Abstract
In this study, we use for the first time the electropenetrography (EPG) technique to characterize and describe the feeding activities of Collaria scenica (Stål) adults and their respective waveforms on wheat, Triticum aestivum L. plants. It comprised of four waveforms, two related to non-feeding (Np and R) and two with feeding activities (CR and I). The Np wave represents the insect resting or walking on the plant surface (75% of the recording time). R wave was associated to sensory evaluation of leaf surface with the tip of the labium (labial dabbing activity—ca. 1% of the recording time). Feeding activities were of relative short duration; cell rupture waveform (CR—ca. 49 min—ca. 15% of the recording time) was highly irregular due to constant movement of the stylets internally in the plant tissue. The waveform I (ca. 28 min—ca. 9% of the recording time) showed stereotypical and repetitive pattern and represents ingestion of the cell contents previously degraded via cell-rupturing activities. The damage caused by C. scenica in wheat leaflets is a result of cell-rupturing activities, generated by action of digestive enzymes plus mechanical action of serrated mandibular stylets. This results in whitish streaks, dots, or spots on the leaflet of wheat plants which cause significant damage and eventually plants’ death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The true bug Collaria scenica (Stål) is a mirid species found in a wide range of hosts, with a greater preference for graminaceous plants (Poaceae), including those cultivated such as wheat, oat, rice, and barley (Carlessi et al. 1999; Salvadori 2000; Ferreira et al. 2001; Goellner and Floss 2001) and pastures (Ferreira et al. 2001). Collaria scenica has been reported in Argentina, Uruguay, Colombia, Venezuela, and Brazil (Briceño 2007); in Brazil, it has been reported mainly in the South Region since the 1980s (Kalvelage 1988; Schuh 2002–2013). This bug is commonly known as “percevejo-raspador” (rasping bug in English) because its feeding causes damage that resembles scraping of the leaf (Carlessi et al. 1999; Ribeiro et al. 2020). Such damage is described as whitish streaks, dots, or spots that occur in leaflets of different stages of the plant development. It results in the insertion of the mouthparts (stylets) in the longitudinal direction of the leaf veins and later ingestion of the cellular content (Auad et al. 2011).
Collaria scenica occurs throughout the year, but its population increases significantly in dry and cold conditions of winter (Gaitán and Riveros 2002), becoming an important pest of winter plants (Naranjo et al. 2013). In some regions of South America, there are reports of C. scenica and other congeneric species, C. oleosa (Distant), causing significant damage to forage grasses (e.g., Urochloa decumbens Stapf, U. brizantha Stapf cv. marandu, Andropogon gayanus Kunth, Digitaria swazilandensis Stent, Panicum maximum Jacq.), and crop plants, such as rice (Oriza sativa L.), wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), and oats (Avena sativa L. and Avena strigosa Schieb.) (Granja and Triana 1998; Melo et al. 2004; Menezes 1990; Silva et al. 1994; Nogueira et al. 2019).
Despite the relevance of C. scenica, sometimes reaching pest status, little is known about its feeding activities. The knowledge of these activities might reveal relevant information of their behavior and potential to cause damage in the attacked cultivated plants. In addition, this knowledge could be employed to development of control strategies and, consequently, improving management programs, since the main control method of this insect is via the use of insecticides (Naranjo et al. 2013).
The electropenetrography technique (EPG) is the most exact and rigorous method to elucidate the feeding behavior of hemipterans (Backus et al. 2019). In this technique, a low electrical current flow through the electrical circuit was created between the insect and food, which allows generating waveforms that represent different stylet activities into the tissue (Walker 2000). This technique has been used for more than 50 years in studies to determine the feeding activities of several species of piercing-sucking insects, including species of the family Miridae (see Lucini and Panizzi 2018a and references therein). Therefore, herein, we used the EPG to evaluate the feeding behavior of adults of C. scenica while feeding on leaf of wheat seedlings, characterizing the waveforms, their proposed biological meanings, and quantification.
