Abstract
Purpose: total removal of spinal hemangioblastomas with satisfactory clinical outcomes remains a challenge. We aimed to evaluate the surgical outcomes of our spinal hemangioblastomas patients, summarize our experiences with this condition and review-related literature. Methods: records of 18 spinal hemangioblastoma patients who underwent microsurgical resection were analyzed retrospectively. Clinical features, surgical procedures and outcomes were reviewed to assess the prognosis of their spinal hemangioblastomas. The McCormick classification method was used to evaluate spinal function and MR scans used to assess location and features of the tumor pre-surgically, tumor recurrence and syringomyelia status post-surgically. Results: total resection of 37 tumors was achieved in all 18 cases. Of those patients, two (11%) were accompanied with von Hippel-Lindau (VHL). Of the 37 tumors, 3 (8.1%) were completely intramedullary, 16 (43.2%) intramedullary–extramedullary and 18 (48.6%) primarily extramedullary. Tumors accompanied with syringomyelia were present in 17 (94.4%) patients. Clinical symptoms such as pain were reduced within 48 h after surgery in 16 patients. Post-operative neurological functions improved in all cases at 3 months post-surgery. Over the subsequent 3–18 month follow-up period, pre-operative symptoms improved in all 18 patients and no tumor recurrence was present. Syringomyelia was reduced or absent within 3–6 months. Conclusions: our results indicated that a complete microscopic resection was effective in eliminating symptoms in these spinal hemangioblastoma patients, who showed a good prognosis after surgery. These improvements in clinical symptoms may be associated with the blood flow changes, but not with reductions and/or eliminations of syrinx after surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinal hemangioblastoma is a highly vascularized benign tumor, which accounts for 2–15% of all primary spinal cord tumors [1]. It is the third most common basis for morbidity in spinal cord tumors, followed only by astrocytoma and ependymoma [2]. Spinal hemangioblastoma can occur sporadically or as a manifestation of von Hippel-Lindau (VHL) disease, which is characterized by numerous lesions that tend to be widely distributed throughout the central nervous system. Approximately, 20–30% of spinal hemangioblastomas are a manifestation of VHL disease [3], which can cause serious neurological damage and a high rate of morbidity. Clinically, microsurgical resection remains the first line of therapy for spinal hemangioblastomas, whereas stereotactic radiosurgery may be an option for patients with incomplete, residual and recurrent tumors, those with unfavorable surgical resection outcomes or those requiring less invasive procedures [4]. When comparing results obtained between sub- versus total-resection of hemangioblastomas, the latter approach has led to better outcomes [5, 6].
Magnetic resonance (MR) imaging represents the most effective tool for the identification and definition of spinal hemangioblastomas [7, 8]. As a result of MR, a substantial increase in the detection rate of this condition has been possible of late [9]. On T1-weighted MR imaging, the tumors appear as bright enhancing lesions, whereas T2-weighted imaging can serve to better define the lesion as well as identify any associated edema and syringomyelia [10]. While the importance of MR in spinal hemangioblastomas is well established, imaging characteristics of some spinal hemangioblastomas are not always obvious, which often causes difficulties in clinical diagnosis and treatment. Therefore, a critical aspect for a more effective use of MR will require the development of detailed MR imaging characteristics such as vascular supply and lesions, as well as the existence of syringomyelia to help with the diagnosis and pre-surgical evaluation of spinal hemangioblastomas.
In this study, we report on 18 cases of spinal hemangioblastomas who underwent surgery in our department and summarize information on their clinical symptoms, surgery and follow-up outcomes. In addition, based on our experience on the outcomes and observations of their MR images, we suggest some guidelines for the pre-surgical evaluation of these spinal hemangioblastomas as based on their MR images.
