Abstract
Dimethyl phthalate (DMP) is one of the most widely used plasticizers. Due to its anthropogenic inputs, DMP has become a serious contaminant in soils and water. Hydroponic experiments were conducted focusing on phytotoxic responses of rice seedlings (Oryza sativa L. cv. XZX 45) to 1,2-dimethyl phthalate. Relative growth rate (RGR) and water use efficiency (WUE) were measured as response endpoints to quantify the effective concentrations (ECs) of DMP to rice seedlings. As a comparison, acute phytotoxicity of dimethyl sulfoxide (DMSO) used as solvent media was also determined. Results showed that acute toxicity of DMP to rice seedlings was evident, but selected endpoints had different responses to DMP exposure. RGR of rice seedlings was more sensitive to change of DMP than WUE. EC-48 h values for 10, 20 and 50 % inhibition of the RGR were estimated to be 6.18, 38.41 and 329.41 μΜ for rice seedlings exposed to DMP, respectively, while smaller ECs were obtained for 96 h exposure. Results from phytotoxicity of DMSO demonstrated that the doses of DMSO used for dissolving DMP had a negligible effect on rice seedlings. In conclusion, DMP is problematic at relatively low concentrations for rice seedlings, and inhibitory effects are highly dependent on response endpoints and the duration of exposure period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters (PAEs) are a class of manufactured chemicals primarily used as non-reactive plasticizers (Rahman and Brazel 2004). They are also widely used in the manufacture of insect repellents, synthetic fibers, cosmetics, personal care products, food packaging, medical devices, pharmaceuticals, paints, and industrial plastics (Hens and Caballos 2003; Hauser et al. 2004; Chen et al. 2008; Zeng et al. 2009; Xia et al. 2011; Wang et al. 2013). The global production of PAEs is approximately 6.0 million tons annually (Mackintosh et al. 2006). In mainland China, the consumption in 2011 is about 2.2 million tons and majority has been used in the production of plastic film (Wang et al. 2013). The large-scale production and widespread application due to anthropogenic activities has resulted in a significant release of PAEs into the environment on a continuous basis (Kong et al. 2012). Indeed, the presence of PAEs has been observed in food, air, water, soil, and sediment worldwide (Xie et al. 2007; Zeng et al. 2009; Srivastava et al. 2010; Wang et al. 2013). For instance, high levels of PAEs in agricultural soils have been detected in the Pearl River Delta (Zeng et al. 2008) and northeast China (Xu et al. 2008).
PAEs are considered to be moderately to highly toxic toward aquatic organisms (Cartwright et al. 2000), soil microbial communities (Jensen et al. 2001), and insects (Liao et al. 2009). Of the 18 commercial PAEs, dimethyl phthalate (DMP), DEP, DBP, BBP, DEHP, and DnOP have been classified as priority pollutants and as endocrine disruptive chemicals (EDCs) by the U.S. Environmental Protection Agency, the European Union, and other international organizations (Liao et al. 2009; Wang et al. 2013). It is known that toxicity and biological effect of PAEs to various animals is well documented. For instance, PAEs exposure has been found to be associated with adverse health outcomes in humans (Lin et al. 2011), including shorter ano-genital distances at birth (Swan 2006), respiratory effects (Jaakkola et al. 2000; Hoppin et al. 2004), and increased waist circumference and insulin resistance (Stahlhut et al. 2007). Epidemiological evidence indicates that boys born to women exposed to PAEs during pregnancy have an increased incidence of congenital genital malformations and spermatogenic dysfunction, signs of a condition referred to as testicular dysgenesis syndrome (Ge et al. 2007). Experimental studies with rats showed that PAEs induced the reproductive toxicity, developmental toxicity, endocrine disruption, and genotoxicity (Lin et al. 2011). For example, high doses of PAEs caused increase in liver weight, peroxisomal proliferation, and skeletal variations (Moorman et al. 2000). Limited studies have been conducted to investigate phytotoxicity of PAEs. The effect of DBP on the synthesis of carotene during seedling growth has been reported (Herring and Bering 1988). It also causes decrease in vitamin C and capsaicin content in capsicum fruits (Capsicum annum) (Yin et al. 2003). DBP even below 1 mg/L had significant effect on the concentration of chlorophyll in Chinese cabbage, and the biomass showed a severe decrease under treatment with more than 30 mg/L of DBP (Liao et al. 2009). However, nothing is known yet about phytotoxicity of DMP.
