Abstract
Diaphorina citri (Kuwayama) is a global pest of citrus that transmits the bacteria associated with the disease, Huanglongbing. Entomopathogenic fungi and the parasitoid Tamarixia radiata (Waterston) are important biological control agents of this pest and likely to interact in D. citri populations. As a basis for interaction studies, we determined the susceptibility of nymphs and adults of D. citri and adults of the parasitoid T. radiata to six fungal isolates from the species Beauveria bassiana s.l. (Bals.-Criv.) Vuill. (isolates B1 and B3), Metarhizium anisopliae s.s. (Metsch.) (Ma129 and Ma65) and Isaria fumosorosea Wize (I2 and Pae). We conducted experiments evaluating infection levels in all three insect groups following inoculation with a series of conidial concentrations (1 × 104–1 × 108 conidia mL−1). Results showed that D. citri nymphs and T. radiata were more susceptible to fungal isolates than D. citri adults. Overall, B. bassiana and M. anisopliae isolates caused the greatest infection compared with I. fumosorosea isolates in all three groups of insects. Isolates B1 (B. bassiana) and Ma129 (M. anisopliae) infected a greater proportion of adults and nymphs of D. citri, respectively. Both isolates of B. bassiana caused greater infection in T. radiata compared with isolates of the other fungal species. We propose that isolates B1 and Ma129 are the strongest candidates for control of D. citri. Our results represent the first report of entomopathogenic fungi infecting T. radiata, and the basis for future studies to design a biological control programme that uses both agents more efficiently against D. citri populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The citrus industry has been severely affected by Huanglongbing (HLB), a disease associated with the bacteria Candidatus Liberibacter spp. This disease is transmitted by the psyllid Diaphorina citri (Kuwayama) (Bové 2006). In Mexico, HLB was first detected in 2009 in the State of Yucatan and currently D. citri populations have been reported in all citrus production regions in Mexico (Trujillo-Arriaga 2010). Although the use of chemical pesticides is the most common control strategy, biological control has also been attempted in Mexico using the eulophid parasitoid Tamarixia radiata (Waterston), the entomopathogenic fungi Isaria fumosorosea Wize and Metarhizium anisopliae (Metsch.) Sorokin (Trujillo-Arriaga 2010, Lezama-Gutiérrez et al 2012).
Entomopathogenic fungi have attributes that make them strong candidates for biological control of this pest. For example, the high infection levels achieved in D. citri (Orduño-Cruz et al 2015a) and the relative ease of mass production, formulation and field release (Moore 1993, Butt et al 2001; Shah & Pell 2003). The susceptibility of D. citri adults to infection by Beauveria bassiana (Bals.-Criv.) Vuill, M. anisopliae, I. fumosorosea and Hirsutella citriformis Kuwayama has been previously demonstrated under laboratory and greenhouse conditions (Avery et al 2009, 2011, Hall et al 2012, Stauderman et al 2012, Gandarilla-Pacheco et al 2013a, Orduño-Cruz et al 2015a). However, once these isolates are released in the field, they are likely to interact with the parasitoid T. radiata. During the selection and development of fungal isolates for use in a biological control programme, any effects on non-target organisms, such as parasitoids and predators, are amongst the most important aspects to consider (Posada & Vega 2005, Lacey et al 2015).
Recently, Orduño-Cruz et al (2015a, b) described extensive in vivo and in vitro experiments to select fungal isolates for biological control of D. citri; as a result, six isolates with the greatest potential for the microbial control of this pest were selected and used in our study. We believe that studying the susceptibility of D. citri and T. radiata to selected fungal isolates (Orduño-Cruz et al 2015a, b) is an important first step in forming a foundation for future studies that would allow us to understand the potential outcomes of the interaction between these two natural enemies in D. citri populations. To achieve this, a series of experiments was done to study the susceptibility of T. radiata, and adults and nymphs of D. citri to the six fungal isolates selected, two from each of the species B. bassiana, M. anisopliae and I. fumosorosea.
