Abstract
This statement paper deals with the key role an neurologist plays in the management of victims of chemical warfare/terrorist attacks. Because terrorist factions have expanded the war zone creating a worldwide risk of terrorist attacks, not only limited to some conflict zones in the Middle East, neurologists in all countries/regions have to be prepared for disaster response. The scope of this paper is to provide guidelines for the neurological management of victims of chemical and biological terrorist attacks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the world has been repeatedly rocked by terrorist attacks. After a long series of worldwide attacks, mostly by Al Qaeda or Islamic State or other jihadist terrorist factions, Belgium was hit by terrorism [1]. Belgium mourned 32 casualties and more than 300 wounded after a series of bombing attacks on Brussels International Airport and the Brussels Metro, March 22, 2016. This was the most deadly attack in Belgium thus far [2].
Thus far, the so-called dirty bombs have not been used, though a terrorist attack with an improvised nuclear device would create political, economic, social, psychological, and environmental havoc around the world. As the threat is global, Nuclear Security Summits have been held (the first summit in April 2010, Washington, D.C., USA) in order to prevent nuclear terrorism and counter nuclear smuggling. The third summit was held in 2016 [3].
On the contrary, both chemical and biological weapons have already been used in acts of terror. The radical religious group Aum Shinrikyo, founded in Japan in the 1980s, used sarin in 1994 as a chemical weapon to poison approximately 600 civilians in Matsumoto city releasing 12 l of sarin, killing seven people. On March 20, 1995, Aum deployed sarin in an even larger terrorist attack on the Tokyo Subway System, which poisoned some 6000 people of which 12 did not survive [4, 5].
In 1984, the Rajneesh movement spreaded salmonella in salad bars at ten restaurants in Oregon to influence a local election, in what is believed to be the first incident of bioterrorism in the United States [6].
Although there is some reasonable risk for terrorist attacks or accidental chemical, biological, radiation, and nuclear incidents, hospitals are not sufficiently prepared to deal with these incidents. Moreover, hospitals themselves are at risk becoming soft targets for terrorist actions [1, 7]. Therefore, awareness among neurologists is essential.
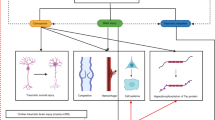
The management of victims of attacks with chemical and biological weapons of mass destruction with neurological symptoms is the focus of this review paper.
Biological weapons
The aim of terrorism is to create panic and social disruption and challenge public health services. Recently, the focus of terrorist factions shifted towards soft targets. Some biological and chemical substances could potentially be used as weapons of mass destruction. The CDC classifies potential bioterrorism agents into three categories based on the agents’ ability to be disseminated, transmitted, the morbidity and mortality rate,… Category A (high risk) agents include anthrax, plague, tularemia, smallpox, the hemorrhagic fever viruses, and botulinum toxin. For the neurologist, anthrax and botulinum toxin are the most relevant [8].
These two agents and Ebola virus were also initially considered for bioterror attacks by the Aum Shinrikyo in Japan, though they eventually chose to use the nerve gas sarin over biological warfare agents [9, 10].
Bacterial warfare: anthrax
Anthrax is caused by Bacillus anthracis, a large, nonmotile, spore-forming, Gram-positive rod. The most serious terrorist threat posed by anthrax is infection by inhalation. For humans, the dose sufficient to kill half of the exposed persons ranges from 2500 to 55,000 inhaled spores [11].
The clinical course of B. anthracis-infection has been studied intensively during two ‘outbreaks’ of this disease. The first took place in 1979 in Yekaterinburg in the former Soviet Union after an accidental release of anthrax spores from a bioweapons facility. This outbreak caused approximately 68 deaths [12].
The second outbreak, consisting of 11 cases, was related to bioterrorism and occurred in 2001 in the USA. The source of the anthrax appeared to be five letters sent through the US Postal Service [13].
In the summer of 2016, a 12-year-old boy in the far north of Russia died in an outbreak of anthrax that experts believe was triggered when unusually warm weather caused the release of the bacteria because of thawing of the permafrost scientists already warned for years before [14, 15].
Infection with B. anthracis is acquired by oral ingestion, by inhalation, or by absorption of the spores through the skin and mucous membranes.
Inhalation anthrax is the most fulminant form of this disease. In a first stage, patients complain of nonspecific flu-like symptoms, e.g., fever, chills, drenching sweats, nausea, vomiting, diarrhea, and abdominal pain, as well as headache, cough, and chest pain (Table 1). In the second stage dyspnea, fever, and shock appear [11].