Toxic effects of chemicals on plants can be measured in different approaches. Trapp et al. (2000) specially developed an acute hydroponic phytotoxicity for chemicals using growth, transpiration, and water use efficiency to evaluate the impacts of pollutants to plants. Stress reduces the water use efficiency (Larcher 1995), and a change in water use efficiency can be used as indicator for chemical stress to the trees on a sublethal level (Trapp et al. 2000). Our objective was to quantify the effective concentrations (ECs) based on different response parameters, using hydroponic experiment with rice seedlings exposed to DMP. Two response endpoints, relative growth rate (RGR) and water use efficiency (WUE), were studied and EC values were estimated using the Levenberg–Marquardt algorithm at the respective time intervals. Comparison of the sensitivities of different response parameters to DMP exposure based on EC values was also performed. Because DMP is non-water soluble, organic solvent dimethyl sulfoxide (DMSO) is required to be an intermediate solvent for dissolving DMP. DMSO is a polar, less-toxic, odorless and an aprotic solvent, which acts as both soft base (sulfoxide sulfur) and hard base (sulfoxide oxygen) (Zhu et al. 2013). Therefore, additional experiments were conducted to clarify the effect of DMSO on rice seedlings. The ECs of DMSO for rice seedlings were also determined using similar exposure regimes and estimation methods. This work was conducted at the College of Environmental Sciences and Engineering, Guilin University of Technology, P. R. China, from October 2013 to April 2014.
Materials and methods
Test chemicals and experiment design
Plant materials and exposure regime were identical to our previous work (Yu et al. 2013). Fifteen-day-old rice seedlings (Oryza sativa L. cv. XZX 45) with similar height and weight was transplanted to a pre-treatment solution containing 1 mM CaCl2 + 2 mM MES-Tris buffer (pH 6.0) for 4 h to clear the cell wall space of ions (Ebbs et al. 2008), and then, ten rice seedlings were transferred into a 50-mL Erlenmeyer flask filled with 50 mL modified ISO 8692 nutrient solution (Yu and Zhang 2013) with addition of 10 µM Fe-EDTA. The plants were first conditioned for 24 h to allow adaptation to the new environmental conditions. The flasks were all wrapped with aluminum foil up to the flask mouth to prevent escape of water, and to inhibit potential growth of algae inside. All flasks were housed in a plant growth chamber with constant temperature of 25 ± 0.5 °C and a relative humidity of 60 ± 2 % under continuous artificial light. Then, the nutrient solution in each flask was replaced by spiked solution, except control.
1,2-Dimethyl phthalate (CAS No. 131-11-3, 99.5 % purity) and DMSO (CAS No. 67-68-5, 99 % purity) were purchased from Sinopharm Chemical Reagent Co., Ltd. Shanghai, PR China. DMP solution was prepared by DMSO. The final DMSO concentration in the experiment media with DMP was maximum 0.002 %. Nominal concentrations were used in this study. Eight different concentrations were used for each testing chemical. Each treatment concentration was conducted in four independent biological replicates. Two test series (48-h and 96-h exposure period) were conducted.
Relative growth rate
Rice seedlings were weighed prior to application and at termination of exposure. The relative growth rate (RGR, %) was calculated using the formula
where M (I) and M (F) are the initial and final weight (g) of rice seedlings, respectively.
Water use efficiency
Water use efficiency (WUE) (mg biomass/mL water) is the ratio between produced biomass and water transpired (Trapp et al. 2000).
where M (I) and M (F) are the initial and final weight (mg) of rice seedlings. V transpired is the transpiration (mL water) of seedlings calculated by the weight loss of the plant-flask system.
Inhibition rate
Percent inhibition rate (IR, %) on each parameter was calculated using the equation
where C is concentration (μΜ), t is time period (h), μ is different measured parameter, i is replicate 1, 2,…, n and j is control 1, 2,…, m.