Materials and methods
Subjects
We evaluated the records of 18 patients with microsurgeries to remove spinal cord hemangioblastomas including 2 patients with VHL disease in our Department of Neurosurgery, at the Tianjin Huanhu Hospital, over the period from January 2012 through January 2017. After obtaining approval from the Institutional Review Board committee at Tianjin Huanhu Hospital (approval number: HHYY2018234), we retrospectively reviewed the patients’ medical records and radiological reports, including clinical characteristics, imaging findings and clinical outcomes.
Clinical evaluation
Neurological functions were evaluated according to classifications of the McCormick method as performed before and at 3 months after surgery. Clinical outcome data were obtained from the medical records and radiological reports at follow-up visits.
Imaging evaluation
Tumor location, distribution and presence/extent of syringomyelia were evaluated in each patient based on their contrast-enhanced T2-weighted MR imaging results (Figs. 1, 2). According to radiological results and intraoperative observations, each tumor was classified as completely intramedullary, intramedullary–extramedullary or primarily extramedullary. Gross-total resection was defined as a complete tumor removal based on operative microscopic findings and post-operative MR images. MR scans were performed within 72 h after surgery and at 3, 6, 12 and 18 months following surgery to assess tumor resection and presence/extent of syringomyelia (Fig. 3).
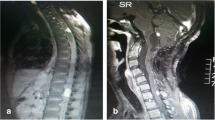
Pre-operative T2-weighted magnetic resonance images from the thoracic spine of a 50-year-old male. Sagittal views (a, b) showing a space-occupying lesion, as well as extensive syringomyelia expanding the spinal cord. Sagittal (c) and axial (d, e) views showing the hemangioblastoma within the spinal cord. The “flow-void” signal as seen in vessels around the lesion (d)
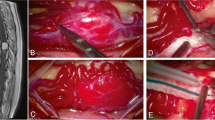
Pre-operative T2-weighted magnetic resonance images from the thoracic spine of a 56-year-old female. Sagittal (a) and coronal (b) views showing a space-occupying lesion posterior to T8 and T9. An extensive syringomyelia expanding the spinal cord was present. c Axial views showing the lesion located in the spinal cord parenchyma. d Tumor and surrounding blood vessels as viewed during surgery
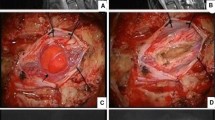
Post-operative T2-weighted magnetic resonance images from the thoracic spine of a 56-year-old female. a The semi-laminar resection and (b) complete tumor resection can be observed with enhanced images within 72 h after the surgery. b Tumor resection was observed at 3 months after surgery. No changes in syringomyelia were observed in this patient at 3 months after surgery
Surgical procedures
All patients underwent MR examination of the entire spinal cord prior to surgery to determine whether typical manifestations of spinal hemangioblastoma were present. Typical manifestations were defined as spinal compression and occupying sites with an obvious T2 signal, accompanied by clear increases in blood vessel “flow-void” signals around the lesions with syringomyelia or total syringomyelia around the lesion. Segment positioning was performed with use of X-rays prior to surgery and methylene blue marking was performed. All patients underwent abdominal B-ultrasound examination and ophthalmoscopy prior to surgery to exclude potential complications of VHL, such as adrenal pheochromocytoma, renal cysts and fundus choroid cysts. Pain relief and dehydration treatment were permitted prior to surgery. No patient underwent pre-operative embolization, external beam radiation therapy or radiosurgery.
After patients were placed in the prone position under general anesthesia, a posterior midline approach was used to expose the target spinous process site as well as the corresponding proximal and distal spinous processes, as identified pre-operatively. An incision of the supraspinous ligament was performed to separate paraspinal muscles. For unilateral lesions <1.5 cm, we generally used hemi-laminectomy. After separating paraspinal muscles, the semi-vertebral plate was removed with use of a drill to the level of the superior and inferior articular processes. The ligamentum flavum within the spinal canal was then removed to expose the dura mater. For larger tumors, we generally used laminectomy/laminotomy. The bilateral paraspinal muscles were separated and secured. A drill with a 1.5 mm bit was used to fracture the bilateral vertebral plates, then vertebral ligaments were severed enabling removal of the vertebral plates and spinous processes. Generally, after incising the dura mater along the median longitudinal direction, it was then secured bilaterally using non-invasive silk thread. Intraoperative neurophysiological monitoring was used during surgery.