Materials and methods
Insect colony and plants
Adults of C. scenica were field-collected on A. sativa and A. strigosa plants and taken to the laboratory of entomology of the Embrapa Trigo located in Passo Fundo, Rio Grande do Sul state, Brazil (latitude 28°15′46″S, longitude 52°24′24″W) and placed in plastic rearing cages (20 × 20 × 25 cm), lined with filter paper. As food source and oviposition substrate, seedlings of spring cereals were provided: black oat (Avena strigosa Schieb.), wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), and rye (Secale cereale L.). Seedlings were replaced three times/week and the leaf sheaths (preferred oviposition site) were inspected to obtain eggs, which were placed in plastic cages (11 × 11 × 3.5 cm), lined with filter paper plus a plastic lid (2-cm diameter) containing moistened cotton. After hatching, nymphs were fed with pieces of leaves of black oat, wheat, barley, and rye, and replaced three times/week until they reached adulthood. The rearing cages were kept in a walk-in chamber (25 ± 2°C temperature, 65 ± 2% relative humidity and 14 h of photophase).
Seeds of the spring cereals, i.e., black oat cv. ‘BRS Neblina,’ barley cv. ‘BRS Quaranta,’ rye cv. ‘BRS Serrano,’ and wheat cv. ‘BRS Reponte,’ were mixed and seeded weekly in plastic pots (200 mL); these plants were used to keep the bug colony in the laboratory. In addition, wheat plants in plastic pots kept in a greenhouse were used in the tillering (seedling) developmental stage (V3 stage) (Large 1954) for the EPG bioassays.
EPG recordings
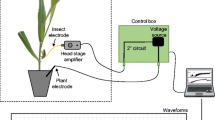
The feeding behavior of adult females of C. scenica on leaflet of wheat seedlings was recorded using a four-channel AC-DC EPG monitor (Backus et al. 2019) which was adjusted to apply an input impedance of 107 Ohms, and 50 mV of alternating current (AC). Waveform rectification and gain (amplitude) were adjusted as necessary. Before wiring, the insects were previously starved for 24 h. After that, insects were separated from colony and placed individually in a test tube; this tube was sunk in a pot containing ice for a few seconds to anesthetize the insects, then they were immobilized using an adhesive tape. The electrode (a piece of gold wire; 3 cm long and 38.1 μm in diameter [Sigma-Aldrich, Saint Louis, MO, USA]) was attached in the insect dorsum using water-based silver glue (white household glue:water:silver flake—1:1:1 [vol:vol:wt]). The wired bug was connected to the EPG probe and the plant electrode (copper wire 3 cm long) was inserted into the soil of the potted plant to close the electrical circuit and start recordings. The feeding activities of C. scenica were recorded continuously for 7 h (9 AM–4 PM) under laboratory conditions with continuous light and temperature of 25 ± 2°C. In total, 15 insects were successfully recorded. The nomenclature, description, and biological meanings of the waveforms recorded for C. scenica were based on EPG studies with plant bugs of the species Lygus spp. (Cervantes et al. 2016). During EPG recordings, we performed visual observations of the insect behavior (e.g., insect walking, resting, stylet movements) and annotated them; this information was later essential to determine the biological meaning of each waveform recorded.
Waveform quantification
EPG waveforms were manually measured using WinDaq Waveform Browser software (DATAQ Instruments, Akron, OH). Four non-sequential EPG variables (Backus et al. 2007) were calculated for each waveform type, as follows: (1) number of waveform events per insect (NWEI); (2) waveform duration per event per insect (WDEI; duration of each individual event of a waveform); (3) waveform duration per insect (WDI; sum of all events of a waveform); and (4) percentage of the recording time (PRT). Herein, the term “event” defines a continuous and uninterrupted occurrence of one waveform type. Descriptive statistics of the EPG variables were performed using the Backus 2.0 program (complete program is available in http://www.crec.ifas.ufl.edu/extension/epg) for Statistical Analysis Software (SAS, Cary, NC).
Results
The overall waveforms structure recorded during feeding activities of C. scenica on wheat seed leaf included both non-feeding and feeding waveforms. Non-feeding included two waveforms visually associated with standing still/walking (Np) and labium tapping on the plant surface (R). Feeding comprised another two waveforms: cell rupturing (CR), and ingestion (I). Electrical characteristics and proposed biological meaning of those waveforms are summarized in Table 1.