Effective concentration
Effective concentration (EC) was determined using different parameters of treated plants as the endpoints of acute phytotoxicity. The EC values at the respective time intervals were estimated by Levenberg–Marquardt algorithm with 95 % confidence intervals using Logistic Model of Origin v. 9.0, which is a commonly used program designed for logistic dose response in Chemistry.
where A 1 is the initial value, A 2 is the final value, X 0 is the central value for EC of the does–response curve, and P is the slope of dose–response curve; f(x) is the function of chemical concentration x; here, it refers to percent inhibition rate for each selected parameter to determine the Levenberg–Marquardt coefficients.
Statistical method
Analysis of variance (ANOVA) and Tukey’s multiple range test was used to determine the statistical significance at 0.01 or 0.05 between the treatments (Sachs 1992).
Results and discussion
Acute phytotoxicity of DMSO to rice seedlings
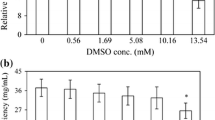
Phytotoxicity of DMSO expressed in terms of RGR and WUE of rice seedlings exposed to DMSO was evident (Table 1). Compared with the non-treated seedlings, 0.35 mM DMSO slightly enhanced both RGR and WUE after 48-h exposure (p > 0.05), which declined at further DMSO concentrations in comparison to control. It is noted that a significant reduction in RGR as well as WUE was detected with rice seedlings exposed to DMSO at greater than or equal to 1.41 mM (p < 0.05), but all plants showed positive growth after 48-h exposure. A different result was obtained in the treatment with 96-h exposure of DMSO, in which decreases in both RGR and WUE were observed with increasing concentration of DMSO. However, both parameters remained slightly changed up to 0.71 mM of DMSO treatment (p > 0.05), compared with the non-treated rice seedlings after 96-h exposure. The RGR and WUE showed a severe decrease under treatment with more than 1.41 mM (p < 0.05) in respect to control. All seedlings showed positive growth after 96-h exposure, except the treatment exposed to 56.42 mM of DMSO.
Acute phytotoxicity of DMP to rice seedlings
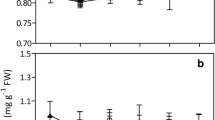
Table 2 shows the changes of selected parameters of rice seedlings exposed to DMP at different exposure periods. A remarkable reduction in RGR was observed (p < 0.05) with all DMP treatments after 48-h exposure in comparison to control, while a significant decrease in WUE was detected with rice seedlings exposed to DMP at greater than or equal to 30.72 μΜ (p < 0.05). When exposed to a period of 96 h, rice seedlings showed different responses to DMP. Compared with non-treated rice seedlings, the RGR decreased significantly with increasing concentrations of DMP treatments (p < 0.05). DMP also caused reduction in WUE, but the decrease was significant (p < 0.05) at 245.74–982.95 μΜ. Our experimental results showed that only the highest DMP dose of 982.95 μΜ yielded negative values of RGR and WUE.
Effective concentrations of DMSO for rice seedlings
Table 3 displays the inhibitory effects of DMSO, as measured by different response variables, on rice seedlings observed at different exposure periods. It is obvious that higher doses of DMSO caused more severe inhibition rates on selected parameters, in which the inhibitory rate of RGR significantly increased to approximately 57.24 % at the treatment of 28.21 mM after 48-h exposure, in comparison with control. It is worthwhile to note that inhibitory effect was also dependent on duration of exposure. Indeed, higher inhibition rates of both RGR and WUE were observed at the treatments of 96-h exposure, especially at the higher doses of DMSO.
For calculation of EC values, the Levenberg–Marquardt algorithm was performed using nonlinear regression. The simulation curves of concentration–response model shown in Fig. 1 indicated that all trends yielded were significant, judged by the critical R for given n (α = 0.05) (Sachs 1992). Therefore, the EC values for different parameters at the respective time intervals can be calculated using the fitting equations. Results of the EC values are depicted in Table 4. It is of interest to note that complete different results were observed using different endpoints to determine the ECs. All EC values based on RGR were smaller than those on WUE, suggesting that RGR of rice seedlings is more sensitive to DMSO exposure than WUE.
Effective concentrations of DMP for rice seedlings
Similarly, a statistical program was used to estimate EC values of DMP for rice seedlings, according to the inhibitory effects of DMP on different variables of plants (Table 5). Therefore, a dose-dependent curve was simulated from the results of percentage of inhibition rate of selected parameters in function of concentrations of DMP (Fig. 2), which evidenced significant response patterns, judged by the critical R for given n (α = 0.05) (Sachs 1992). It is obvious that the smaller EC value was obtained using RGR than using WUE. This suggested that exposure of DMP caused more seriously inhibitory effect on RGR than that on WUE.