Overall, 18 operations for the resection of 37 spinal cord hemangioblastomas were performed within the 18 patients of this report. A single tumor was present in 16 cases, with locations at cervical segments in 10 cases and thoracic segments in 6 cases. Multiple tumors were present in 2 cases with VHL disease, with either 3 or 18 tumors being distributed from cervical segments to the conus medullaris. No post-operative external-beam radiation therapy or stereotactic radiosurgery were performed in any of the patients.
Results
Patient population and clinical characteristics
A total of 18 patients, including 10 males and 8 females, underwent microsurgical treatment for intraspinal hemangioblastomas (Table 1). The mean + SD age at first surgery was 42.3 ± 16.7 (16–65 years). As summarized in Table 1, the main clinical symptoms included sensory changes in 18 patients (100%), pain in 17 (94.4%), motor deficits in 3 (16.7%) and bladder/bowel dysfunction in 1 (5.6%). A total of 18 operations for the resection of 37 spinal cord hemangioblastomas had been performed. Two patients (11.1%) met the criteria for VHL disease. Among those with VHL disease, a total of 21 hemangioblastomas were removed. In ten patients (55.6%), the hemangioblastomas were located in the cervical spine, six (33.3%) in the thoracic spine, one (5.6%) in both cervical and thoracic spines and one in both thoracic and lumbar spines. Of the 37 tumors, 3 (8.1%) were completely intramedullary, 16 (43.2%) were intramedullary–extramedullary and 18 (48.6%) were primarily extramedullary, which were from 1 patient with 18 spinal cord hemangioblastomas attached to the cauda equina. The mean ± SD tumor size was 2278.9 ± 1113.2 mm3 and ranged from 673.5 to 4008 mm3. Tumors were accompanied by syringomyelia in 17 patients (94.4%).
Clinical outcome
Neurological functions in these patients were evaluated at 3 months after the surgery with use of the McCormick classification method and compared with that obtained before surgery (Table 2). Overall, the median preoperative McCormick grade was II, whereas the median grade at 3 months after surgery was I. For the 11 patients at grade II before surgery, all showed improved neurological functions with a McCormick score of I. One patient with a score of II failed to show any improvement in neurological functions at 3 months after the surgery. For the four patients with grade III before surgery, their functions improved to grades I or II. For the two patients with grade IV before the surgery, their neurological functions improved to grades I and II at 3 months after the surgery.
Follow-up
Symptoms such as pain were reduced in all patients within 48–72 h after surgery. Enhanced MR examinations were performed at 72 h after surgery in each patient. Total tumor resections were achieved in all 18 patients with no secondary lesions, such as spinal cord hemorrhage and/or edema being present. For the 17 of 18 patients with syringomyelia, the syringomyelia was found to be reduced in one patient and no change in the remaining 16 patients at 72 h after surgery. Symptoms were found to continue to improve during the inpatient stay. All patients underwent follow-up examinations at 3, 6, 12 and 18 months after the surgery (Table 3). As compared to their symptoms prior to the surgery, 17 showed improved symptoms at 3 months and all patients reported that their symptoms have been improved when assessed at 6, 12 and 18 months after surgery. MR imaging results showed no tumor recurrence in any of the patients as determined at any of the follow-up visits. In the 17 patients with syringomyelia, 10 showed a reduction in syringomyelia size at the 3-month follow-up visit. Although the remaining seven patients showed no change in syringomyelia at this 3-month follow-up, their symptoms improved. As compared to images obtained at the 3-month follow-up, six patients showed no change, eight a reduction and three an absence of syringomyelia when assessed at 6 months. As compared to this 6-month assessment, 14 out of the 17 patients showed no change and 3 showed an absence of syringomyelia status at their 12- and 18-month follow-up visits. Interestingly, each patient exhibited changes in syringomyelia status at varying times after surgery (Fig. 4). For some patients (n = 7), no changes in syringomyelia were observed at 3 months after surgery but could be reduced, absent or unchanged at 6 months after surgery. Some patients (n = 10) showed relatively rapid reductions in syringomyelia at 3 months after surgery and their status was observed to be further reduced, absent or unchanged at 6 months after surgery. Overall, the status of syringomyelia was stabilized by 12 and 18 months after surgery.
