. The control area is covered by shrubs and native grasses (such as Cartamus oxycanta, Alhaji camelorum, Achillea millefoleoum, Chicorium intybus, and Astragalus officinalis). The soils were classified as Typic Calcixerepts (TC), located in 45°06′ E; 37°33′ N, Typic Haploxerepts (TH), located in 45°06′ E; 37°36′ N, and Fluaquentic Endoaquepts (FE), located in 45°08′ E; 37°38′ N, for both wastewater-irrigated soil and reference soil. Twenty-six surface soil samples (0–30 cm) for each soil type (13 samples for the wastewater-irrigated and 13 samples for the well water-irrigated soils) were analyzed. In each soil type, the samples (wastewater irrigated and control soils) were selected in similar slope, aspect, and parent materials.
Analytical methods
Mineralogical analysis
The analysis methods of Kunze (1965) and Mehra and Jackson (1960) used for mineralogical analysis. Sub samples were treated with 30% H2O2 and Na-dithionite-citrate-bicarbonate to remove organic matter and Fe oxides, followed by separation of the size fraction. Orientated clay samples were subjected to Mg, Mg plus ethylene glycol-solvated, and K saturation and heat of 550°C. The scanning operation was performed by X-ray diffraction technique using 2θ from 3° to 30° and CuKα radiation source. The percentage of clay minerals was estimated semi-quantitatively through Biscaye methods (1965).
Physicochemical properties
Particle-size distribution was determined using the pipette methods as described by Day (1966). The soil pH was determined in saturated paste and used the saturated extract to measure electrical conductivity (EC). Calcium carbonate equilibrium (CCE) was estimated using acid neutralization (Nelson 1982). Soil organic carbon (SOC) was measured by dichromate oxidation (Nelson and Sommers 1982); total soil N by the Kjeldahl methods (Bremner 1982); soil available phosphorus by 0.5 M NaHCO3 (Olsen and Sommers 1982); and Soil available K and cation exchange capacity (CEC) by 1 N ammonium acetate at pH 7 and 8.2, respectively (Thomas 1982).
DTPA-extractable Zn, Cu, Cd, and Pb determined with the method described by Lindsay and Norvell (1978). Total trace metal concentrations were extracted using a mixture of concentrated (the equa regia digest method) HCl–HNO3 as HCl: HNO3 ration of 3:1 (Baker and Amacher 1982). The soil extracts were analyzed by an atomic absorption spectrophotometer (Shimadzu AA-6300). The results for each parameter were recorded as the mean content of the each soil solum. The ranges of studied heavy metals values were compared to the maximum permissible limits reported by Kabata-Pendias and Mukherjee (2007). Relative enrichment (RE) or depletion (RD) of each parameter in the wastewater-irrigated soil was measured through calculating the ratio of the mean results from wastewater-irrigated soil to the mean from the reference soil (Taylor et al. 2010).
The pH, EC, total dissolved soluble (TDS), total hardness, and dissolved metals in wastewater and well water samples were determined soon after sampling according to standard methods (Eaton et al. 1995). Statistical analyses were performed using the Statview software.
Estimation of trace metal concentration factors
Concentration factor (CF) for total element was calculated as the concentration of each metal in the soil divided by concentration of that metal in the corresponding control (Kabata-Pendias and Mukherjee 2007). Two common approaches to exhibiting the extent of the metals was calculated (1) using the geoaccumulation index (Igeo) as proposed by Müller (1969). Müller (1969) proposed the degree of metal concentration in the seven enrichment categories as described in Table 1. This index is expressed as:
where Cn is the measured value of the element in the enriched point, Bn is the background value of element, and 1.5 is the correction factor of background which is contributed to lithogenic effects. (2) contamination degree (Cd) of Abrahim (2005) and Abrahim and Parker (2008) which is a modified and generalised form of the Hakanson (1980) equation for the calculation of the overall degree of contamination. This equation calculated as follows:
where CF = contamination factor, n = number of investigated trace metals, and i = ith the metals. The index was classified in some gradations (Table 1). The values of trace metals in reference soils (control) were considered as background in the studied region.