Comparison to other findings
An important step involved in assessing the environmental safety and ecological risk of different chemicals is to determine the effective concentration, in which plant assays are required to measure ultimate adverse responses of plants to target chemicals. In the literature review of Nellessen and Fletcher (1993), responses of plants to organic chemicals were grouped in three categories: unique features, common features and growth parameters. It is known that different response endpoints such as hoot length, biomass production, percentage germination, and root growth were often selected for phytotoxic determination (Sánchez-Pardo and Zornoza 2014). In the present study, RGR and WUE were selected as sensitive variables to determine phytotoxicity of DMP to rice seedlings. Although a dose-dependent decline in both parameters was observed in DMP-treated rice seedlings, RGR and WUE showed different responses to DMP exposure. Indeed, a clear difference in the inhibitory effect on RGR and WUE was observed. This is probably due to the fact that DMP resulted in more adverse damage on biomass growth of plants than transpiration. It is obvious when levels of chemicals accumulated in plant materials are below toxicity threshold, plants are able to maintain their normal functioning (Yu 2014). However, if phyto-assimilation rate of chemicals is slower than accumulation in plants, phytotoxic effects could appear. It is worthwhile to note that only the highest DMP dose of 982.95 μΜ yielded negative values of RGR and WUE at the treatments of 96-h exposure, suggesting that (1) phyto-removal of DMP into plant materials from hydroponic solution was apparent; (2) phyto-degradation of DMP might be a process involved and the velocity was highly dependent on the DMP doses supplied; (3) plant materials are also a sink for bioaccumulation of DMP in rice seedlings, this caused, as consequence, phytotoxicity to rice seedlings.
Liao et al. (2009) investigated effects of DBP on Chinese cabbage at the morphological and proteome-level changes, in which 50 mg/L of DBP resulted in etiolation of leaves with decrease in biomass and content of chlorophyll after 42 days exposure. Additionally, phyto-transport of DBP from low parts into upper parts of plants was evident, and DBP accumulation in plant materials showed dose-dependent manner (Liao et al. 2009). In the proteomic analysis, three increasing protein spots were observed, which are responsible for biosynthesis of fatty acids, signal transduction of phototropic response, and nitrate assimilation in plant cells, respectively (Liao et al. 2009). Growth inhibition of DBP on algae (Pavlova viridis) was also detected (Xiao et al. 2013), where 5.0 mg/L of DBP killed the algae cells completely, and the content of chlorophyll and carotenoids significantly decreased with increasing concentrations of DBP.
In this study, DMSO was used as solvent media to dissolve DMP. The maximum concentration of DMSO was approximately 0.002 % (<0.35 mM). Results from phytotoxicity of DMSO showed that such a dose was unable to cause significant impact on rice seedlings. Estimation of EC10 also provided evidence to confirm that involvement of DMSO in phytotoxicity of DMP to rice seedlings is negligible. It is conclude that adverse responses of rice seedlings exposed to DMP observed is chiefly resulted from DMP rather than a joint toxicity of DMSO and DMP.
The EC is defined by the concentration of a chemical, which produces percentage inhibition of the maximum possible response for that chemical. In the case of this study, toxic responses include 10, 20, and 50 % inhibition of the RGR and WUE. It is of interest to note that using RGR as a variable always yielded lower EC values than using WUE for both testing chemicals at any exposure period, suggesting that RGR is more sensitive than WUE. For instance, the EC10-48 h for WUE was more than a factor of 2 higher than that obtained for RGR at the treatment with DMP. However, a much higher variation in EC values between WUE and RGR was observed at the treatment of 96-h exposure, implying that phytotoxicity of DMP increased with exposure time. This gives rise to conclusion that phyto-assimilation of DMP may be a very slow process in rice seedlings. For phytoremediation projects, phyto-accumulation of DMP by rice seedlings largely occurs in rice seedlings during a short-term exposure. RGR of rice seedlings is a sensitive bioindicator for risk assessment of DMP.