Chest radiography show typical mediastinal changes and pleural (bloody) effusions.
In the Russian outbreak, the mean duration of the first stage was 3–4 days, and the median duration of the second stage before death was only 1 day [12].
Untreated, mortality reaches 95%. Among the confirmed inhalational cases from the second outbreak in the USA, mortality rate was 45% [13].
Anthrax and the neurologist
Meningitis due to infection with Bacillus anthracis is commonly considered an infrequent manifestation of the disease but one associated with high case fatality. The frequency of anthrax meningitis, however, seems to be underestimated.
The index case of the 2001 outbreak of inhalational anthrax in the USA was associated with meningitis clearly demonstrated by the presence of typical Gram-positive rods in CSF [16].
Of the other patients involved in this outbreak 20% developed meningitis [13].
The most common neurological manifestations were headache and confusion.
In the Russian experience, hemorrhagic meningitis was found in as much as 50% of the autopsy cases [12].
Meningitis presents with fever, headache, nausea, vomiting, and altered mental status. Clinical signs include meningeal signs, long-tract signs, hyperreflexia, seizures, myoclonus, fasciculations, rigidity, stupor, or coma. Cerebrospinal fluid (CSF) shows neutrophilic pleocytosis often greater than 500 ml, an elevated erythrocyte count, and elevated protein, corresponding with hemorrhagic type of meningitis [17].
Gram-stain shows copious large Gram-positive rods with or without endospores. In the majority of patients, blood cultures are positive. CT or MRI of the brain reveals diffuse cerebral edema, prominent leptomeningeal enhancement, and focal bleeding (intraparenchymatous, subarachnoidal, or intraventricular) [18].
Treatment of B. anthracis consists of a multi-drug regimen. Because of the high frequency of meningitis in anthrax infection, agents that penetrate the blood–brain barrier, such as high-dose penicillin, are preferred for patients with inhalational anthrax associated with meningitis in association with one or two other antibiotics, e.g., chloramphenicol, erythromycin, streptomycin, ciprofloxacin, and other quinolones. B. anthracis is resistant to ceftriaxone and other 3rd generation cephalosporins. Doxycycline and clindamycin should be avoided in cases of anthrax meningoencephalitis because of the poor blood–brain barrier penetrance [19, 20].
Studies showed that patients treated with three or more recommended antimicrobials had a favorable outcome compared to those who received one or two antimicrobials (survival 75% versus only 16%). Treatment duration is long: the original recommendation from the CDC was a 60-day course of antimicrobials. The same applies for post-exposure profylaxis.
In anthrax meningitis steroids demonstrated to improve survival, although experience is very limited [21].
In case of mass casualty incidents and shortage of resources (e.g., antibiotics) or limited access to diagnostics (e.g., spinal tap analysis), standards of care recommendations for diagnosis and treatment are summarized by CDC [22].
Katharios-Lanwermeyer and co-workers developed an easy-to-use assessment tool for screening patients for meningitis during mass casualty incidents, based on bedside available clinical data: severe headache, altered mental status, meningeal signs and other neurological signs [23].
Differential diagnosis of meningitis in the wake of terrorism includes the plague (Yersinia pestis), tularemia (Francisella tularensis), Q fever (Coxiella burnetii), brucellosis (Brucella species, and smallpox, besides the regular community-acquired causes of meningitis [11].
Biotoxin warfare: botulinum toxin
Clostridium botulinum was discovered after an outbreak at a funeral dinner with smoked ham by Emile Pierre van Ermengem, Professor of bacteriology at the University of Ghent. He originally named the bacteria Bacillus botulinus because of its pathological association with sausages (“botulus” in Latin) [24, 25].
Neurologists recognize the beneficial effects of botulinum toxin as it is used as a treatment for several medical conditions characterized by muscle hyperactivity/spasm. These conditions include blepharospasm, strabismus, cervical dystonia, spastic dysphonia, limb spasticity associated with underlying neurological problems, tremors, migraine,… [25, 26]
However, used as a warfare agent, most likely via aerosol, it has the potential detrimental effects as it is 15,000 times more lethal than the highly potent chemical agent VX and 100,000 times more lethal than sarin [27].
Only a single gram of crystallized botulinum toxin can kill more than 1 million people [28].
Permanent damage to the nerve synapse and thus irreversible neuromuscular blockade could be induced by botulinum toxin in a sufficient dosage.
Incubation time following ingestion is 12–36 h, following inhalation usually 18–72 h.
Botulism is characterized by its acute, afebrile, descending, symmetric, flaccid paralysis that always begins in the bulbar musculature [29].