Non-feeding waveforms (Np and R)
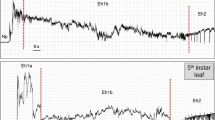
Both waveforms occurred interspersed with each other and represent activities on which the bug was not with the stylets (mouthparts) inserted into the plant tissue. Waveform Np (baseline of the recording) presented either a flat line appearance (observed when insect was standing still) or irregular peaks randomly distributed (when insect was walking) (Fig. 1). Waveform R was characterized by a sudden voltage rise, followed by an irregular and high-amplitude peak, often showing long flat plateaus (Fig. 1).
Feeding waveforms (CR and I)
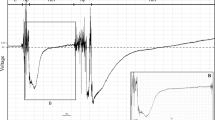
The CR waveform always started with a high-amplitude spike positively (Fig. 2(a–c)) or negatively going oriented (the highest in each feeding event). Following the initial peak, CR presented a highly irregular waveform appearance (i.e., without a clear pattern) (Fig. 2(d)) greatly variable in appearance among insects and even within the same individual insect recorded. The fine structure of CR showed that this waveform is in general composed by sections with almost flat appearance (i.e., very low frequency and amplitude) (Fig. 2(d)), but sometimes some sections with variable frequency (sometimes with high-frequency) and amplitude are also recorded (Fig. 2(c); first CR event). In several cases, multiple CR events were recorded in a short period of time, which was marked by brief stylet withdrawal backing to baseline (Np waveform) followed by immediate stylet penetration (CR waveform). In contrast, the I wave was a stereotypical waveform with a very regular pattern of waves without peaks (Fig. 2(e)) with a fairly frequency of 4 Hz.
Overview of a feeding section recorded from Collaria scenica adults on leaf of wheat seedlings (a). Initial stylet insertion recorded after a Np event (b). Overview of multiple cell-rupturing (CR) events (c). Fine structure of waveform CR (d). Fine structure of sustained ingestion waveform I (e). a has Windaq compression 100 (20s vertical/division), gain 16×; b has compression 2 (0.4 s vertical/div.), gain 8×; C has compression 5 (1 s vertical/div.), gain 16×; d and e have compression 2 (0.4 s vertical/div.), gain 64×
Waveform quantification
Number of waveform events per insect (NWEI), waveform duration per event per insect (WDEI), and waveform duration per insect (WDI; overall) were calculated (Table 2). The waveform Np presented the largest waveform duration of each event and overall per insect among all waveforms recorded, representing 75% of the recording time. CR activity had the second longest overall duration (ca. 49 min), by far, caused by a larger numbers (NWEI) of short-duration (WDEI) events (Table 2). Ingestion waveform (I) presented the third longer overall waveform duration (ca. 28 min), which was caused by a combination of few numbers (NWEI) of intermediate-duration (WDEI) feeding events. Labial dabbing (R) represented the shortest event and overall duration per insect, ca. 1% of the recording time (Table 2).
Discussion
In this study, we used for the first time the EPG technique to characterize and describe the feeding activities C. scenica adults and their respective waveforms. The feeding activities of C. scenica comprised four waveforms, two related to non-feeding (Np and R) and two with feeding activities (CR and I). Waveform Np represents the insect resting or walking on the plant surface. These activities are well known and discussed for several species of hemipterans, including plant bugs (e.g., Cervantes et al. 2016). Waveform R was associated with sensory evaluation where the bug touches the wheat leaf surface with the tip of the labium before inserting its stylets into the plant tissue (named labial dabbing activity). To our knowledge, this sensory activity is reported for the first time for a plant bug species.
The labial behavior has been reported in the pentatomids Nezara viridula L. (Mitchell et al. 2018), and Piezodorus guildinii (Westwood) (Lucini et al. 2016), which was recorded before stylet insertion into the plant tissue. In contrast, for the pentatomid Halyomorpha halys (Stål), Serteyn et al. (2020) described a different tapping activity which they called “epidermal scanning.” This behavior represents the bug dragging its labium on plant surface and probably involving a slight breakage of the epidermal cells to select a penetration site. Thus, R waveform of C. scenica is probably related to sensory evaluation of the tissue before insertion of the stylets. However, our observations cannot ensure whether the bug use the labium only to touch the plant surface or if they slightly break the plant cuticle to perform epidermal scanning while search a suitable place to penetrate their stylets.