Reliability of EC estimation using Levenberg–Marquardt algorithm
The EC values in this study were estimated by Levenberg–Marquardt algorithm. Although all fits using nonlinear regression were significant, as indicated by the high regression coefficient R 2, several unusual EC values have been found. For instance, the estimated EC10-48 h value for WUE at the treatment with DMSO was 7.96 mM, while 7.05 mM DMSO yielded 12 % inhibition on WUE from experimental measurements. This is largely due to the fact that the data of WUE from experimental observation were only in a range of −4.54 to 36.86 %. Such data led to an unreliable simulation curve and hence to large errors in EC estimation. A similar result was also found in the 48-h DMP treatment. However, it is observed that DMSO and DMP caused more serious inhibition on WUE, when increasing exposure period. The estimated EC-96 h values for both variables were much closer to the results of experimental measurements. It is to suggest that the reliability of using Levenberg–Marquardt algorithm to estimate EC values is largely dependent on experimental observations of variables, which must be distributed homogeneously in the range of 0–100 %; otherwise, this method will lead to a large error in EC estimation.
Conclusion
Results from our experiments indicated that acute phytotoxicity of DMP on rice seedlings was apparent. Responses of RGR and WUE to DMP exposure showed the dose-dependent decline. The estimation of EC values revealed that RGR of rice seedlings was more susceptible to change of DMP than WUE. Further research on the molecular mechanisms involved in metabolic responses of DMP to plants is required to improve our understandings.
References
Cartwright CD, Thompson IP, Burns RG (2000) Degradation and impact of phthalate plasticizers on soil microbial communities. Environ Toxicol Chem 19:1253–1261
Chen ML, Chen JS, Tang CL, Mao IF (2008) The internal exposure of Taiwanese to phthalate—an evidence of intensive sue of plastic materials. Environ Inter 34:79–85
Ebbs SD, Piccinin RC, Goodger JQD, Kolev SD, Woodrow IE, Baker AJM (2008) Transport of ferrocyanide by two eucalypt species and sorghum. Int J Phytoremediat 10:343–357
Ge RS, Chen GR, Tanrikut C, Hardy MP (2007) Phthalate ester toxicity in Leydig cells: developmental timing and dosage considerations. Reprod Toxicol 23:366–373
Hauser R, Duty S, Godfrey-Bailey L, Calafat AM (2004) Medications as a source of human exposure to phthalates. Environ Health Perspect 112:751–753
Hens GA, Caballos AMP (2003) Social and economic interest in the control of phthalic acid esters. Trend Anal Chem 22:847–857
Herring R, Bering CL (1988) Effects of phthalate esters on plant seedlings and reversal by a soil microorganism. Bull Environ Contam Toxicol 40:626–632
Hoppin JA, Ulmer R, London SJ (2004) Phthalate exposure and pulmonary function. Environ Health Perspect 112:571–574
Jaakkola JJ, Verkasalo PK, Jaakkola N (2000) Plastic wall materials in the home and respiratory health in young children. Am J Public Health 90:797–799
Jensen J, Langevelde JV, Pritzl G, Krogh PH (2001) Effects of di(2-ethylhexyl) phthalate and dibutyl phthalate on the collembolan Folsomia Fimetaria. Environ Toxicol Chem 20:1085–1091
Kong SF, Ji YQ, Liu LL, Chen L, Zhao XY, Wang JJ, Bai ZP, Sun ZR (2012) Diversities of phthalate esters in suburban agricultural soils and wasteland soil appeared with urbanization in China. Environ Pollut 170:161–168
Larcher W (1995) Physiological plant ecology, 3rd edn. Springer, Berlin
Liao CS, Yen JH, Wang YS (2009) Growth inhibition in Chinese cabbage (Brassica rapa var. chinensis) growth exposed to di-n-butyl phthalate. J Hazard Mater 163:625–631
Lin S, Ku HY, Su PH, Chen JW, Huang PC, Angerer J, Wang SL (2011) Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere 82:947–955
Mackintosh CE, Maldonado JA, Ikonomou MG, Gobas FAPC (2006) Sorption of phthalate esters and PCBs in a marine ecosystem. Environ Sci Technol 40:3481–3488