Results and discussion
Properties of wastewater and well water
The results of the chemical analysis of wastewater and well water were illustrated in Table 2. The mean of chemical parameters of wastewater were significantly (P ≤ 0.05) higher than those of well water. Based on WHO guideline (2006), the pH, nitrate, and phosphorus of wastewater were above the acceptable range for agricultural practices, which could influenced on soil quality. The EC, TDS, and hardness were in the permissible levels (WHO 2006) in the used wastewater for irrigation.
The exceptional of Zn, concentration of the trace metals were upon their maximum acceptable levels (FAO 1992) for the applied wastewater. Comparing with well water, wastewater concentrated 26 and 29 time higher value of Zn and Cu, respectively, which could highlighted a considerable risk for accumulation of two elements at toxic value in the soil through time. This trend may be attributed to the discharge of trace metals by some industrial unites contain urban and sub-urban like batteries and paint. Cadmium and lead were not determinable in well water through spectrophotometery.
Clay mineralogy
Illite, smectite, kaolinite, and chlorite were known as the major clay minerals in both wastewater-irrigated and control soils (Table 3). With the exception of smectite, the relative abundance of clay mineral followed almost of the same pattern for both the wastewater and well water irrigated soils. For instance, long-term wastewater irrigation did not appear any modification in the XRD patterns of kaolinite and chlorite, either in the intensity or in the position of peaks their. This trend is logical in arid and semi-arid environments, where the presence of high alkaline and calcareous material along with the limited weathering and leaching process led to the stable pattern for the two minerals (Dixon and Schulz 2002). Fluaquentic Endoaquepts indicated a visible increase of smectite in response to wastewater irrigation and agricultural practice. This soil in consistent with a cumulative environment [a process of soil formation whereby mineral particles are added to the surface of a soil solum by hydrology, human agents, or eolian (Gregorich et al. 2002)] and lowland unite which received fine particle carried in suspension from other position. Consequently, the characteristics were probably subject to accumulation of smectite in the soil. However, neoformation of smectite from soil solution is not ruled out in Fluaquentic Endoaquepts where high pH and concentration of basic compound is highlight.
Physicochemical characteristics
Mean (±SD) content of the selected physicochemical properties is summarized in Table 4. Distribution of sand and silt content indicated that there were no significant differences between the wastewater-irrigated and the control soils. Using wastewater irrigation improved 3% (RE of 1.03) to 17% (RE of 1.17) compared to their control soils. This can be attributed to two pathways: (1) accelerated alteration induced by using long-term wastewater irrigation and (2) the clay addition through the applied wastewater. These results are comparable to the data found by Mathan (1994) and Wang et al. (2003).
The pH values displayed a slight change with a mean 0.1–0.2 unite for most of the soils following long-term wastewater irrigation. In the alkaline and calcareous soils, decrease in soil pH can be viewed as a positive change and any increase in soil pH, in contrary, can be viewed as a negative change (Smith and Doran 1996, De Clerck et al. 2003). Using this interpretation, we found that 67% of the soils had negative modification in the mean pH and 37% had positive modification through the decrease of pH with wastewater. Nevertheless, the range of pH was in an optimal condition (6–7.5) for general plant growth and microbial activity (Smith and Doran 1996) in most of the studied soils. Salinity highlighted a marked negative aspect in soil quality by wastewater irrigation system as reflected in EC (Table 4). By the average, an increase of 87% (RE of 1.76) to 157% (RE of 2.6) was occurred in soil EC through wastewater irrigation compared with the control. This trend, linked with high level of soluble salts in raw wastewater as recorded by its EC and TDS (Table 2), is known as a natural process in arid and semi-arid regions, where irrigation and anthropeogenic activities have been associated with an increase of soluble salts (Walker and Lin 2008). Continuous wastewater irrigation manifested a marginal increase in soil CCE content (a mean rise of 2–13%) as compared with the adjoining control soil (Table 4). Such pattern could be ascribed to greater level of carbonates in the wastewater than that of well water (Table 2) as represented by their hardness. The wastewater system induced to some increase in soil CEC (a mean rise of 10–12%) compared with control soils, mainly as result of increasing the combination of soil organic matter and clay content in wastewater-irrigated soils (Table 4).