The latter cardinal early clinical manifestations of botulism include blurred vision, ptosis, diplopia, dysarthria, dysphonia, and dysphagia. Bilateral facial palsy is common [11].
Botulism has also a delayed initiation of action which results in descending paralysis after involvement of the major cranial nerve palsies as mentioned above. Severe weakness tends to occur by day 4 after exposure. Paralysis of the diaphragm and intercostal muscles leads to respiratory failure and the need for mechanical ventilation.
Autonomic nervous system dysfunction is frequent and is demonstrated by absent sympathetic skin response as well as significantly decreased heart rate variation
Patients remain fully conscious, as the toxin does not penetrate the blood brain barrier.
Ascending weakness as seen typically in Guillain–Barré–Strohldisease has not been reported.
Differential diagnosis includes Miller–Fisher disease, myasthenia gravis, pontine stroke drug intoxication (e.g., atropine), poliomyelitis, tick paralysis, diphtheria, and seafood toxin effect. CSF analysis is normal.
The only specific treatment for botulism is passive immunization with an equine antitoxin.
When treating secondary infections, aminoglycosides and clindamycin should be avoided as they may exacerbate the existing neuromuscular blockade [29]. In the case of a bioterrorist attack, a large number of patients would need ICU care and mechanical ventilation for several weeks. Shortage of available ICU beds and ventilators could be catastrophic by day 4 after exposure [11, 25].
Biotoxin warfare: seafood neurotoxins
There are two naturally occurring seafood neurotoxins: tetrodotoxin (TTX) and saxitoxin. TTX is a heat and acid-stable, alkaloid toxin produced by marine bacteria and microalgae and is toxic for humans via the ingestion of contaminated puffer fish believed to be confined to regions of the South East Asia. However, recent studies demonstrated the presence of TTX in the United Kingdom waters and the Dutch Eastern Scheldt, contaminating mussels and oysters and causing the Dutch Food and Wares Authority to ban the sale of oysters and mussels from a part of the Eastern Scheldt in June 2016.
The use as a bioterrorism weapon is rather unlikely, though there is a potential risk that contaminated fish or shell fish banned for human consumption could enter the food chain by malicious traders [30–32].
Ingestion of only about 2 mg of TTX (about 50–100 g of puffer fish) could be fatal by blocking the voltage-activated sodium channels, terminating nerve conduction and muscle action potentials and leading to progressive paralysis and respiratory failure [33].
Numbness and tingling are often prominent; mild peripheral weakness may appear within minutes to a few hours after ingestion, followed by an ascending paralysis. Bulbar symptoms, hypersalivation, and sweating are common. Other clinical symptoms include hypotension, hypertension, convulsions, and cardiac arrhythmias. The patients remain fully conscious. There is no specific antidote for TTX or saxitoxin: anticholinesterases and naloxone are ineffective. Activated charcoal of gastric lavage could reduce the severity of poisoning.
Supportive treatment including mechanical ventilation might be needed for as long as 2 weeks [11, 34].
Viral warfare: ZIKA
Zika virus infection, transmitted mainly by the bite of female Aedes mosquitoes, has been known in Africa and Asia since many decades, but only recently it is considered as an emerging infection as severe neurological sequelae have been linked to Zika infection [35, 36].
The majority of patients infected with Zika are asymptomatic. Fortunately, the cardinal symptoms are aspecific and mild: mild fever, skin rashes, conjunctivitis (nonpurulent), muscle and joint pain, back pain, or a headache are described. It is indistinguishable from the symptoms of other arboviral diseases such as dengue and chikungunya [35].
However, both Guillain–Barré syndrome (first reported in 2013 during a Zika outbreak in French Polynesia) and microcephaly (first reported in Brazil in 2015 in infected pregnant women) have been postulated to be caused by Zika virus although the evidence is limited and debate is still ongoing [35, 37].
Used as biological warfare, the transmission is easily and potential victims would be at random, leading to possible fear and panic, especially in pregnant women as is demonstrated by the concerns and fears of the participants and spectators of the Olympicin Rio, Brazil, 2016. Therefore, CDC laid down guidelines for pregnant ladies [38].
Zika infection can be transmitted by viraemic travelers (by sexual intercourse and by blood donations), and of course by infected Aedes aegypti mosquitos introduced by terrorists in the country they want to destabilize. Due to global warmth and changing climate, European states become more vulnerable to Aedes which can become endemic as eradication has been shown to be very difficult, but not impossible [39, 40].
However, the extent of an outbreak is poorly predictable and the public health effects are only long term and as such less attractive to terrorists.