Material and Methods
Fungal isolates and insect colonies
Six fungal isolates were used (Table 1), all of which have been deposited in the Culture Collection of the Insect Pathology Laboratory, Colegio de Postgraduados, Campus Montecillo, Texcoco, Mexico.
For all experiments nymphs and adults of D. citri and adults of T. radiata were provided by the Centro Nacional de Referencia en Control Biológico (CNRCB), SENASICA, Tecoman, Colima, Mexico. Nymphs of D. citri were used to maintain the T. radiata colony. Both colonies were maintained under greenhouse conditions at the CNRCB facilities with 27 ± 2°C, at 60–80% RH.
The D. citri colony was maintained on one-year-old Murraya paniculata (L.) Jack plants inside steel cages (70 × 70 × 70 cm) covered with insect proof mesh. Plants containing mostly 4th instar nymphs were selected; from these, leaflets bearing large numbers of nymphs were removed, placed in an icebox and transported to the laboratory, where groups of 15 nymphs were separated using a fine camel hair brush under the stereomicroscope and placed in the experimental units (60 mm diameter Petri dishes). For D. citri adults, insects (~7 d old) were captured from the cages using a mouth aspirator and placed, in groups of 15, inside the glass vials (27 mm diameter and 50 mm height) with 1 mm orifices in the lid to allow ventilation. Vials were then placed in an icebox and transported to the laboratory.
Adult parasitoids were collected from M. paniculata plants containing parasitized 4th and 5th instar nymphs (parasitoids in the pre-pupal stage). Leaflets were removed and placed in an icebox and transported to the laboratory where they were incubated in darkness at 27°C at 60% RH until adult parasitoid emergence. Parasitoids were collected using a mouth aspirator and deposited in glass vials, in groups of 15, as described above and kept in an icebox until needed.
Production of fungal conidial suspensions
Monosporic versions of each isolate were used throughout, and each isolate was not sub-cultured more than three times before retrieving a new vial from −80°C storage. Each isolate was cultured on Sabouraud dextrose agar (SDA) in 90 mm diameter Petri dishes. Inoculated SDA dishes were incubated at 25°C in complete darkness for 20 days until sporulation was abundant. Under sterile laminar flow conditions, conidia from each isolate were harvested using a sterile steel spatula to scrape them into 30 mL glass tubes with 20 mL of 0.03% Tween 80 solution. Conidial suspensions of each isolate were vortexed for 5 min and then filtered using sterile muslin cloths into new 30 mL glass tubes. From each stock suspension, 10 μL were taken, suspended in 990 μL of 0.03% Tween 80 solution and used to estimate conidial concentration of the stock suspension. Materials from the stock suspensions were then serially diluted to produce a range of five different conidial concentrations: 1 × 104, 1 × 105, 1 × 106, 1 × 107 and 1 × 108 conidia mL−1. All conidial suspensions were maintained at 4°C for no longer than 24 h prior to use. Germination in conidial suspensions was assessed prior to experiments, and was always above 95%.
Experimental design for assays of fungi against D. citri and T. radiata
All insects were inoculated in groups of 15 using a spray tower (glass cylinder of 51 cm diameter and 20 cm height) fitted with a cone spray nozzle (Spraying Systems Co. Wheaton, IL, USA) attached to an air compressor at 20 psi. Nymphs of D. citri, adults of D. citri and adults of T. radiata were assayed separately, but all isolates were applied against each target on the same day.
For each isolate, groups of 4th instar nymphs of D. citri were placed on filter paper located in the base of 60 mm diameter Petri dishes. Six different dishes were treated, each with either a different conidial concentration (five) or just the carrier, 0.03% Tween 80, as a control, for a total of 36 Petri dishes for the six isolates in total. For each conidial concentration, 1.2 mL of the suspension (or 0.03% Tween 80 for the control) was sprayed. For each isolate, the control treatment was sprayed first followed by the lowest concentration and so on ending with the highest concentration. Between each concentration of the same isolate, the nozzle and the tower were cleaned by spraying 5 mL of 70% ethanol, followed by two washes of 5 mL of sterile distilled water. Between isolates, 5 mL of 5% sodium hypochlorite were sprayed between the ethanol and the distilled water. Before each spraying, any liquid within the tower was removed using clean paper towels.