Discussion
As spinal hemangioblastoma is a rare, benign tumor, reports on its clinical characteristics, imaging findings, surgical procedures and outcomes are limited. Consistent with a previous study [5], sensory changes and pain were the major symptoms observed in our patients, followed by motor deficits, bladder/bowel dysfunction and other varied symptoms. Approximately, 20–30% of spinal hemangioblastomas are a manifestation of VHL disease [3]. While there is one report indicating that 5/9 of their patients (55%) with spinal hemangioblastomas were affected by VHL disease [11], in our current study, only 2/18 patients (11%) had VHL. Thus, this percent may vary due to sample size and/or other factors. In slightly more than half of our patients, the hemangioblastomas were located in the cervical spine (55.6%), followed by thoracic spine (33.3%) with one patient (5.6%) in both cervical and thoracic spines and 1 (5.6%) in both thoracic and lumbar spines. Similarly, the most common tumor location was also found to be in the cervical spine, followed by the thoracic and lumbar spines as reported by Siller et al. [12].
As reported in the literature, most hemangioblastomas (92.6–95%) occur in the posterior region of the denticulate ligament [10, 12], of which 66% are located in the posterior root entry zone. We also observed a similar phenomenon, in that all 39 tumors were located in the posterior region of the denticulate ligament and were unilateral. Only 28/39 (71.9%) of the tumors were located in the posterior root entry zone. The exact reasons for this phenomenon remain unclear, but may be related to the source of tumor cells. Pathologically, hemangioblastoma has been shown to be comprised of three main components: matrix, endothelial cells and mast cells. Initially, it was believed that tumors were primarily from mast cells, however, subsequent studies have shown that hemangioblastoma in the central nervous system may originate from mesoderm cell residues [13,14,15]. The overall evidence regarding the bases of these tumors is not particularly robust. Accordingly, the exact source of tumor cells remains controversial. Recent findings as generated from molecular pathological analyses have suggested that an allelic deletion of the VHL gene may be present in all three cells of hemangioblastoma [13]. As these three cells fail to display heterogeneity at the genetic level, it is not yet possible to determine the origin of the tumor.
MR remains the primary diagnostic tool for spinal hemangioblastoma and has substantially enhanced the ability to effectively diagnose this disease. As a highly vascularized lesion, hemangioblastoma is often manifested as showing a highly uniform enhancement, usually with an increased degree of “brightness”. In T1, the signal is uniform, while in T2, the signal often shows characteristic abnormalities, generally consisting of an obvious vascular flow void shadow in or around the tumor body. Thus, as compared to T1, the diagnostic value of the T2 sequence is more reliable. Moreover, T2 images of hemangioblastoma display other features, such as an expansion of the myelocoele in adjacent segments of the tumor or the presence of total syringomyelia. However, such features may not be present in all patients. In our study, 17/18 patients (94.4%) had syrinx, which is similar to that of a recent study which reported that 22 tumors (81.5%) were found to be accompanied by peri-tumoral edema and/or syringomyelia [12]. However, in another report, only 30% of their patients showed syringomyelia [16].