Viral warfare: viral hemorrhagic fevers
Filoviridae such as Marburg and Ebola virus are a greater threat and, therefore, are classified as Category A biological warfare agents by the CDC: authorities fear outbreaks of, e.g., Ebola in Europe or the United States by means of viraemic travelers. In fact, filoviridae could be turned into a biological weapon. Since airport exit and entry screening have been implemented and ill travelers and persons who reported a high risk for exposure to Ebola were denied boarding, no international air traveler from West-African countries has been reported as symptomatic with Ebola [41–43].
However, there is still a threat. In 1993, the Japanese Aum Shinrikyo cult sent a team to Africa aiming at collecting samples of Ebola-infected patients for use as a biological weapon. In a recent review, Cenciarelli and co-workers discuss the possible worst case scenarios of terrorist attacks using weaponized Ebola in Underground stations, on cruise ships and in airports [44].
In fact, terrorist actions using human carriers (on a suicide mission) infected with Ebola virus to intentionally disseminate the virus through different scenarios are a real threat. Recent articles suggest the possibility for the Islamic State to send operative groups into an Ebola outbreak area to intentionally expose themselves to the virus [44, 45].
Ebola poses serious concerns about the safety of doctors and paramedics involved in the treatment of Ebola-infected patients and the possibility of devastating health services. Ebola was discovered back in 1976 by Peter Piot and Guido van der Groen from the Antwerp-based Prince Leopold Institute of Tropical Medicine, when analyzing an outbreak of hemorrhagic fever in Yambuku, Republic of Congo, involving a mission outpost run by Flemish nuns—the Sisters of the Sacred Heart of Our Lady of ‘s Gravenwezel. Two Flemish missionary sisters were the first European victims of this disease, among more than 200 congolese victims.
Later outbreaks with a mean case fatality rate of 79% disrupted health care services as many health care workers contracted the disease [46, 47]. Use of barrier protection and surgical masks is adequate to halt most nosocomial transmission of Ebola viruses, but still health care workers get infected when caring for patients [48, 49].
For neurologists, only two recent case reports are both interesting and intriguing, both originating from the Sierra Leona (West-Africa) outbreak in 2015. One is the case of a 39-year-old nurse from Scotland who had assisted in direct patient care and was diagnosed with Ebola on her return to Glasgow. She was intensively treated. However, 9 months later she was readmitted with progressive meningoencephalitis, cranial neuropathies (bilateral VI, left partial III, left VII palsies), cerebellar signs and radiculopathy. MRI showed enhanced leptomeningeal staining of the brainstem, cranial nerves, cauda equina and conus medullaris and the left cerebellar hemisphere. Both CSF and blood tested positive for virus RNA. The authors state that ongoing vigilance for relapsed Ebola virus infection of survivors is essential [49]. Another case is a case of encephalitis probably as the presentation of the original illness [50]. These cases are unique thus far as only autopsy cases on Marburg virus-infected patients have demonstrated the presence of encephalitis [51].
Chemical warfare
The first large-scale use of chemical warfare agents was during World War I (1914–1918). During the great war, gases such as chlorine (first used on april 22, 1915 in Ypres), phosgene, and mustard gas (first used in 1917) were deployed causing a new and complex public health threat that endangered not only soldiers and civilians on the battlefield but also health workers (e.g., ambulance personnel).
Although approximately 30% of all war casualties were victims of gas exposure, more than 80% of these were by mustard gas alone [52].
Chemical warfare: nerve agents
Chemical warfare nerve agents (NAs) are a group of organophosphorous compounds that have played an important role in terroristic attacks during the last few decades. They were originally used in agriculture, but there are more and more cases in which its misuse leads to various (neurologic) symptoms and eventually death [53, 54].
The NAs can be classified into G and V agents. The G agents include Tabun, Sarin and Cyclosarin. The V agents include VE, VM, VG, VR and VX. In the 1930s, IG Farben industries first produced the G agents (G stands for Germany). The V agents were produced later after World War II, with the V standing for the victory of the allied forces. Later on, the usage of NAs became more and more common. A first example is the Iran–Iraq conflict in 1983–1988 where NAs, especially sarin, were used against Iranian troops and even civilians. In the terrorist attacks in 1994 in Matsumoto, Japan and Tokyo subways Sarin was used to poison 6100 people, resulting in 18 mortalities [11, 53, 55].
NAs are not actually gasses, because they are liquid at room temperature. They are also tasteless, odorless and volatile, which makes them unrecognizable and even more dangerously. The G agents spread very rapidly and are active in the environment several hours after release of the agent. They rapidly spread on the skin and release only 30 min after contact with clothing. The V agents are oilier and thus take much longer to evaporate [11, 53].
