Abstract
Panonychus citri (citrus red mite) is a devastating pest of citrus orchards. The conventional chemical acaricides have been strongly forbidden for the management of agricultural insect pests in China. Therefore, we evaluated the susceptibility of adult and nymphs P. citri in laboratory against eight isolates of four fungal species, Akanthomyces lecanii, Metarhizium anisopliae, Beauveria bassiana and Aschersonia aleyrodis. Each citrus seedling having 40 adults (2-d-old) and nymphs (on separate plants) were sprayed with isolates at the concentration of 104 ~ 108 conidia mLˉ1 whereas controlled seedlings were sprayed with 0.02% Tween-80. After 9 days of fungal exposure, the four fungal isolates caused more than 50% mortality of mites, such as; 85.6%, 87.9%, 64.6% and 79.7% by A. lecanii (V3450), B. bassiana (BFZ0409), M. anisopliae (MFZ0706) and A. aleyrodis (AsG0910), respectively. The nymphal mites were less susceptible to applied fungi compared to adults. The LC50s of the tested isolates were determined by the fitted time-concentration-mortality relationships, which declined over days after spray. LT50s were decreased with a high concentration of isolates. After the 9-d inoculation, two isolates of B. bassiana (BFZ0409 and D1344) and one isolate of A. lecanii (V3450) were highly effective at the minimal dose of LC50 of 104 conidia mLˉ1 and are promising candidates to control mites, as compared to other tested fungal isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mites belonging to Arachnida class (Ruppert et al. 2004) consist of four stages, i.e. egg, larva, nymph and adult. Nymphs (protonymph, deutonymph, and tritonymph) and adult are most feeding and damaging stages. There are more than 50,000 species of mites, which are predatory, parasitic, saprophagous, herbivores, necrophagous, fungivores, coprophagous as well as phoretic species (Dhooria 2016).
Mites that attack citrus plantations worldwide include Panonychus citri McGregor, Eotetranychus kankitus Ehara, Polyphagotarsonemus latus Banks, Tetranychus kanzawai Kishida, Phyllocoptruta oleivora Ashmead, as well as Brevipalpus yothersi Baker (Al-Azzazy 2016; Vechia et al. 2018; Zhou et al. 1999). Among them, Panonychus citri (citrus red mite) (CRM) is more harmful to fresh citrus shoots (Haiyuan 1996; Li 1990). Adult and nymph nurture and survive on the delicate plant leaves through sucking (Kranz et al. 1977), and develop lighter grey spots on leaves, that may hinder the process of photosynthesis (Kennett et al. 1999). High infestations cause early leaf dropping as well as shoot dieback, and weaken the plant vigor, along with feeding and damaging the fresh fruits (Jamieson et al. 2005; Kranz et al. 1977), and destroys the citrus plantation in China (Li et al. 1990; Yang et al. 2009).
A considerable number of acaricides have been used to control mites in China. Therefore, mite pests―including P. citri―become resistant to various insecticides due to long-term usage (Gerson and Cohen 1989; Meng et al. 2000). It is easy for CRM to become resistant to insecticides, and since the 1970s, citrus mites have become resistant to organophosphates and organochlorines, e.g. dipterex, dimethoate and chlorodifon (Gerson and Cohen 1989). Likewise, P. citri has developed resistance to pyridaben, abamectin and dicofol up to 11.2, 13.4 and 23 fold, respectively, in China (Meng et al. 2000). Therefore, conventional chemical acaricides have been strongly forbidden for the management of agricultural insect pests in China (Yu 2001). However, many other alternative control measures are being considered against mites in the world (Idrees et al. 2016). Predators and entomopathogens are being used as biological agents to control mites on broad-spectrum (Chandler et al. 2000; Jamieson et al. 2005; Paz et al. 2007; Poinar Jr and Poinar 1998; Shi and Feng 2004; Van der Geest et al. 2000). Therefore, entomopathogenic fungi are being applied as biopesticides for the management of mites (Chandler et al. 2000).
Entomopathogenic hyphomycetes infecting citrus mites have been described, especially on citrus plantation such as Beauveria bassiana, Akanthomyces (Lecanicillium) lecanii (Kepler et al. 2017), Metarhizium anisopliae and Cordyceps (Isaria) fumosorosea (Kepler et al. 2017) are well known microbial agents (Martins et al. 2016; Roberts and Leger 2004) and are being applied in pests control (Qasim et al. 2018; Wraight et al. 2000). These entomopathogens strongly respond against various mite pest across the globe in different ecosystems (Chandler et al. 2000). Recently, it has been observed that entomopathogenic fungi infect the various stages of spider mites (Alves et al. 2002; Shi et al. 2008a; Wekesa et al. 2005, 2006) as well as ectoparasitic mites (Shaw et al. 2002). Some other fungal isolates had been exploited against CRM, which caused significant mortality of mites. Meira geulakonigii (Boekhout, Scorzetti, Gerson & Sztejnberg) was applied on seedlings of sour orange at the rate of 2 × 108 conidia mLˉ1, which caused 75% mortality of P. citri within 1 week (Sztejnberg et al. 2004). While Paz et al. (2007) claimed that M. geulakonigii caused 63% mortality of P. citri with the dose of 1 × 108 conidia mLˉ1 within a week. Furthermore, M. argovae (Boekhout, Scorzetti, Gerson & Sztejnberg) and Acaromyces ingoldii (Boekhout, Scorzetti, Gerson & Sztejnberg) resulted in the death of 59% and 58% CRM population, respectively. Whereas, Puspitarini et al. (2011) reported that more than 40% population of P. citri collected from natural citrus plantation was infected with Hirsutella sp. in Indonesia.