After treatment, nymphs were transferred to leaflets of M. paniculata using a fine camel hair brush; nymphs from each replicate group were placed on a different leaflet. The petiole of the leaflet was inserted into a 1.5 mL Eppendorf tube containing sterile tap water to ensure the leaflet remained turgid during incubation. Each leaflet bearing nymphs was placed individually inside a 200 mL cylindrical plastic container. On one side of the container, a 3 cm diameter hole was made and covered with insect-proof mesh to allow ventilation. On the other side of the container, a 1.3 cm diameter hole was made. The 1.5 mL Eppendorf tube containing the M. paniculata leaflet was inserted through this hole, leaving the leaflet inside the plastic container in a horizontal position, and the tip of the Eppendorf tube outside the plastic container. A paper towel moistened with 0.5 mL of sterile distilled water was placed inside the plastic container to maintain a relative humidity of approximately 60% within. All plastic containers were incubated at 25°C in a light regime of 12:12 for 7 days. Each day, D. citri mortality was recorded. Dead nymphs were incubated on sterile moistened filter paper in 60 mm diameter Petri dishes; any observed sporulation confirmed fungal infection as the cause of mortality.
Experiments with adult D. citri and adults of the parasitoid T. radiata were conducted the same as the nymph assays described above with the following exceptions. Prior to inoculation, all groups of adult D. citri and T. radiata were exposed to 2°C for 5 min to reduce their mobility during spraying. Inoculated adults were transferred to clean leaflets of M. paniculata as described for nymphs but the leaflets were transferred to a different type of transparent cylindrical plastic containers (5 cm diameter × 3 cm high) that had a hermetically sealed lid. The container had a 3 cm diameter hole covered with inset-proof mesh to allow ventilation. A paper towel moistened with 5 mL of sterile distilled water was placed in the base of each container to produce a relative humidity of approximately 60%. All plastic containers were incubated at 25°C in a 12:12 light regime for 7 days for T. radiata and 15 days for D. citri adults. Confirmation of fungal infection was made in the same way as described for D. citri nymphs above. Tamarixia radiata adults were provided with a drop of honey every day as food.
All experiments with the three group of insects were conducted using a completely randomized design where all treatments were made on the same day and the complete experiment was replicated on three separate occasions. For each group of insects and occasion (replicate), 36 Petri dishes were used representing six Petri dishes (five conidial concentrations and a control) for each of the six isolates. Therefore, 108 Petri dishes were used for each group of insects, for a total of 324 Petri dishes for the complete experiment involving all three groups of insects.
Statistical analyses
By testing serially increasing conidial concentrations of each isolate, we hoped to be able to determine the lethal median concentration (LC50) for each isolate and insect group. It has been reported that for an accurate LC50 estimation, the range of doses tested should produce a response between 25 and 75% in the target insect (Inglis et al 2012); however, some of the isolates we tested did not cause more than 50% infection at the highest conidial concentrations. Therefore, we analysed our data using a different approach and it is described below. Statistical analyses for the two developmental stages of D. citri and the parasitoid were similar. Dead insects in all fungal treatments showed sporulation, therefore, data analysed represents number of infected insects. For the analysis, infection values representing the final cumulative data at the end of the incubation time were used. Data were analysed using logistic regression, assuming a binomial distribution, where infection represented the proportion of the total number of insects tested. The main effects and interactions for the factorial set of treatments defined by fungal species (combining the two isolates per species) and conidial concentration (combining all isolates) were estimated and tested. Nested contrasts were incorporated to compare between isolates within each fungal species and their interactions with conidial concentration was assessed. In each experiment (replicate), a control treatment was included for each isolate tested. Total control mortalities were 8.8, 4.4 and 12.5% for D. citri nymphs, adults and T. radiata, respectively. None of the dead control insects sporulated confirming no cross contamination between fungal and control treatments had occurred; therefore, control data were excluded from the analyses. All the statistical analyses were done using the statistical package GenStat v. 8.0 (Payne et al 2005). For the three groups of insects, data from the three repetitions (occasions) were compared and no significant differences were found (P > 0.05), which allowed these data for treatment effect comparisons to be pooled.