Their mechanism of action is through the inhibition of cholinesterase, which leads to an accumulation of acetylcholine in the synaptic clefts up to toxic doses. The consequence is an overstimulation of the cholinergic pathway and a desensitization of the cholinergic receptor site [11, 53, 55–58]
The clinical presentation of NAs-induced symptoms can be divided into acute and late complications. Acutely, high doses of NAs can cause important neurological manifestations such as fatigue, muscle weakness, flaccid paralysis, generalized fasciculations and seizures.
If persons are exposed to less higher doses of NAs, they may still experience symptoms such as headache, dizziness, restlessness, anxiety, mental confusion, ataxia, irritability, insomnia, bad dreams, depression, forgetfulness, impaired judgment and lack of concentration.
There has also been a report of an intermediate syndrome (IMS) that occurs 24–96 h after exposure. IMS leads to reversible weakness in proximal (chest) muscles and cranial nerve palsies. The etiology of IMS is not completely clear yet, but a theory lies in delayed AChE inhibition.
Two to four weeks after exposure, sensory and motor disorder of the peripheral nervous system can occur, defined as organophosphate-induced delay neuropathy or OPIDN. It is characterized by progressive weakness, impaired reflexes and distal paraesthesias. Most victims present with cholinergic irritation: nose secretion, increased salivation, pharyngitis and laryngitis. This is followed by paralysis of the leg muscles which can persist for 1–2 months. The most probable cause is the degeneration of myelin and axons.
The base for treatment is atropine, a parasympatholytic and competitive antagonist of ACh on muscarinic receptors and thus only effective on the muscarinic signs, like bronchoconstriction, bradycardia and smooth muscle contraction. A dose of 2 mg is recommended.
The second known group is the oximes. They reactivate the phosphorylated cholinesterase enzyme by breaking the nerve-enzyme bond, which ensures an almost complete reverse of the effects of the NAs [11, 53, 54, 57, 58].
Galantamine, donepezil and rivastigmine, acting as centrally cholinesterase inhibitors, have also been mentioned as potential antidotes for nerve agents, but the potential use is considered to be limited thus far [59, 60].
The specific treatment for convulsions consists of administrating a benzodiazepine, such as diazepam, lorazepam and midazolam. The most commonly used is diazepam with a dose for children of 0.2–0.5 mg/kg and for adults 5–10 mg. However, other research has shown that midazolam is more rapid in stopping an NA-induced seizure. It should be administered in doses of 1.5 mg/kg [11, 53, 61, 62].
The neurologist and the management of victims of chemical or bioterrorism
Early recognition of an increase or clustering of disease syndromes that might represent a disease outbreak is essential. After 9/11 public health officers were alerted and implemented a syndromic surveillance system in hospital emergency departments (EDs) to identify a large-scale bioterrorist event and other health conditions related to the terrorist attacks [63, 64].
In overt terrorist attacks like the Sarin attack in Tokyo or sending an anthrax-containing letter to a person, the outbreak of neurological disease is clear so medical response is fast and adequate.
In covert terrorist attacks, however, cases of very rare (infectious) diseases, normally not occurring in the region, in individuals who did not travel to epidemic regions, should be reported to public health officers. A thorough epidemiological survey is warranted in all patients. Immediate reporting can reveal a cluster of patients and thus a possible covert terrorist attack.
Discussion
As one of Europe’s most densely populated countries with multiple nuclear installations and a prominent petrochemical industry, Belgium is at some reasonable risk for terrorist attacks or accidental chemical, biological, radiation, and nuclear (CBRN) incidents. Brussels is considered the de facto capital of the European Union, hosting the institutions of the European Union within its European Quarter as well as many consulates, embassies or the seats of inter-governmental organizations and interest groups. Belgium is also home to the headquarters of the North Atlantic Treaty Organization (NATO) in Brussels and of the Supreme Headquarters Allied Powers Europe (SHAPE). Other international organizations like the World Health Organization, Unesco, UNICEF, United Nations, World Bank, and many others have also an office in Brussels.
Therefore, Belgium would be also at risk for terrorist attacks. In the recent metro bombing (March 2016) in Brussels, the European Quarter was targeted.
Not only Belgian emergency services and hospitals should be prepared to deal with a variety of potential incidents and threats. Because most bioterrorism agents can directly or indirectly affect the nervous system, neurologists play a key role in identifying potential bioterrorist attacks.