The present research was conducted to test the lethal response of P. citri to the four hypocrealean fungi with a complementary log-log (CLL) model, and this model was selected to confirm an apparent trend of fungal toxicity under the binary effect of concentration and time. Moreover, the comparative effectiveness of all isolates was assessed at different concentrations as well as fungal exposure.
Material and methods
Source of fungal isolates and conidia preparation
Eight isolates of four species were used in this study, i.e. A. (L.) lecanii (Vl6063, V3450, Vp28 and V09); B. bassiana (D1344, BFZ0409); M. anisopliae (MFZ0706) and A. aleyrodis (AsG0910), and all of the isolates were procured from different labs (Table 1). All fungal isolates were cultured on the Petri plates of Sabouraud dextrose agar (SDA), under the conditions of 25 ± 1 °C, 80 ± 5% R.H., and 14:10 Light: Dark (L:D) photoperiod. Conidia of all isolates were collected separately according to the method of Ye et al. (2005). Then the suspension was prepared into universal flasks, having 3 mm glass beads, by using 10 mL deionized water as well as 0.02% (v/v) Tween®-80 (Fluka). After that, suspensions were homogenized by shaking tubes on vortex for 5 min, and concentration was determined by using a Neubauer hemocytometer (model 1103) (Goettel and Inglis 1997). However, conidial viability of all isolates was observed before each bioassay, according to Wang et al. (2004). The conidial viability of suspensions was consistently high in all bioassays being greater than 98.3 ± 0.52%.
CRM rearing
Colonies of P. citri were established from individuals collected from citrus seedlings (Citrus sinesis Osbeck) in a greenhouse (25 ± 2 °C) of Fujian Agriculture and Forestry University, Fuzhou, China and reared on citrus plants. After that, bioassays were done against both stages of mite, nymph and adult. For this purpose, 20 healthy adult females were collected from the population, and kept separately on fresh seedlings for 24 h, to get a homogeneous batch of eggs, and at last uniform aged nymphs. The vigorous nymphs (24 h after hatching from eggs of uniform age in the same growth chamber) were transferred onto detached citrus twigs with two leaves on a sponge containing a rhizocaline (100 g mLˉ1) for bioassays. To collect particular aged adults for further experiment, 50 quiescent deutonymphs (second stage of nymph of mite) were taken from the seedlings and transferred onto detached citrus twigs with two leaves on a sponge containing a rhizocaline of 100 g mLˉ1, under the conditions of 25 ± 1 °C, 12:12 L: D as well as 80 ± 5% RH. After that, nymphs and adult females were collected under a stereo-microscope (Nikon SX-45-TR) and then transferred onto new twigs of healthy citrus leaves, and these twigs were maintained the glass pot (12 × 9 cm).
Bioassays
We assayed for the biocontrol potential of eight isolates from four fungal species mentioned above against the nymphs and adults of P. citri in a lamp-chimney-caged seedling bioassay system. A 3.0 mL spore suspension (1.0 × 104 to 1.0 × 108 conidia mLˉ1) (of each fungal species) was sprayed into a chamber having infested twigs with a gas sprayer (Preval Sprayer, NY, USA, Vapor Pressure 4.018, Vapor Density 1.8) as fungal treatment whereas 3.0 mL 0.02% (v/v) Tween®-80 was sprayed on controlled twigs. After that, the top of lamp-chimney-cage was covered with a water-proof mesh film up to initial 24 h to maintain relative humidity, for the conidial germination. While inner wet filter paper lined on the Petri dish was changed daily (surviving mites on the filter paper dropping from the twigs were transferred onto the leaves of the twigs again). All observations (counting of dead and alive for nymph and adult) were done daily for 9 days with the help of a 10-fold hand magnifier. Later on, these mite cadavers were transferred into moist Petri dishes for fungal growths up to 2–3 days and followed by the verification of fungal infection, under the stereomicroscope (Nikon SX-45-TR) at 50X magnifications. Then the particular individuals, having fungal outgrowths, were counted as dead as a consequence of the tested isolates. All bioassays were repeated for five times with 40 female adult or nymphal mites for every treatment or blank control in each repeat.