Compared to control soils, long-term continuous wastewater irrigation caused a positive change in SOC from mean of 13% (RE of 1.13) to 44% (RE of 1.44) for most of the studied soils, which can attributed to its direct input by the used wastewater. A considerable improve in soil quality was observed following wastewater irrigation with regard to nutrients (NPK) due to, probably, the combination of their addition through wastewater and interaction between the used wastewater and its receiving soils. In this context, orders were as FE> TH> TC for total N (a rise of 10–120%), TH> TC> FE for available P (a rise of 270–300%), and FE> TC> TH for K available (a rise of 2–53%). Overall, available P and K, EC, and total N were the major chemical factors affecting on soil quality response to wastewater irrigation.
Trace metal
Irrigation with wastewater was related to a significant build-up in both DTPA-extractable and total trace metals including Zn, Cu, Cd, and Pb for the most of the examined soils compared with control soils (Table 5).
In general, the accumulation of DTPA-extractable metals for the different soils were in sequence FE> TC> TH and for the different elements were as Zn> Pb> Cu> Cd in both wastewater-irrigated soils and control soils (Fig. 1a, b). By the mean, long-term wastewater irrigation enriched 3.5–7, 1.8–3.4, 2.4–3.5, 3–3.5 time greater amounts of DTPA-extractable Zn, Pb, Cu, and Cd, respectively, than those of control soil. This data is in line with Masto et al. (2009) who found that soils more than 20 years sewage irrigation resulted a significant build-up of DTPA extractable Zn (314%), Cu (102%), Fe (715%), Cd (203%), and Ni (1358%) when compared with the adjacent rain-fed reference. The highest accumulation of DTPA-extractable metals resulting from continuous irrigation with wastewater was associated with Fluvaquentic Endoaquepts, where the greatest clay content and smectite were occurred. The clay minerals of smectite which posses’ greater cation exchange capacity have a higher affinity for DTPA-extractable metals (Dixon and Schulz 2002). This means that the greater clay and smectite content, and corresponding greater surface along with CEC attributed to the highest amounts of DTPA-extractable Zn, Pb, Cu, and Cd in Fluvaquentic Endoaquepts as compared with other soils.
Comparing to the adjacent control soils, the total forms of examined metals were associated to a significant accumulation in wastewater irrigation system as illustrated in Table 5. In the light of this, mean concentration of total-Zn, Cu, Cd, Pb in wastewater irrigation region was 3.6–7, 2.8–4.9, 4.4–4.7, and 1.8–2.3 times higher, respectively, than those of control soils. In spite of this pattern, the concentration of all analyzed metals were below their maximum permissible limits (Zn < 300 mg kg−1, Cu < 100 mg kg−1, Cd < 5 mg kg−1, and Pb < 100 mg kg−1) as described by Kabata-Pendias and Mukherjee (2007). For different sub-groups of soils, the spatial distribution of the metals were in order FE> TC> TH (Fig. 2a, b) as results of: (1) the load rate of the received wastewater at the flooding irrigation system; (2) impact of the applied wastewater with its receiving soil. In fact, It seem that Fluvaquentic Endoaquepts served as a collector of the material transported from the other area, following receives the larger amount of wastewater and the metals than the other soil over time.
Fine particle-size fraction (<0.002 mm), organic matter, and calcium carbonates were appeared as the main agents in retaining the total trace metals as reflected in significant positive correlation between the agents and the metals (Table 6). Such trend comes as no surprise because the agents due to containing compounds with large surface area like clay minerals, iron and manganese oxy-hydroxides, and humic acids have a high tendency for adsorption and transport trace heavy metals (Bradl 2004, Dragovic et al. 2008). These relationships were not significant in the control soils (Table 6), meaning that the dynamic of heavy metals had a dissimilar trend in wastewater-irrigated soils and well water-irrigated soils. Positive correlation among total content of trace metal indicated that the metals are originating from the same pollution source (Table 6).