Early recognition of an increase or clustering of disease syndromes that might represent a disease outbreak is essential. After 9/11 public health officers were alerted and implemented a syndromic surveillance system in hospital emergency departments (EDs) to identify a large-scale bioterrorist event and other health conditions related to the terrorist attacks. The most important issue is to be aware of the clinical patterns and to be capable to make a distinction of syndromes initiated by bioterrorism from naturally occurring diseases.
In general, neurological symptoms tend to manifest somewhat later on in case of a biological attack, as compared to chemical weapons [65].
However, prompt death might occur following exposure to both botulinum toxin, tetrodotoxin, saxitoxin, and chemical nerve agents. If death does not occur on the scene, agents as anthrax and botulinum toxic and sea food toxins can pose a severe burden on hospitals and the health care system as long ICU stay is needed.
The imminent threat of (bio)terrorism warrants neurologists to be thoroughly instructed and trained in recognizing and managing terrorism victims.
Although different surveillance systems were deployed as a means of detecting outbreaks, including bioterrorist attacks, the correct early recognizing and management of bioterrorism induced diseases will rely on the individual physicians’ awareness and ability to correct diagnosis.
Educational intervention can improve physicians’ diagnosis and treatment [66, 67]. Hopefully this manuscript will also provide a better bioterrorism awareness of neurologists working in a challenging world.
References
De Cauwer H, Somville F, Sabbe M, Mortelmans LJ (2017) Hospitals: soft target for terrorism? Prehospital disaster medicine. Prehosp Disaster Med 32(1):94–100
Bommen Zaventem bevatten spijkers. http://www.hln.be/hln/nl/957/Binnenland/article/detail/2654459/2016/03/22/Bommen-Zaventem-bevatten-spijkers.dhtml. Assessed 4th July 2016
Foreign Press Center Briefing: Preview of the 2016 Nuclear Security Summit. http://www.nss2016.org/news/2016/3/30/fpc-briefing-3-29-16. Assessed 4th July 2016
Okudera H, Morita H, Iwashita T, Shibata T, Otagiri T, Kobayashi S, Yanagisawa N (1997) Unexpected nerve gas exposure in the city of Matsumoto: report of rescue activity in the first sarin gas terrorism. Am J Emerg Med 15(5):527–528
Asai Y, Arnold JL (2003) Terrorism in Japan. Prehosp Disaster Med 18(2):106–114
Terrorism in the United States. https://en.wikipedia.org/wiki/Terrorism_in_the_United_States. Assessed 4th July 2016
Mortelmans LJ, Van Boxstael S, De Cauwer HG, Sabbe MB (2014) Belgian Society of Emergency and Disaster Medicine (BeSEDiM) study. Preparedness of Belgian civil hospitals for chemical, biological, radiation, and nuclear incidents: are we there yet? Eur J Emerg Med 21(4):296–300
CDC Working Group (2000) Biological and chemical terrorism: strategic plan for preparedness and response. Recommendations of the CDC Strategic Planning Workgroup. MMWR Recomm Rep 49:1–14
Barras V, Greub G (2014) History of biological warfare and bioterrorism. Clin Microbiol Infect 20(6):497–502
Cenciarelli O, Gabbarini V, Pietropaoli S, Malizia A, Tamburrini A, Ludovici GM et al (2015) Viral bioterrorism: Learning the lesson of Ebola virus in West Africa 2013–2015. Virus Res 210:318–326
Bus KM, Bleck TP (2012) Treatment of neuroterrorism. Neurotherapeutics. 9(1):139–157
Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH (1993) Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci USA 90(6):2291–2294
Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M et al (2001) Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 7(6):933–944
Revich BA, Podolnaya MA (2011) Thawing of permafrost may disturb historic cattle burial grounds in East Siberia. Glob Health Action. doi:10.3402/gha.v4i0.8482
Anthrax outbreak triggered by climate change kills boy in Arctic Circle. https://www.theguardian.com/world/2016/aug/01/anthrax-outbreak-climate-change-arctic-circle-russia. Assessed 31 Aug 2016
Bush LM, Abrams BH, Beall A, Johnson CC (2001) Index case of fatal inhalation anthrax due to bioterrorism in the United States. N Engl J Med 345:1607–1610
Lanska DJ (2002) Anthrax meningoencephalitis. Neurology 59:327–334