Moorman WJ, Ahlers HW, Chapin RE, Daston GP, Foster PM, Kavlock RJ, Morawetz JS, Schnorr TM, Schrader SM (2000) Prioritization of NTP reproductive toxicants for field studies. Reprod Toxicol 14:293–301
Nellessen JE, Fletcher JS (1993) Assessment of published literature pertaining to the uptake/accumulation, translocation, adhesion and biotransformation of organic chemicals by vascular plants. Environ Toxicol Chem 12:2045–2052
Rahman M, Brazel CS (2004) The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Polym Sci 29:1223–1248
Sachs L (1992) Angewandte Statistik. Springer, Berlin
Sánchez-Pardo B, Zornoza P (2014) Mitigation of Custress by legume–Rhizobium symbiosis in white lupin and soy bean plants. Ecotoxicol Environ Saf 102:1–5
Srivastava A, Sharma VP, Tripathi R, Kumar R, Patel DK, Mathur PK (2010) Occurrence of phthalic acid esters in Gomti River sediment, India. Environ Monit Assess 169:397–406
Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH (2007) Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US males. Environ Health Perspect 115:876–882
Swan SH (2006) Prenatal phthalate exposure and ano-genital distance in male infants. Environ Health Perspect 114:A88–A89
Trapp S, Zambrano KC, Kusk KO, Karlson U (2000) A phytotoxicity test using transpiration of willows. Arch Environ Contam Toxicol 39:154–160
Wang J, Luo YM, Teng Y, Ma WT, Christie P, Li ZG (2013) Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use plastic film. Environ Pollut 180:265–273
Xia XH, Yang LY, Bu QW, Liu RM (2011) Levels, distribution, and health risk of phthalate esters in urban soils of Beijing, China. J Environ Qual 40:1643–1651
Xiao AF, Yang HL, An M, Duan SS (2013) Toxicity and interference effects of dibutyl phthalate on Pavlova viridis. Ecol Sci 32:429–433 (in Chinese)
Xie Z, Ebinghaus R, Temme C, Lohmann R, Caba A, Ruck W (2007) Occurrence and air–sea exchange of phthalates in the Arctic. Environ Sci Technol 41:4555–4560
Xu G, Li FS, Wang QH (2008) Occurrence and degradation characteristics of dibutyl phthalate (DBP) and di-(2-ethylhexyl) phthalate (DEHP) in typical agricultural soils of China. Sci Total Environ 393:333–340
Yin R, Lin XG, Wang SG, Zhang HY (2003) Effect of DBP/DEHP in vegetable planted soil on the quality of capsicum fruit. Chemosphere 50:801–805
Yu XZ (2014) Uptake, assimilation and toxicity of cyanogenic compounds in plants: facts and fiction. Int J Environ Sci Technol. doi:10.1007/s13762-014-0571-6
Yu XZ, Zhang FZ (2013) Effects of exogenous thiocyanate on mineral nutrients, antioxidative responses and free amino acids in rice seedlings. Ecotoxicology 22:752–760
Yu XZ, Zhang FZ, Peng XY (2013) Effects of inhibitors on ferricyanide uptake and assimilation by plants. Int J Environ Sci Technol 10:125–132
Zeng F, Cui KY, Xie ZY, Wu LN, Liu M, Sun GQ, Lin YJ, Luo DL, Zeng ZX (2008) Phthalate esters (PAEs): emerging organic contaminants in agricultural soils in peri-urban areas around Guangzhou, China. Environ Pollut 156:425–434
Zeng F, Cui KY, Xie ZY, Wu LN, Luo DL, Chen LX, Lin YJ, Liu M, Sun GX (2009) Distribution of phthalate esters in urban soils of subtropical city, Guangzhou, China. J Hazard Mater 164:1171–1178
Zhu P, Chen Y, Wang LY, Qian GR, Zhou M, Zhou J (2013) A novel approach to separation of waste printed circuit boards using dimethyl sulfoxide. Int J Environ Sci Technol 10:175–180
Acknowledgments
This work was financially supported by the research foundations from Guilin University of Technology (Grant No.: GUTRC2011007) and The Guangxi Key Laboratory of Theory and Technology for Environmental Pollution Control (Grant No.: 1201Z029).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yue, DM., Yu, XZ. & Li, YH. Quantification of effective concentrations of 1,2-dimethyl phthalate (DMP) to rice seedlings. Int. J. Environ. Sci. Technol. 12, 3009–3016 (2015). https://doi.org/10.1007/s13762-014-0704-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0704-y