Pain and sensory disturbances are the first symptoms of spinal hemangioblastoma, often manifested as segmental. In later stages, myelopathy-like manifestations, such as hyperreflexia, scoliosis, urinary incontinence and limb weakness often develop. The bases for these symptoms include peri-tumoral edema, in addition to direct tumor compression, with the most notable contributing factor being the formation of syringomyelia [17, 18]. Opinions vary as to the mechanisms of syringomyelia formation. Most investigators believe that it is the increased blood flow rates and volumes resulting in increased venous pressure of fluid in the spinal vein which are responsible for the gradual formation of syringomyelia in these highly vascularized tumors [19]. In our report, 17/18 patients showed obvious syringomyelia. The range of syringomyelia was significantly larger than the segment where the tumor was located, with some extensive syringomyelia expanding the spinal cord. Moreover, when compared with other spinal cord tumors, we found that intramedullary glioma often produced local segmental syringomyelia, while compression resulting from schwannomas and meningioma rarely generate syringomyelia. Such findings indicate that a hemangioblastoma generated syringomyelia is not only the result of compression and local stimulation of the tumor but is also likely due to systemic problems associated with the entire spinal cord. For example, changes in blood perfusion and increases in hydrostatic pressure of the entire spinal cord may represent contributing factors.
Although syringomyelia can contribute to the symptoms of spinal hemangioblastoma, the symptom improvement after surgery may not be directly related with the post-operative recovery of the syrinx. In our study, we found that 16/18 patients had symptoms immediately relieved and/or absent within 48 h after the surgery. Although MR imaging was not conducted at 48 h after surgery, no obvious changes were observed in syringomyelia in 16 patients at 72 h after surgery when MR images were taken. One patient showed a reduction in the size of their syrinx. Moreover, follow-up MRI images revealed that syringomyelia was reduced in 10 of 17 patients and unchanged in the remaining seven patients at 3 months after surgery, which suggests that symptom recovery is faster than the reduction of syrinx. Therefore, we considered that improvements in symptoms may not be related with syringomyelia, whereas, blood flow changes after surgery may result in the elimination of symptoms. Using spinal angiography to examine blood distribution in spinal cord can help to support our hypothesis. Unfortunately, spinal angiography was not performed for our patients after surgery.
Currently, the established and most effective treatment for spinal hemangioblastoma is surgical resection, as applied for sporadic cases or in cases of VHL syndrome [20, 21]. Whether asymptomatic spinal hemangioblastomas require surgery is controversial and necessitates consideration of two factors. The first being the growth expectation and prognosis of the tumor and the second being the complications and safety of the surgery. With regard to the first factor, it has been suggested that in cases with a tumor volume >500 mm3 and neurological symptoms which may occur over a short period of time, the lesion should be treated surgically. This protocol is based upon observations resulting from multiple groups of patients with spinal hemangioblastoma [22]. For patients with smaller tumor volumes (<500 mm3), no obvious recent tumor growth or clinical symptoms, a more conservative treatment is recommended. Of the patients in our group, 12 had clinical symptoms and 6 a progressively enlarged tumor over the 2 years of follow-up. Accordingly, surgical treatment was performed on all 18 patients. When evaluating tumor volume in the 18 patients of our group, lesion volumes were >500 mm3, with the average volume being 2461.76 mm3 and clinical symptoms were observed in all cases. It was not possible to determine whether clinical symptoms were present for lesions <500 mm3.