Results
Susceptibility of D. citri and T. radiata to entomopathogenic fungi
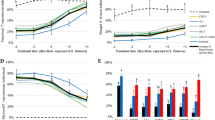
When data from the two isolates and conidia concentrations for each fungal species were combined for comparison, no significant differences amongst them were found for D. citri nymphs (F2, 58 = 2.71, P = 0.075), but significant differences were found for D. citri adults (F2, 58 = 8.21, P < 0.001) and T. radiata adults (F2, 58 = 7.69, P = 0.001). Overall, the greatest proportion becoming infected was achieved by B. bassiana and M. anisopliae isolates followed by the I. fumosorosea isolates for D. citri nymphs (Fig 1A), adults (Fig 2A) and T. radiata adults (Fig 3A). Significant differences were found amongst the five conidial concentrations for D. citri nymphs (F4, 58 = 8.70, P < 0.001), D. citri adults (F4, 58 = 10.18, P < 0.001) and T. radiata adults (F4, 58 = 3.23, P = 0.018). The proportion of insects infected increased with the increase in conidial concentration and, although this trend was consistent for all fungal isolates when tested in D. citri nymphs (F8, 58 = 1.63, P = 0.138, Fig 1A) and T. radiata adults (F8, 58 = 1.20, P = 0.315, Fig 3A), it was not for D. citri adults, specifically for the I. fumosorosea isolates (Fig 2A), resulting in a significant interaction between conidial concentration and fungal species (F8, 58 = 2.31, P = 0.032).
Proportion of Diaphorina citri nymphs becoming infected following inoculation with five different conidial concentrations of isolates from three species of entomopathogenic fungi: A, two isolates of Beauveria bassiana B1 and B3; B, two isolates of Metarhizium anisopliae Ma65 and Ma129 and; C two isolates of Isaria fumosorosea I2 and Pae D. Error bars represent 95% confidence intervals back-transformed from the logistic scale.
Proportion of Diaphorina citri adults becoming infected following inoculation with five different conidial concentrations of isolates from three species of entomopathogenic fungi: A, two isolates of Beauveria bassiana B1 and B3; B, two isolates of Metarhizium anisopliae Ma65 and Ma129 and; C two isolates of Isaria fumosorosea I2 and Pae D. Error bars represent 95% confidence intervals back-transformed from the logistic scale.
Proportion of Tamarixia radiata adults becoming infected following inoculation with five different conidial concentrations of isolates from three species of entomopathogenic fungi fungi: A, two isolates of Beauveria bassiana B1 and B3; B, two isolates of Metarhizium anisopliae Ma65 and Ma129 and; C two isolates of Isaria fumosorosea I2 and Pae D. Error bars represent 95% confidence intervals back-transformed from the logistic scale.