Typically, the total values of the trace metals were considerable greater in topsoil than in subsoil mainly at wastewater-irrigated soils (data not shown), suggesting that the metals concentrating in the topsoil was due to their strong affinities with the soil solid fraction (Kabata-Pendias and Mukherjee 2007). The ration of the metal content in topsoil to that in the corresponding subsoil enrichment (RTE) ranged from 1.25 to 2.3 and 1.3 to 2.6 for wastewater-irrigated and well water-irrigated soils, respectively (Table 7). Based RTE values, the mobility sequence of Cd> Zn> Pb> Cu was observed for wastewater-irrigated region whereas it was found as Cd> Cu> Zn> Pb for the control region (Table 7). The pathways highlighted the different responses of the monitoring soils to the trace metals, reflected both the two face agreement and disagreement with other authors (Camobreco et al. 1996; Haiyan and Stuanes 2003).
Estimation of pollution scales of total trace metals
For estimating contamination impact of the metal, the two indices of geoaccumulation index (Igeo) and degree of contamination (Cd) were assessed. The mean Igeo values of Zn, Cu, Cd, and Pb were ranged as 0.4–0.7, 0.3–0.5, 0.5–0.6, and 0.1–0.2, respectively, for the examined soils (Table 8). By the pattern, the soils are categorized as uncontaminated (class 1, 0 < Igeo ≤ 1) regarding to the analyzed trace metals. However, Pb and Cu appear to be at least Igeo values, whereas Zn and Cd reflect the greatest Igeo values for all the soils. The spatial distribution of Igeo values were in sequence Zn> Cd> Cu> Pb for Typic Calcixerepts, Cd> Zn> Cu> Pb for Typic Haploxerepts, and Zn> Cd> Cu> Pb for Fluvaquentic Endoaquepts. The data can be as result of the variability of soil properties (mainly the <0.002 mm fraction, SOC, and calcium carbonates as the major contributor in retaining the metals as discussed before) and their different impacts on the used wastewater.
Degree of contamination (Cd) and contamination factor (Cf) for the four studied elements (total Zn, Cu, Cd, and Pb) were in the ranges of 3–5 and 1.8–6.8, respectively, as showed in Table 8. The Cf values in the three soils were in the sequence of EF (4.9) > TC (4.1) > TH (3.2), suggesting that the soils are classified as moderate degree to high degree of contaminating (Table 9). Indeed, the value of Cd led to 67% of soils to high degree of contaminating and 37% to moderate degree of contaminating (Fig. 3). The load and contamination rates of the trace metals in Fluvaquentic Endoaquepts found to be a considerable increase compared to other soils with respect to Igeo, Cf, and Cd indices. Differences in the soil properties such as content of fine particle fraction, organic matter, and clay mineralogy at Fluvaquentic Endoaquepts in comparison to other soils can be an acceptable reason for accumulating the studied trace metal in the soil. It is widely reported that fine particle-size and organic fractions exhibited great tendency for adsorption of heavy metals (Bradl 2004, Dragovic et al. 2008). In this context, the affinity of these fractions is strongly influenced by their electrochemical properties and is closely related to the specific surface area and cation exchange capacity (CEC) of them (Kabata-Pendias and Mukherjee 2007).