In 17/18 of our patients, clinical symptoms were resolved within 3 weeks after surgery, neurological functions improved, and the patients returned to work. These findings were similar to that reported in the literature where recovery rates for spinal hemangioblastoma were found to be >96% [23, 24], with no new neurological symptoms being observed after surgery and most patients returning to work. A number of notable characteristics regarding the tumors in our group of patients should be specified. First, most tumors were located on the dorsal side of the spinal cord, making them easily accessible for surgery. Second, due to the lack of hemangioblastoma homology and spinal nerve tissue development, a clear boundary was present between the tumor and spinal cord, even when the tumor was located in the spinal cord, a relationship that was confirmed in our surgery. In this way, although the tumor was closely adherent to the surrounding tissues, the presence of a clear border enabled a complete removal of the tumor with minimal damage to the spinal cord. Third, while an abundant blood supply to the tumors was present, this blood supply originated from a single source. Tumors are generally located on the dorsolateral side of the spinal cord and their blood supply is typically from the ipsilateral root artery and anterior spinal artery. Early control of the blood supply to the tumor in surgery makes it easier to remove, however, severing of the tumor drainage vein should be conservative after cutting off the arterial blood supply. The artery ipsilateral to the tumor can be removed, but electrocoagulation should be avoided, to prevent any disruption in the venous return to the spinal cord. Fourth, it may not be necessary to treat the syringomyelia in these cases. Syringomyelia is often spontaneously relieved or dissipates within a few weeks after surgery and its clinical symptoms usually dissipate within 1 week after surgery. Fifth, there is currently no literature supporting use of pre-operative embolization for spinal hemangioblastoma [25]. This treatment only increases the risk of surgery and offers no overall benefit for the surgical procedure. No pre-operative spinal angiography was performed on the patients of our study. As based upon the literature reviewed, all points listed above were confirmed from information as derived from up to 20 years of follow-up studies on spinal hemangioblastoma.
Recently, evidence regarding the natural history of hemangioblastoma has been revealed from findings generated in conservative follow-ups and treatments of patients with hemangioblastoma as determined at several different medical centers. Three forms of tumor growth have been identified [26]. The first is saltatory growth, which consists of rapid growth during its initial (certain) stage, followed by a stable stage, and then a second growth at a later stage. Linear growth is the second form and involves a steady growth of the tumor at a certain rate. Finally, exponential growth, involves an ever-increasing tumor growth rate. Among these three, saltatory growth is the most common form of tumor growth [27], and one with an initial growth period that can be followed by a silent period lasting for several months to several years. There were nine patients that we followed for 1–3 years prior to surgery, and nine patients followed for 3 years before the recurrence and re-surgery. All showed saltatory growth, with tumors often growing rapidly in the initial period followed by a silent period. No cases of continued growth were observed in our 18 cases.
Our sample size was relatively small, as spinal hemangioblastoma is rare disease. With a larger sample size, it would be possible to identify potential risk factors that affect prognosis of spinal hemangioblastoma, surgical outcomes and extent of the syringomyelia. In addition, the tumor growth pattern within our study population was not completely captured. With better understanding of the tumor growth pattern, we could better determine the necessity and predict the time for surgery. Moreover, it would be possible to better understand the association between tumor size and patients’ symptoms, as well as to provide a better prediction regarding the outcome of patients without surgery.
In conclusion, spinal hemangioblastoma represents a relatively rare tumor that can usually be diagnosed with use of MR at qualified medical centers. In this study, we reviewed 18 cases of spinal hemangioblastoma that were subjected to microsurgery in our hospital. We found their surgical outcome to be satisfactory as indicated by improved neurological functions and no tumor recurrence as observed within 18 months of follow-up. The findings that symptoms were quickly relieved after surgery suggests that these symptoms may result from an abnormal distribution of blood flow. Based on our observations, we propose that a hemangioblastoma generated syringomyelia is not only the result of compression and local stimulation of the tumor but is also likely to result from systemic problems associated with the entire spinal cord. Moreover, our study showed that the recovery of syringomyelia is not directly related with improvements in clinical symptoms. Clinical symptoms can be relieved within 48 h to 2 weeks after surgery, whereas the reduction and elimination of syrinx were not observed until 3–6 months after surgery. We also propose that, in addition to the clinical characteristics, surgical indications for spinal hemangioblastoma may depend on MR characteristics which include: (1) expansion of the central duct (syringomyelia) throughout the entire spinal cord or at multiple segments of the spinal cord, effects which remain difficult to explain, (2) enhanced lesions which are obvious and (3) a “flow-void” signal which can be observed in vessels around the lesion in T2-weighted MR images. However, due to the limited case numbers, no definitive conclusion can be derived from our study. Therefore, while the results of this review provide new and important information regarding guidance on surgical indications for spinal hemangioblastoma, there remains a number of controversial issues which will require further study and assessment.