When a comparison between isolates for each fungal species was made for D. citri nymphs, no significant differences were found between the B. bassiana isolates (F1, 58 = 2.51, P = 0.119) (Fig 1B) or between the I. fumosorosea isolates (F1, 58 = 0.31, P = 0.578) (Fig 1D), but significant differences were found between the two M. anisopliae isolates (F1, 58 = 6.06, P = 0.017). The M. anisopliae isolate Ma129, consistently caused greater infection in nymphs compared with isolate Ma65 at all conidial concentrations except at the 1 × 107 conidia mL−1, in which Ma65 achieved 100% infection (Fig 1C). For D. citri adults, significant differences were found between the B. bassiana isolates (F1, 58 = 7.40, P = 0.009). Isolate B1, consistently caused greater infection compared with isolate B3 at all concentrations except the lowest (Fig 2B). As a result, a significant interaction between isolate and conidial concentration was obtained (F4, 58 = 3.90, P = 0.007). The M. anisopliae isolate Ma129, consistently caused greater infection in adults compared with isolate Ma65 (F1, 58 = 5.64, P = 0.021) (Fig 2C), and this was regardless of the conidial concentration (F4, 58 = 2.02, P = 0.104). No differences were found between the I. fumosorosea isolates (F1, 58 = 0.41, P = 0.522), nor an interaction with conidial concentration (F4, 58 = 1.36, P = 0.260). The proportion becoming infected was never greater than 0.3 (aprox. 30%) for the two I. fumosoresa isolates at all conidial concentrations (Fig 2D). For T. radiata adults, no differences were found between B. bassiana (F1, 58 = 0.03, P = 0.866) (Fig 3B), M. anisopliae (F1, 58 = 1.10, P = 0.298) (Fig 3C) or I. fumosorosea isolates (F1, 58 = 2.33, P = 0.133) (Fig 3D). The M. anisopliae and I. fumosorosea isolates never achieved infection levels above 0.6 (aprox. 60%) at any conidial concentration (Fig 2B and C). Only B. bassiana caused greater proportions of infection, especially at the highest conidial concentrations (1 × 107 and 1 × 108 conidia mL−1), in which the proportions of infection ranged between 0.7 and 0.9 (Fig 3B).
Discussion
The three groups of insects we tested here were susceptible to all fungal isolates. Although there are previous reports of fungi infecting adults (e.g. Stauderman et al 2012, Orduño-Cruz et al 2015a) and nymphs of D. citri (Ferreira Pinto et al 2012, Lezama-Gutiérrez et al 2012, Gandarilla-Pacheco et al 2013b), our results represent the first report of entompathogenic fungi infecting the parasitoid T. radiata. Nymphs of D. citri were the most susceptible to fungal infection, followed by T. radiata adults and D. citri adults as the least susceptible. Infection levels in D. citri nymphs were similar amongst all three species of fungi and amongst isolates within each species; however, only the B. bassiana and M. anisopliae isolates achieved 100% infection rates at the highest conidia concentration compared with I. fumosorosea which, at the highest conidia concentration, caused only 70% infection. Our results showed that both developmental stages of D. citri were less susceptible to I. fumosorosea isolates than seen by Stauderman et al (2012), who reported 100% mortality rates in D. citri adults. These authors exposed adults of D. citri to conidia-contaminated grapefruit leaves for 12 days, which means that the insects were continually exposed to the fungi, substantially increasing the number of conidia attached to the insects compared with our study; we exposed the insects to a direct spray of 1.2 mL of conidial suspension and then transferred inoculated insects to clean arenas where they would not acquire additional fungal propagules during incubation. The effect of the method of application can also be observed in the results reported by Orduño-Cruz et al (2015a), where 0.3 μL of a 1 × 108 conidia mL−1 of isolates B1 (B. bassiana) and Ma65 (M. anisopliae) were deposited on D. citri adults, resulting in 85 and 97% infection, respectively. With our method, 75 and 42% infection in D. citri adults was achieved for isolates B1 and Ma65, respectively, at 1 × 108 conidia mL−1. It is likely that by using a spraying technique for fungal delivery, a different number of conidia reached the insect compared to a topical method (Orduño-Cruz et al 2015a). Quantifying the number of conidia per cm2 would have provided some insights into these differences. Unfortunately, we have been unable to obtain these values. While performing preliminary experiments to define the method for the formal experiments, we attempted to quantify the number of conidia per cm2 by placing a 1-cm diameter 2% water-agar plug on a glass slide adjacent to the targets during spraying. However, we could only determine an increase in the number of conidia in relation to concentrations above 1 × 106 conidia mL−1; no conidia could be quantified at 1 × 104 and 1 × 105 conidia mL−1. The fact that we obtained infection at these lower concentrations indicates that conidia were being delivered, even though they were not quantifiable on the agar. We also tested larger volumes of conidial suspensions to increase the likelihood of recovery of conidia at the lowest concentrations, but control mortality of parasitoids then exceeded 50% (data not shown). While new methods to quantify conidial deposition would be helpful in interpretation, our current results are still valid.