Kim HJ, Jun WB, Lee SH, Rho MH (2001) CT and MR findings of anthrax meningoencephalitis: report of two cases and review of the literature. AJNR Am J Neuroradiol 22:1303–1305
Sejvar JJ, Tenover FC, Stephens DS (2005) Management of anthrax meningitis. Lancet Infect Dis 5(5):287–295
Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM et al (1999) Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 281(18):1735–1745
Dixon TC, Meselson M, Guillemin J, Hanna PC (1999) Anthrax. N Engl J Med 341:815–826
Bower WA, Hendricks K, Pillai S, Guarnizo J, Meaney-Delman D (2015) Centers for Disease Control and Prevention (CDC). Clinical framework and medical countermeasure use during an anthrax mass-casualty incident. MMWR Recomm Rep 64(4):1–22
Katharios-Lanwermeyer S, Holty JE, Person M, Sejvar J, Haberling D, Tubbs H et al (2016) Identifying meningitis during an anthrax mass casualty incident: systematic review of systemic anthrax since 1880. Clin Infect Dis 62(12):1537–1545
Erbguth FJ (2004) Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov Disord 19(Suppl 8):S2–S6
Caya JG, Agni R, Miller JE (2004) Clostridium botulinum and the clinical laboratorian: a detailed review of botulism, including biological warfare ramifications of botulinum toxin. Arch Pathol Lab Med 128(6):653–662
Panicker JN, Muthane UB (2003) Botulinum toxins: pharmacology and its current therapeutic evidence for use. Neurol India 51(4):455–460
Franz DR, Jahrling PB, Friedlander AM et al (1997) Clinical recognition and management of patients exposed to biological warfare agents. JAMA 278:399–411
Martin CO, Adams HP Jr (2003) Neurological aspects of biological and chemical terrorism: a review for neurologists. Arch Neurol 60:21–25
Arnon SS, Schechter R, Inglesby TV et al (2001) Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059–1070
Noguchi T, Onuki K, Arakawa O (2011) Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. ISRN Toxicol 2011:276939
Turner AD, Higgins C, Higman W, Hungerford J (2015) Potential threats posed by tetrodotoxins in UK waters: examination of detection methodology used in their control. Mar Drugs 13(12):7357–7376
Food Safety Agency seeks to avert mussel scare. http://deredactie.be/cm/vrtnieuws.english/Health%2Band%2BEnvironment/1.2696432. Assessed 21 August 2016
Cole JB, Heegaard WG, Deeds JR, McGrath SC, Handy SM (2015) Centers for Disease Control and Prevention (CDC). Tetrodotoxin poisoning outbreak from imported dried puffer fish–Minneapolis, Minnesota, 2014. MMWR Morb Mortal Wkly Rep 63(51):1222–1225
Bane V, Lehane M, Dikshit M, O’Riordan A, Furey A (2014) Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins (Basel) 6(2):693–755
Hajra A, Bandyopadhyay D, Hajra SK (2016) Zika virus: a global threat to humanity: a comprehensive review and current developments. N Am J Med Sci 8(3):123–128
Centers for disease control and prevention. Transmission & risks. http://www.cdc.gov/zika/transmission/index.html. Assessed 05 Sept 2016
Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S et al (2016) Zika virus as a cause of neurologic disorders. N Engl J Med 374(16):1506–1509
Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM et al (2016) Interim guidelines for pregnant women during a Zika virus outbreak-United States, 2016. MMWR Morb Mortal Wkly Rep 65:30–33
Baldacchino F, Caputo B, Chandre F, Drago A, Della Torre A, Montarsi F, Rizzoli A (2015) Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Manag Sci 71(11):1471–1485
Scholte E, Den Hartog W, Dik M, Schoelitsz B, Brooks M, Schaffner F, Foussadier R, Braks M, Beeuwkes J (2010) Introduction and control of three invasive mosquito species in the Netherlands, July–October 2010. Euro Surveill 15(45)
Brown CM, Aranas AE, Benenson GA, Brunette G, Cetron M, Chen TH et al (2014) Centers for Disease Control and Prevention (CDC). Airport exit and entry screening for Ebola—August–November 10, 2014. MMWR Morb Mortal Wkly Rep 63(49):1163–1167
Strauss S (2014) Ebola research fueled by bioterrorism threat. CMAJ 186(16):1206
Bray M (2003) Defense against filoviruses used as biological weapons. Antiviral Res 57(1–2):53–60
Cenciarelli O, Gabbarini V, Pietropaoli S, Malizia A, Tamburrini A, Ludovici GM et al (2015) Viral bioterrorism: Learning the lesson of Ebola virus in West Africa 2013–2015. Virus Res 210:318–326