The feeding activities of C. scenica on wheat leaflet presented, in general, a very short duration; about one quarter of the recording time is represented by feeding behaviors. This result is similar to the one observed for the plant bug Lygus lineolaris (Palisot de Beauvois) and Lygus hesperus Knight (Cervantes et al. 2016). The CR waveform showed a highly irregular appearance without any resemblance to ingestion waves. This irregularity is result of the constant movement of the stylets internally in the plant tissue. CR waveform from C. scenica strongly resembles the pattern of the analogous waveform CR described for L. lineolaris and L. hesperus, using the same input impedance (Ri level) and applied signal, which encompasses stylet movements and enzymatic salivation into the plant tissue, but with little or no ingestion activity (Cervantes et al. 2016).
Phytophagous mirids do not secrete a salivary sheath as part of their feeding activities; in fact, they are exclusively cell-rupturing feeders, employing the macerate-and-flush tactic (Miles and Taylor 1994; Backus et al. 2005). In this tactic, the cell degradation involves some cell laceration caused by the slow, but continuous, movements of the stylets, and primarily by the secretion of watery saliva highly concentrated in digestive enzymes, i.e., saliva-induced maceration. These activities result in the formation of a slurry of plant cell contents that were ingested afterwards (Backus et al. 2005). Visual observations from C. scenica showed that during CR waveform the bug moved their stylets into the tissue often following the veins of the leaf (longitudinally) (see video recording in Electronic Supplementary Material). Thus, CR waveform of C. scenica probably combines some stylet laceration plus enzymatic salivation to liquefy the plant tissue to create a slurry for ingestion.
(MP4 31990 kb)
In general, multiple cell rupture events (CR wave) were performed lonely (i.e., CR events were followed by periods of walking or resting behaviors) before ingesting the cell contents (waveform I). According to Cervantes et al. (2016), after injecting saliva into the plant tissue during these multiple lone CR events, the bug (Lygus) presumably waits the enzymatic degradation of the cells during non-feeding activity before ingestion. Thus, it is plausible that this similar behavior might be occurring in C. scenica.
In L. lineolaris and L. hesperus, Cervantes et al. (2016) reported for the first time a transition waveform (T wave) in a cell rupture feeder, which was recorded after CR waveform and always preceding the I waveform. The authors suggested that T wave presumably represents the taste/test of the plant tissue previously degraded to obtain the optimum level of cell liquefaction prior to ingestion. However, for C. scenica, a transition waveform was not observed. Perhaps, the electrical characteristics (low Ri level applied [107 Ohms]) applied in this study might have masked the recording of this waveform, since in Lygus spp., the transition wave was much more visible when applied a high Ri level (109 Ohms). Additional studies, applying higher Ri levels, are required to determine whether C. scenica produces a transition waveform.
The waveform I was always recorded after one or multiple CR events. The stereotypical and repetitive pattern of I wave is probably related to the cibarial pumping (Dugravot et al. 2008), and it has strong similarities with sustained ingestion waveforms recorded for several heteropterans species (e.g., Backus et al. 2013; Cervantes et al. 2016; Lucini and Panizzi 2018b). Therefore, we propose that the I waveform of adult C. scenica represents ingestion of the cell contents previously degraded via cell-rupturing activities.
The damage caused by the feeding activities of sucking insects is a sum of many factors that include frequency (number of times) and duration of stylet activities, depth reached by stylets, salivation, saliva composition, and susceptibility of plant tissues to stylet activities (Cooper and Spurgeon 2013). Our results show that the damage caused by C. scenica in wheat leaflets is a result of the cell-rupturing activities, probably generated by the action of digestive enzymes and enhanced by the mechanical action of the mandibular stylets that contain multiple “teeth” at their tip, which break the cells (Fig. 3). In hemipterans, the tip of their mandibular stylets is often barbed, because they are the principal structure used by the insect to penetrate the tissue (Chapman 1998). In addition, the ingestion of the cell contents further evidences the damage to plant tissue. The feeding activities of C. scenica cause whitish streaks, dots, or spots that occur on the leaflet of the plants (Fig. 4), which might cause significant damage and even cause the death of the attacked plants.