Isolate B1 caused greater infection in D. citri adults compared with the other isolates. Interestingly, this isolate was originally isolated from an infected D. citri adult (Orduño-Cruz et al 2015b), which is in line with some reports suggesting that entomopathogenic fungi may be more virulent when applied against individuals of the same host species from which they were originally isolated (Goettel 1994, Cabanillas & Jones 2009). However, when tested against nymphs, it did not cause the greatest infection levels, suggesting that virulence could not only be dependent on the host species but also the developmental stage. Although this confirms previous reports (Petersen-Silva et al 2015), our results need further confirmation by evaluation of a greater number of isolates and hosts.
From a practical point of view, our data and previously reported data (e.g. Avery et al 2011, Stauderman et al 2012, Gandarilla-Pacheco et al 2013a, Cortez-Madrigal et al 2014) show that entomopathogenic fungi have great potential for biological control of D. citri. Control efforts with fungi should focus on nymphs, which are more susceptible to fungal infection than adults. Furthermore, nymphs lack mobility and are gregarious on young leaflets making them a better target for biological control (Fernández & Miranda, 2005). However, we cannot ignore the importance of adult D. citri which, being a very active stage, are more effective at disseminating Candidatus Liberibacter spp., the bacteria associated with the disease known as HLB. Therefore, using isolates with the potential to infect both developmental stages is also very important. Recently, Orduño-Cruz et al (2016) using some of the isolates studied here (Ma65 and B1) reported that D. citri adults carrying the bacterium Candidatus Liberibacter asiaticus were more susceptible to fungal infection compared to bacterium-free D. citri, representing a possibility to achieve greater mortalities in D. citri adults vectoring the bacterium and potentially reducing the possibility of HLB transmission.
The ability to infect the target pest insect is just one biological attribute of entomopathogenic fungi that requires evaluation before field release. One of the most important attributes to be considered is their impact on non-target organisms (Khan et al 2012). In this respect, the parasitoid T. radiata is an important biological control agent of D. citri (Chen & Stansly 2014) and it is very likely that both organisms (fungus and parasitoid) will interact with each other once released in the field. Therefore, it is important to study the potential outcomes of these interactions in order to use them both efficiently to reduce D. citri populations in the field. As a first step to achieving this, we studied the relative susceptibility of T. radiata and D. citri to the same fungal isolates. Our results showed that all isolates infected the parasitoids and that the B. bassiana isolates caused the greatest infection levels followed by the M. anisopliae and I. fumosorosea (Fig 3). Although it cannot be confirmed statistically, our results suggest that the parasitoids were less susceptible than D. citri nymphs but more susceptible than D. citri adults. However, the infection levels obtained after insects had been inoculated in the laboratory (physiological hosts) are not necessarily reproducible in the field (ecological hosts) (Vega et al 2012). Therefore, great caution should be taken before extrapolating laboratory results to the field environment (Hajek & Goettel 2007). More accurate information about the susceptibility of non-target organisms would be obtained by performing semi-field and field experiments as well as laboratory experiments (Hajek & Goettel 2007).
Despite the fact that all fungal isolates infected D. citri and its parasitoid, which may cause a conflict when selecting isolates for field applications, we consider that the combined use of both biological control agents could still be possible. As Goettel (1994) states, although some insects can be infected in the laboratory, the lack of reports of natural infection in the field suggests that field infection is unlikely. We are currently assessing these interactions between the two biological control agents in more detail within D. citri populations using isolates Ma129 and B1.
In summary, our data suggest that the six fungal isolates tested here could infect both D. citri and T. radiata. We propose isolates Ma129 and B1 as strong candidates for the control of D. citri. However, their use in combination with T. radiata although potentially possible, still requires further research to design a biological control programme that makes the most of both agents. For example, studies on the effect of a time separation between releases, on the order of inoculation and on the ability of the parasitoid to discriminate between infected and healthy hosts.