Dvorin, T. (2014). Could ISIS target the West with Ebola? Arutz Sheva. IsraelNational News. http://www.israelnationalnews.com/News/News.aspx/186020#.VDvayfldWSo. Assessed 22 Aug 2016
Unieke videobeelden hoe ebola ontdekt werd. http://www.hln.be/hln/nl/957/Binnenland/article/detail/2096411/2014/10/21/Unieke-videobeelden-hoe-ebola-ontdekt-werd.dhtml. Assessed 5 Sept 2016
Rosello A, Mossoko M, Flasche S, Van Hoek AJ, Mbala P, Camacho A et al (2015) Ebola virus disease in the Democratic Republic of the Congo, 1976–2014. eLife 4:e09015
Osterholm MT, Moore KA, Kelley NS, Brosseau LM, Wong G, Murphy FA et al (2015) Transmission of Ebola viruses: what we know and what we do not know. MBio 6(2):e00137
Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A et al. (2016) Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 30(388):498–503
Howlett P, Brown C, Helderman T, Brooks T, Lisk D, Deen G, Solbrig M, Lado M, Leone S (2016) Ebola virus disease complicated by late-onset encephalitis and polyarthritis. Emerg Infect Dis 22(1):150–152
Jacob H (1971) The neuropathology of the Marburg disease in man. In: Martini GA, Siegert R (eds) Marburg virus disease. Springer, Berlin, pp P54–P61
Fitzgerald GJ (2008) Chemical warfare and medical response during World War I. Am J Public Health 98(4):611–625
Moshiri M, Darchini-Maragheh E, Balali-Mood M (2012) Advances in toxicology and medical treatment of chemical warfare nerve agents. Daru 20(1):81
Weissman BA, Raveh L (2011) Multifunctional drugs as novel antidotes for organophosphates’ poisoning. Toxicology 290(2–3):149–155
Balali-Mood M, Balali-Mood K (2008) Neurotoxic disorders of organophosphorus compounds and their managements. Arch Iran Med 11(1):65–89
Raveh L, Eisenkraft A, Weissman BA (2014) Caramiphen edisylate: an optimal antidote against organophosphate poisoning. Toxicology 325:115–124
Cannard K (2006) The acute treatment of nerve agent exposure. J Neurol Sci 249(1):86–94
Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY (2012) Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc Chem Res 45(5):756–766
Aracava Y, Pereira EF, Akkerman M, Adler M, Albuquerque EX (2009) Effectiveness of donepezil, rivastigmine, and (±)huperzine A in counteracting the acute toxicity of organophosphorus nerve agents: comparison with galantamine. J Pharmacol Exp Ther 331(3):1014–1024
Lavon O, Eisenkraft A, Blanca M, Raveh L, Ramaty E, Krivoy A et al (2015) Is rivastigmine safe as pretreatment against nerve agents poisoning? A pharmacological, physiological and cognitive assessment in healthy young adult volunteers. Neurotoxicology 49:36–44
Marrs TC (2004) The role of diazepam in the treatment of nerve agent poisoning in a civilian population. Toxicol Rev 23(3):145–157
Gilat E, Kadar T, Levy A, Rabinovitz I, Cohen G, Kapon Y et al (2005) Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: an electrographic, behavioral, and histological study in freely moving rats. Toxicol Appl Pharmacol 209(1):74–85
Heffernan R, Mostashari F, Das D, Besculides M, Rodriguez C, Greenko J et al (2004) New York City syndromic surveillance systems. MMWR Suppl 53:23–27
Das D, Weiss D, Mostashari F, Treadwell T, McQuiston J, Hutwagner L et al (2003) Enhanced drop-in syndromic surveillance in New York City following September 11, 2001. J Urban Health 80(2 Suppl 1):i76–i88
Donaghy M (2006) Neurologists and the threat of bioterrorism. J Neurol Sci 249:55–62
Casebeer L, Andolsek K, Abdolrasulnia M, Green J, Weissman N, Pryor E et al (2006) Evaluation of an online bioterrorism continuing medical education course. J Contin Educ Health Prof 26(2):137–144
Cosgrove SE, Perl TM, Song X, Sisson SD (2005) Ability of physicians to diagnose and manage illness due to category A bioterrorism agents. Arch Intern Med 165(17):2002–2006
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors report no disclosure nor conflict of interest relevant to the manuscript. All authors report no financial disclosure.
Ethical approval
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
None.
Rights and permissions
About this article
Cite this article
De Cauwer, H., Somville, F.J.M.P. & Joillet, M. Neurological aspects of chemical and biological terrorism: guidelines for neurologists. Acta Neurol Belg 117, 603–611 (2017). https://doi.org/10.1007/s13760-017-0774-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-017-0774-y