Histogram of the degree of contamination (Cd) for the four analyzed trace metals (Zn, Cu, Cd, and Pb) in the examined soils. The horizontal lines shown boundaries between moderate degree of contamination and high degree of contaminate as was represented by Abrahim and Parker (2008)
Conclusion
It can be concluded that irrigation with wastewater manifested into significant improve, positively, the organic carbon and nutrients input (total N and P and K available). In contrary, a significant build up of salinity and trace metal appeared as unfavorable effects of wastewater after four decades of operation. On an average, wastewater-irrigated soils enriched DTPA-extractable Zn (250–570%), Cu (140–200%), Cd (220–250%), and Pb (70–240%) as well as total pools of Zn (260–580%), Cu (180–390%), Cd (240–470%), and Pb (80–130%) when compared to the control soils for the different sub-groups of Inceptisols. Based on geoaccumulation index, the examined soils are suggested to be uncontaminated regarding to the four measured metals (Zn, Cu, Cd, and Pb) whereas they are categorized as moderate degree of contamination (TH) and high degree of contaminating (TC and EF) by degree of contamination. It found that Zn, Cu, Cd, and Pb are mainly associated with the different soil fractions which retain those metals in the sequence of fine particle-size fraction (<0.002 mm) > organic carbon > calcium carbonate. Although the trace metal levels were below the maximum accepted limits, periodic analysis of wastewater is necessary prior to irrigation to keep of landscape and agriculture health.
References
Abrahim GMS (2005) Holocene sediments of Tamaki Estuary: characterisation and impact of recent human activity on an urban estuary in Auckland, New Zealand. Ph.D. thesis, University of Auckland, Auckland, New Zealand, 361 p
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238
Arienzoa M, Christena EW, Quaylea W, Kumarc A (2009) A review of the fate of potassium in the soil–plant system after land application of wastewaters. J Hazard Mater 164:415–422
Baker DE, Amacher MC (1982) Nickel, copper, zinc and cadmium. In: Page AL et al (eds) Methods of soil analysis. Part 2. American Society of Agronomy, Madison, pp 323–336
Biscaye P (1965) Mineralogy and sedimentation of recent deep sea clay in the Atlantic Ocean and adjacent seas and oceans. Geo Soc Am Bull 76:803–832
Bradl HB (2004) Adsorption of heavy metal ions on soils and soil constituents. J Coll Inter 277:1–18
Bremner JM (1982) Total Nitrogen. In: Page AL et al (eds) Methods of soil analysis. Part 2. American Society of Agronomy, Madison, pp 595–624
Camobreco VJ, Richards BK, Steenhuis TS, Peverly JH, McBride MB (1996) Movement of heavy metals through undisturbed and homogenized soil columns. Soil Sci 161:740–750
Day PR (1966) Particle fractionation and particle-size analysis. In: Black CA (ed) Methods of soil analysis. Part I. American Society of Agronomy, Madison, pp 545–566
De Clerck F, Singer MJ, Lindert P (2003) A 60-year history of California soil quality using paired samples. Geoderma 114:215–230
Dixon JB, Schulz DG (2002) Soil mineralogy with environmental applications. Soil science American Society, Madison
Dragovic S, Mihailovic N, Gajic B (2008) Heavy metals in soils: distribution, relationship with soil characteristics and radionuclids and multivariate assessment of contamination sources. Chemosphere 72:491–495
Eaton AD, Clesceri LS, Greenberg AE (1995) Standard methods for the examination of water and wastewater, 19.th edn. American Public Health Association, Washington
FAO (1992) Wastewater treatment and use in agriculture. Irrigation and drainage paper, No 47. FAO, Rome, Italy
Gregorich EG, Turchenek LW, Carter MR, Angers DA (2002) Soil and environmental science dictionary. CRC Press, Florida
Haiyan W, Stuanes AO (2003) Heavy metal pollution in air-water-soil-plant system of Zhuzhou city, Hunan Province. Water Air Soil Poll 147:79–107
Hakanson L (1980) Ecological risk index for aquatic pollution control, a sedimentological approach. Water Res 14:975–1001
Kabata-Pendias A, Mukherjee AB (2007) Trace elements in soils and plants. Springer, Berlin