References
Avery PB, Hunter WB, Hall DG, Jackson MA, Powell CA, Rogers ME (2009) Diaphorina citri (Hemiptera: Psyllidae) infection and dissemination of the entomopathogenic fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. Fla Entomol 92:608–618
Avery PB, Wekesa VW, Hunter WB, Hall DG, McKensie CL, Osborne LS, Powell CA, Rogers ME (2011) Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocon Sci Technol 21:1065–1078
Bové JM (2006) Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88:7–37
Butt TM, Jackson CW, Magan N (2001) Introduction—fungal biological control agents: progress, problems, potential. In: Butt TM, Jackson CW, Magan N (eds) Fungi as biocontrol agents: progress, Problems and Potential. CAB International, Wallingford, pp 1–8
Cabanillas HE, Jones WA (2009) Pathogenicity of Isaria sp. (Hypocreales: Clavicipitaceae) against the sweet potato whitefly B biotype, Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot 28:333–337
Chen X, Stansly PA (2014) Biology of Tamarixia radiata (hymenoptera: Eulophidae), parasitoid of the citrus greening disease vector Diaphorina citri (Hemiptera: Psylloidea): a mini review. Fla Entomol 97:1404–1413
Cortez-Madrigal H, Sánchez-Saavedra JM, Díaz-Godínez G, Mora-Aguilera G (2014) Enzymatic activity and pathogenicity of entomopathogenic fungi from Central and Southeastern Mexico to Diaphorina citri (Hemiptera: Psyllidae). Southwest Entomol 39:491–502
Fernández M, Miranda I (2005) Comportamiento de Diaphorina citri Kuwayama (Hemiptera:Psyllidae) Parte I: Características morfológicas, incidencia y enemigos naturales asociados. Rev Prot Veg 2:27–31
Ferreira Pinto AP, Batista Filho A, Marcondes de Almeida JE, Marchizeli Wenzel I (2012) Beauveria bassiana pathogenicity to Diaphorina citri and compatibility of the fungus with phytosanitary products. Pesq Agrop Brasileira 47:1673–1680
Gandarilla-Pacheco FL, Galán-Wong LJ, López-Arroyo JI, Rodríguez-Guerra R, Quintero-Zapata I (2013a) Optimization of pathogenicity tests for selection of native isolates of entomopathogenic fungi isolated from citrus-growing areas of México on adults of Diaphorina ciri Kuwayama (Hemiptera: Liviidae). Fla Entomol 96:187–195
Gandarilla-Pacheco FL, López-Arroyo JI, Galán-Wong LJ, Quintero-Zapata I (2013b) Patogenicity of native entomopathogenic fungi from the Mexican citrus-growing area against Diaphorina citri Kuwayama (Hemiptera: Liviidae). Southwest Entomol 38:325–338
Goettel MS (1994) Host range and specificity in relation to safety of exotic fungi. In “VI th International Colloquium on Invertebrate Pathology and Microbial Control, XXVII th Annual Meeting of the Society for Invertebrate Pathology, Montpellier, France 28 August–2 September, 1994,” pp. 325–329
Hajek AE, Goettel MS (2007) Guidelines for evaluating effects of entomopathogens on non-target organisms. In: Lacey LA, Kaya HK (eds) Field manual of techniques in invertebrate pathology. Springer, Dordrecht, pp 815–833
Hall DG, Hentz MG, Meyer JM, Kriss AB, Gottwald TR, Boucias DG (2012) Observations on the entomopathogenic fungus Hirsutella citriformis attacking adult Diaphorina citri (Hemiptera: Psyllidae) in managed citrus grove. BioControl 57:663–675
Ibarra-Cortés KH, Guzmán-Franco AW, González-Hernández H, Suarez-Espinosa J, Baverstock J (2013) Selection of a fungal isolate for the control of the pink hibiscus mealybug Maconellicoccus hirsutus. Pest Manag Sci 69:874–882
Inglis GD, Enkerli J, Goettel MS (2012) Laboratory techniques used for entomopathogenic fungi: Hypocreales. In: Lacey LA (ed) Manual of techniques in invertebrate pathology, Second edn. Academic Press Inc., San Diego, pp 189–253