In conclusion, this study used for the first time the EPG technique to evaluate and characterize the waveforms related to feeding behavior of adults of C. scenica on leaf of wheat plants. Results showed that bugs use the cell rupture feeding strategy to degrade the plant tissue, via maceration-and-flush tactic (slowly cell laceration plus saliva-induced enzymes), to later ingest the degraded cell contents. The knowledge obtained with this study serves as groundwork for future quantitative studies for this insect, such as evaluating the feeding behavior on different host plants (e.g., different spring cereals), and the effect of tolerant/resistant cultivars and chemical compounds, aiming to improve the management tactics to control this pest.
References
Auad AM, Pimenta DS, Silva DM, Monteiro PH, Resende TT (2011) Collaria oleosa (Hemiptera: Miridae) on Brachiaria ruziziensis and Penissetum purpureum (Poaceae): Characterization of injury and biological aspects. Rev Colomb Entomol 37:244–248
Backus EA, Cervantes FA, Guedes RNC, Li AY, Wayadande AC (2019) AC–DC electropenetrography for in-depth studies of feeding and oviposition behaviors. Ann Entomol Soc Am 112:236–248
Backus EA, Cline AR, Ellerseick MR, Serrano MS (2007) Lygus hesperus (Hemiptera: Miridae) feeding on cotton: new methods and parameters for analysis of nonsequential electrical penetration graph data. Ann Entomol Soc Am 100:296–310
Backus EA, Rangasamy M, Stamm M, McAuslane HJ, Cherry R (2013) Waveform library for chinch bugs (Hemiptera: Heteroptera: Blissidae): characterization of electrical penetration graph waveforms at multiple input impedances. Ann Entomol Soc Am 106:524–539
Backus EA, Serrano MS, Ranger CM (2005) Mechanisms of hopperburn: an overview of insect taxonomy, behavior, and physiology. Annu Rev Entomol 50:125–151
Briceño A (2007) Otra chinche más en el pasto kikuyo del Estado Mérida. Bol Divulg 60:31–32
Carlessi LR, Corseuil E, Salvadori JR (1999) Aspectos biológicos e morfométricos de Collaria scenica (Stal)(Hemiptera: Miridae) em trigo. An Soc Entomol Bras 28:65–73
Cervantes FA, Backus EA, Godfrey L, Akbar W, Clark TL (2016) Characterization of an EPG waveform library for adult Lygus lineolaris and Lygus hesperus (Hemiptera: Miridae) feeding on cotton squares. Ann Entomol Soc Am 109:684–697
Cooper WR, Spurgeon DW (2013) Feeding injury to cotton caused by Lygus hesperus (Hemiptera: Miridae) nymphs and prereproductive adults. Environ Entomol 42:967–972
Chapman RF (1998) The insects: structure and function, 4th edn. Cambridge University Press, Cambridge, p 770
Dugravot S, Backus EA, Reardon BJ, Miller TA (2008) Correlations of cibarial muscle activities of Homalodisca spp. sharpshooters (Hemiptera: Cicadellidae) with EPG ingestion waveform and excretion. J Insect Physiol 54:1467–1147
Ferreira PSF, Silva ER, Coelho LBN (2001) Miridae (Heteroptera) fitófagos e predadores de Minas Gerais, com ênfase em espécies com potencial econômico. Iheringia 91:159–169
Gaitán CAR, Riveros GD (2002) El chinche de los pastos (Collaria scenica): Una amenaza para la producción lechera en la Sabana de Bogotá y valles de Ubaté y Chiquinquirá. Instituto Colombiano Agropecuário, Colombia, p 22
Goellner CI, Floss E (2001) Insetos-pragas da cultura da aveia: Biologia controle e manejo. Universidade de Passo Fundo, Passo Fundo, p 98
Granja EM, Triana NDCB (1998) La chinche de los pastos Collaria scenica Stal. en la Sabana de Bogotá. Bogotá, Colombia, p 66
Kalvelage H (1988) Collaria scenica (Stal, 1859) (Hemiptera: Miridae): Praga de gramíneas forrageiras na Região do Planalto Catarinense, Brasil. An Soc Entomol Bras 17:221–222
Large EC (1954) Growth stages in cereals. Illustration of the Feekes scale. Plant Pathol 3:128–129
Lucini T, Panizzi A (2018a) Electropenetrography (EPG): a breakthrough tool unveiling stink bug (Pentatomidae) feeding on plants. Neotrop Entomol 47:6–18