References
Mandigo CE, Ogden AT, Angevine PD, McCormick PC (2009) Operative management of spinal hemangioblastoma. Neurosurgery 65(6):1166–1177. https://doi.org/10.1227/01.NEU.0000359306.74674.C4
Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D (2001) Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery 48(1):55–62 (discussion 62–53)
Pietila TA, Stendel R, Schilling A, Krznaric I, Brock M (2000) Surgical treatment of spinal hemangioblastomas. Acta Neurochir (Wien) 142(8):879–886
Pan J, Jabarkheel R, Huang Y, Ho A, Chang SD (2018) Stereotactic radiosurgery for central nervous system hemangioblastoma: systematic review and meta-analysis. J Neurooncol 137(1):11–22. https://doi.org/10.1007/s11060-017-2697-0
Deng X, Wang K, Wu L, Yang C, Yang T, Zhao L, Yang J, Wang G, Fang J, Xu Y (2014) Intraspinal hemangioblastomas: analysis of 92 cases in a single institution: clinical article. J Neurosurg Spine 21(2):260–269. https://doi.org/10.3171/2014.1.SPINE13866
Dwarakanath S, Sharma BS, Mahapatra AK (2008) Intraspinal hemangioblastoma: analysis of 22 cases. J Clin Neurosci 15(12):1366–1369. https://doi.org/10.1016/j.jocn.2007.04.014
Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH (2003) von Hippel-Lindau disease. Lancet 361(9374):2059–2067. https://doi.org/10.1016/S0140-6736(03)13643-4
Rebner M, Gebarski SS (1985) Magnetic resonance imaging of spinal-cord hemangioblastoma. AJNR Am J Neuroradiol 6(2):287–289
McCormick PC, Post KD, Stein BM (1990) Intradural extramedullary tumors in adults. Neurosurg Clin N Am 1(3):591–608
Lonser RR, Weil RJ, Wanebo JE, DeVroom HL, Oldfield EH (2003) Surgical management of spinal cord hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg 98(1):106–116. https://doi.org/10.3171/jns.2003.98.1.0106
Na JH, Kim HS, Eoh W, Kim JH, Kim JS, Kim ES (2007) Spinal cord hemangioblastoma : diagnosis and clinical outcome after surgical treatment. J Korean Neurosurg Soc 42(6):436–440. https://doi.org/10.3340/jkns.2007.42.6.436
Siller S, Szelenyi A, Herlitz L, Tonn JC, Zausinger S (2017) Spinal cord hemangioblastomas: significance of intraoperative neurophysiological monitoring for resection and long-term outcome. J Neurosurg Spine 26(4):483–493. https://doi.org/10.3171/2016.8.SPINE16595
Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, Sohn T, Kim SH, Lubensky IA, Vortmeyer AO, Rodgers GP, Oldfield EH, Lonser RR (2007) von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med 4(2):e60. https://doi.org/10.1371/journal.pmed.0040060
Vortmeyer AO, Frank S, Jeong SY, Yuan K, Ikejiri B, Lee YS, Bhowmick D, Lonser RR, Smith R, Rodgers G, Oldfield EH, Zhuang Z (2003) Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel-Lindau disease. Cancer Res 63(21):7051–7055
Ang SO, Chen H, Gordeuk VR, Sergueeva AI, Polyakova LA, Miasnikova GY, Kralovics R, Stockton DW, Prchal JT (2002) Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells Mol Dis 28(1):57–62
Bostrom A, Hans FJ, Reinacher PC, Krings T, Burgel U, Gilsbach JM, Reinges MH (2008) Intramedullary hemangioblastomas: timing of surgery, microsurgical technique and follow-up in 23 patients. Eur Spine J 17(6):882–886. https://doi.org/10.1007/s00586-008-0658-1
Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH (2006) Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease: implications for treatment. J Neurosurg 105(2):248–255. https://doi.org/10.3171/jns.2006.105.2.248
Imagama S, Ito Z, Wakao N, Sakai Y, Kato F, Yukawa Y, Sato K, Ando K, Hirano K, Tauchi R, Muramoto A, Hashizume Y, Matsuyama Y, Ishiguro N (2011) Differentiation of localization of spinal hemangioblastomas based on imaging and pathological findings. Eur Spine J 20(8):1377–1384. https://doi.org/10.1007/s00586-011-1814-6
Hayashi N, Matsumae M, Yatsushiro S, Hirayama A, Abdullah A, Kuroda K (2015) Quantitative analysis of cerebrospinal fluid pressure gradients in healthy volunteers and patients with normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 55(8):657–662. https://doi.org/10.2176/nmc.oa.2014-0339
Mitha AP, Turner JD, Abla AA, Vishteh AG, Spetzler RF (2011) Outcomes following resection of intramedullary spinal cord cavernous malformations: a 25-year experience. J Neurosurg Spine 14(5):605–611. https://doi.org/10.3171/2011.1.SPINE10454
Glasker S, Klingler JH, Muller K, Wurtenberger C, Hader C, Zentner J, Neumann HP, Velthoven VV (2010) Essentials and pitfalls in the treatment of CNS hemangioblastomas and von Hippel-Lindau disease. Cent Eur Neurosurg 71(2):80–87. https://doi.org/10.1055/s-0029-1234040
Kim TY, Yoon DH, Shin HC, Kim KN, Yi S, Oh JK, Ha Y (2012) Spinal cord hemangioblastomas in von hippel-lindau disease: management of asymptomatic and symptomatic tumors. Yonsei Med J 53(6):1073–1080. https://doi.org/10.3349/ymj.2012.53.6.1073
Lonser RR (2014) Surgical management of sporadic spinal cord hemangioblastomas. World Neurosurg 82(5):632–633. https://doi.org/10.1016/j.wneu.2014.08.026
Bamps S, Calenbergh FV, Vleeschouwer SD, Loon JV, Sciot R, Legius E, Goffin J (2013) What the neurosurgeon should know about hemangioblastoma, both sporadic and in Von Hippel-Lindau disease: a literature review. Surg Neurol Int 4:145. https://doi.org/10.4103/2152-7806.121110
Ampie L, Choy W, Khanna R, Smith ZA, Dahdaleh NS, Parsa AT, Bloch O (2016) Role of preoperative embolization for intradural spinal hemangioblastomas. J Clin Neurosci 24:83–87. https://doi.org/10.1016/j.jocn.2015.09.006
Lonser RR, Butman JA, Huntoon K, Asthagiri AR, Wu T, Bakhtian KD, Chew EY, Zhuang Z, Linehan WM, Oldfield EH (2014) Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. J Neurosurg 120(5):1055–1062. https://doi.org/10.3171/2014.1.JNS131431
Feletti A, Anglani M, Scarpa B, Schiavi F, Boaretto F, Zovato S, Taschin E, Gardi M, Zanoletti E, Piermarocchi S, Murgia A, Pavesi G, Opocher G (2016) Von Hippel-Lindau disease: an evaluation of natural history and functional disability. Neuro Oncol 18(7):1011–1020. https://doi.org/10.1093/neuonc/nov313
Funding
This study was supported by National Science Foundation of Tianjin City, China, to Dr. Yang Nan (Grant No. 18JCZDJC98600) and Health and Family Planning Commission in Binhai New District of Tianjin City, China to Dr. Yang Nan (Grant No. 2018BWKZ003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Zhang, L., Wang, H. et al. Spinal hemangioblastoma: surgical procedures, outcomes and review of the literature. Acta Neurol Belg 121, 973–981 (2021). https://doi.org/10.1007/s13760-020-01420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-020-01420-4