Khan S, Guo L, Maimaiti Y, Mijit M, Qiu D (2012) Entomopathogenic fungi as microbial biocontrol agent. Mol Plant Breed 3:63–79
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41
Lezama-Gutiérrez R, Molina-Ochoa J, Chávez-Flores O, Ángel-Sahagún CA, Skoda SR, Reyes-Martínez G, Barba-Reynoso M, Rebolledo-Domínguez O, Ruíz-Aguilar GML, Foster JE (2012) Use of the entomopathogenic fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to control Diaphorina citri (Hemiptera: Psyllidae) in Persian lime under field conditions. Int J Trop Insect Sci 32:39–44
Moore DPC (1993) The potential of mycoinsecticides. Biocontrol News Inf 14:31–40
Orduño-Cruz N, Guzmán-Franco AW, Rodríguez-Leyva E, Alatorre-Rosas R, González-Hernández H, Mora-Aguilera G (2015a) In vivo selection of entomopathogenic fungal isolates for control of Diaphorina citri (Hemiptera: Liviidae). Biol Control 90:1–5
Orduño-Cruz N, Guzmán-Franco AW, Rodríguez-Leyva E, Alatorre-Rosas R, González-Hernández H, Mora-Aguilera G, Rodriguez-Maciel JC (2015b) In vitro selection of a fungal pathogen for use against Diaphorina citri. Biol Control 90:6–15
Orduño-Cruz N, Guzmán-Franco AW, Rodríguez-Leyva E (2016) Diaphorina citri populations carrying the bacterial plant pathogen, Candidatus Liberibacter asiaticus, are more susceptible to infection by entomopathogenic fungi than bacteria-free populations. Agric For Entomol 18:95–98
Payne RW, Murray DM, Harding SA, Baird DB, Soutar DM (2005) GenStat for windows (8th edition) introduction. VSN international, Hemel Hempstead
Petersen-Silva R, Inácio L, Henriques J, Naves P, Sousa E, Pugade-Villara J (2015) Susceptibility of larvae and adults of Monochamus galloprovincialis to entomopathogenic fungi under controlled conditions. Int J Pest Manag 61:106–112
Posada FJ, Vega FE (2005) A new method to evaluate the biocontrol potential of single spore isolates of fungal entomopathogens. J Insect Sci 5:37, 10 pp
Shah PA, Pell JK (2003) Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol 61:413–423
Stauderman K, Avery P, Aristizábal L, Arthurs S (2012) Evaluation of I. fumosorosea (Hypocreales: Cordycipitaceae) for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci Tech 22:747–761
Trujillo-Arriaga J (2010) Situación actual, regulación y manejo del HLB en México. Memorias del Segundo Taller Internacional del HLB, Mérida
Vega FE, Meyling NV, Luangsa-ard JJ, Blackell M (2012) Fungal Entomopathogens. In: Vega FE, Kaya HK (eds) Inset pathology, Second edn. Academic Press Inc., San Diego, pp 171–220
Acknowledgements
KHIC received a scholarship from Consejo Nacional de Ciencia y Tecnología (CONACYT) Mexico for her PhD. This research was partially supported by the Fidecomiso 2013 - Colegio de Postgraduados. We are grateful to Cesar Hugo Arredondo Bernal MSc. and personnel at the Centro Nacional de Referencia de Control Biologico for providing the insects. We also thank Dr. Roberto Lezama-Gutierrez for allowing us to use the incubators in his laboratory for the experiments reported here.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Marcos R Faria – Embrapa
Rights and permissions
About this article
Cite this article
Ibarra-Cortés, K.H., Guzmán-Franco, A.W., González-Hernández, H. et al. Susceptibility of Diaphorina citri (Hemiptera: Liviidae) and Its Parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) to Entomopathogenic Fungi under Laboratory Conditions. Neotrop Entomol 47, 131–138 (2018). https://doi.org/10.1007/s13744-017-0539-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0539-6