Keeley GM, Quin BF (1979) The effects of irrigation with meatworks-fellmongery effluent on water quality in the unsaturated zone and shallow aquifer, prog. Water Technol 11:369–386
Kunze G (1965) Pretreatment for mineralogical analysis. In: Klute A (ed) Methods of soil analysis. Part 1. American Society of Agronomy, Madison, pp 210–221
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Mapanda F, Mangwayana EN, Nyamangara J, Giller KE (2005) The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric Ecosyst Environ 107:151–165
Masto RE, Chhonkar PK, Singh D, Patra AK (2009) Changes in soil quality indicators under long-term sewage irrigation in a sub-tropical environment. Environ Geol 56:1237–1243
Mathan KK (1994) Studies on the influence of long-term municipal sewage-effluent irrigation on soil physical properties. Bioresour Tech 48:275–276
Mehra O, Jackson M (1960) Iron oxide removal from soils and clays by a dithionite citrate system with sodium bicarbonate. Clays Clay Miner 7:317–327
Müller G (1969) Index of geoaccumulation in the sediments of the Rhine River. GeoJournal 2:108–118
Nelson RE (1982) Carbonate and gypsum. In: Page AL (ed) Methods of soil analysis. Part 2. American Society of Agronomy, Madison, pp 181–199
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL (ed) Methods of soil analysis, part 2. American Society of Agronomy, Madison, pp 539–580
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL et al (eds) Methods of soil analysis. Part 2. American Society of Agronomy, Madison, pp 403–430
Pedrero F, Kalavrouziotis I, Alarcon JJ, Koukoulakis P, Asano T (2010) Use of treated municipal wastewater in irrigated agriculture-review of some practices in Spain and Greece. Agric Water Manage 97:1233–1241
Qadir M, Sharma BR, Bruggeman A, Choukr-Allah R, Karajeh F (2007) Nonconventional water resources and opportunities for water augmentation to achieve food security in water scarce countries. Agric Water Manage 87:2–22
Qadir M, Wichelns D, Raschid-Sally L, McCornick PG, Drechsel P, Bahri A, Minhas PS (2010) The challenges of wastewater irrigation in developing countries. Agric Water Manage 97:561–568
Radcliffe JC (2004) Water recycling in Australia. Australia Academic of Technology Science and Engineer, Parkville
Smith JL, Doran JW (1996) Measurements and use of pH and electrical conductivity for soil quality analysis. In: Methods for assessing soil quality. SSSA special publication 49, Soil Science Society of America, Madison, Wisconsin, USA, pp 169–185
Soil Survey Staff (2003) Soil survey manual. Agric handbook No. 19. U.S. Government Printing Office, Washington, DC
Soil Survey Staff (2010) Keys to soil taxonomy. United States Department of Agriculture, Natural Resources Conservation Service, Washington, DC
Taylor MD, Kim ND, Hill RB, Chapman R (2010) A review of soil quality indicators and five key issues after 12 yr soil quality monitoring in the Waikato region. Soil Use Manage 26:212–224
Thomas GW (1982) Exchangeable cations. In: Page AL et al (eds) Methods of soil analysis. Part 2. American Society of Agronomy, Madison, pp 159–166
Walker C, Lin HS (2008) Soil property changes after four decades of wastewater irrigation: a landscape perspective. Catena 73:63–75
Wang Z, Chang A, Wu L, Crowley D (2003) Assessing the soil quality of long-term reclaimed wastewater-irrigated cropland. Geoderma 114:261–278
WHO (2006) Guidelines for the safe use of wastewater, excreta and grey water, part II, Geneva, Switzerland
Xu J, Wu L, Chang AC, Zhang Y (2010) Impact of long-term reclaimed wastewater irrigation on agricultural soils: a preliminary assessment. J Hazard Mater 183:780–786
Acknowledgments
This study was supported by the Urmia University. The authors are thankful to the staff of Vice Chancellor Research Office for their cooperation and assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezapour, S., Samadi, A. Soil quality response to long-term wastewater irrigation in Inceptisols from a semi-arid environment. Nutr Cycl Agroecosyst 91, 269–280 (2011). https://doi.org/10.1007/s10705-011-9460-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-011-9460-2