Lucini T, Panizzi AR (2018b) EPG monitoring of the Neotropical brown-stink bug, Euschistus heros (F.), on soybean pods: an electrical penetration graph-histology analysis. J Insect Sci 18(5):1–14
Lucini T, Panizzi AR, Backus EA (2016) Characterization of an EPG waveform library for redbanded stink bug, Piezodorus guildinii (Hemiptera: Pentatomidae), on soybean plants. Ann Entomol Soc Am 109:198–210
Melo MC, Dellapé PM, Carpintero DL, Coscarón MDC (2004) Reduviidae, Miridae y Lygaeoidea (Hemiptera) recolectados en Colonia Carlos Pellegrini (Esteros de Iberá, Corrientes, Argentina). Rev Soc Entomol Arg 63:59–67
Menezes M (1990) Collaria oleosa (Distant, 1883) (Hemiptera: Miridae), a new pest of forage grasses in southeastern Bahia, Brazil. Agrotrópica 2:113–118
Miles PW, Taylor GS (1994) ‘Osmotic pump’ feeding by coreids. Entomol Exp Appl 73:163–173
Mitchell PL, Cooke SB, Smaniotto LF (2018) Probing behavior of Nezara viridula on soybean: Characterization and comparison of electrical penetration graph (EPG) waveforms on vegetative and reproductive plant structures. J Agric Urban Entomol 34:19–43
Naranjo N, Montero DA, Sáenz A (2013) Control de la chinche de los pastos Collaria scenica (Hemiptera: Miridae) con nematodos entomopatógenos en invernadero. Rev Bras Ciênc Agrár 8:90–94
Nogueira BCF, Coelho LA, Martins DS, Barcellos BD, Sartori SR, Ferreira PSF (2019) Associações de percevejos mirídeos (Hemiptera: Miridae) com plantas no Brasil. Biológico 81:1–30
Ribeiro LK, Tokarski A, Rech C, Lara CA, Nardi C (2020) New record of Microtechnites bractatus (Say) (Hemiptera: Miridae) infesting Crotalaria spp. and injuries of Miridae in cultivated plants in the State of Paraná, Brazil. Rev Bras Entomol 64:e20200027
Salvadori JR (2000) Pragas da cultura da cevada. Embrapa Trigo, Passo Fundo, p 48
Schuh RT (2002–2013) On-line systematic catalog of plant bugs (Insecta: Heteroptera: Miridae). <http://research.amnh.org/pbi/catalog/>, Accessed 24 Nov 2020.
Serteyn L, Ponnet L, Backus EA, Francis F (2020) Characterization of electropenetrography waveforms for the invasive heteropteran pest, Halyomorpha halys, on Vicia faba leaves. Arthropod Plant Interact 14:113–126
Silva DB, Alves RT, Ferreira PSF, Camargo AJA (1994) Collaria oleosa (Distant, 1883) (Heteroptera: Miridae), uma praga potencial na cultura do trigo na região dos cerrados. Pesqui Agropec Bras 29:2007–2012
Walker GP (2000) A beginner’s guide to electronic monitoring of homopteran probing behavior. In: Walker GP, Backus EA (eds) Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Entomological Society of America, Lanham, pp 14–40
Funding
This study was partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant 302293/2017-5 to ARP, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) by a post doctorate scholarship to TL (process number 88887.371769/2019-00). We also thank the Embrapa Trigo, Passo Fundo, RS for the support and for providing facilities.
Author information
Authors and Affiliations
Contributions
CR and TL co-planned, analyzed, and co-wrote the manuscript; ARP co-planned and co-wrote the manuscript; CN co-wrote the manuscript.
Corresponding author
Ethics declarations
Research activity registered at the Sisgen databank under number AA93702. Registered at the Publication Committee of Embrapa Trigo under number 21205.002125/2020-08.
Conflict of interest
The authors declare no competing interests.
Additional information
Edited by Fábio S Nascimento
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Rech, C., Lucini, T., Panizzi, A.R. et al. Feeding Behavior of Collaria scenica (Stål) (Hemiptera: Miridae) on Wheat Plants: an EPG Waveform Characterization. Neotrop Entomol 50, 366–373 (2021). https://doi.org/10.1007/s13744-021-00859-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-021-00859-1