Data analysis
The serial time-concentration-mortality was designed according to the description of Preisler and Robertson (1989) as well as Robertson and Preisler (1992). Data were analyzed by using the complementary log-log model (CLL model), and cumulative mortality was estimated (Nowierski et al. 1996; Wang et al. 2004). Controls were adjusted according to the model, described by Christensen and Chen (1985), and Robertson and Preisler (1992). Similarly, the particular formulae of Robertson and Preisler (1992) were used for the values of LC50 (or LC90) whereas LT50 values were estimated by linear interpolation (Feng et al. 1998; Nowierski et al. 1996). Mortality was adjusted according to the method of Nowierski et al. (1996).
The procedures, including modeling, estimation of time and concentration-effect parameters for the CLL models, test for goodness of fit, and estimation of virulence indices (LC50) using the parameters were analyzed using DPS data processing system software (Feng et al. 1998; Tang and Feng 2007).
Results
CRM infected by fungal pathogens
Early mycosis-caused deaths of CRM (nymphs and adults) began from 3-d inoculation, and the infected nymph and female adults became lethargic before death. The mycosed dead bodies indicated subtle fungal out-growths on the confined citrus leaves, perhaps, due to less humidity. However, all nymphs and adults became well infected after being transferred into moist petri dishes, within 3 days. All fungal infected mites (adult and nymphs) produced proper mycelia and conidia in the petri dishes, and, which mean all of eight strains, were capable of infecting both stages of the mites.
Mortality of CRM by fungal isolates
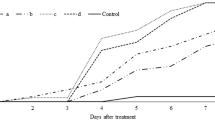
The cumulative mite mortalities by different concentrations of all fungal isolates are presented in Figs. 1 and 2. The trends of the observed mite mortalities were depended on both concentration and time. After 4 days of fungal application, there was considerable mortality of both stages (nymph and adults). After the ninth day of fungal application at the concentration of 108 against nymphal mite population, maximum mortality was 70.7% by BFZ0409 and D1344 (B. bassiana), whereas least mortality was 46.5% by AsG0910 (A. aerlodis) (Fig. 2). Similarly, after 9-d fungal exposure at the highest concentration against adult mites, BFZ0409 (B. bassiana) caused maximum mortality (87.9%), followed by V3450 (A. lecanii) with 85.6% mortality. The least mortality (51.9%) was presented by Vl6063 (A. lecanii). On the other hand, after 9-d of observation in the controlled treatment, the maximum mortality was 5.4% and 6.9% for nymphal and adult mites, respectively (Fig. 1).
The results of time-concentration-mortality modeling of nymph and adult female mites infected by eight isolates simulated by CLL model are shown in Figs. 1 and 2. The t-tests for all parameters estimated were significant (P < 0.01). Hosmer–Lemeshow statistic \( \overset{\wedge }{C} \) (a grouped Pearson’s χ2, i.e. modified Pearson’s χ2 by Nowierski et al. (1996) for the heterogeneity of the goodness of fit were non-significant for all eight fungal isolates (P < 0.05, Table 2). The data of the eight fungal isolates against the nymphs and females was fitted well to the CLL model. The slope values (β), the parameters from the maximum likelihood estimation in the CLL model indicated the rate of the proportion of CRM mortality as a function of log (spores concentrations of each suspension). Significant relationships between proportion mortality and log dose were found in all eight fungal isolates considered (P < 0.01, Table 2). The mortality and treatment had a strong relationship, as illustrated by the magnitude of the slope values (P = 0.05 by DMRT, Table 3). The fitted parameter β represented the slope values of the fitted curve, with the range from 0.15 to 0.49 and 0.16 to 0.36 against nymphal and adult mites, respectively. Isolates with a larger magnitude of slope values caused CRM mortality at a faster rate. Two isolate of A. lecanii (Vp28 (β = 0.49) and V3450 (β = 0.36)) were found to have the most substantial magnitude of slope value among all strains, examined for the impact on nymphal and adult mites, respectively (Table 2). The fitted parameters indicated that the concentration and time affected the efficiency of the tested isolates. The estimated parameters of 4-d spaying (γ4) were the largest for most tested isolates (Vl6063, Vp28, V09, BFZ009), indicating the estimate of latent periods for these microbial agent tested (Christensen and Chen 1985).
Based on the cumulative relationships of the fungal isolates against the mites determined by the fitted β and γj, the values of LC50s and associated confidence 95% intervals were computed as a function of the post-spray days (Tables 3 and 4). After 9 days of fungal exposure to adult mites, two isolates of B. bassiana (BFZ0409 and D1344) and one isolate of A. lecanii (V3450) showed the highest virulence at the least LC50 value (104 conidia mLˉ1). On the other hand, two isolates of A. lecanii (Vl6063 and V09) showed the least virulence at higher LC50s of 106 conidia mLˉ1, respectively. However, at the same fungal and time exposure, the LC50s values were higher for all isolates against nymphal mites. For example, least LC50 value was 3.25 × 105 conidia mLˉ1 for BFZ0409 (B. bassiana), and higher LC50 value was 6.59 × 108 conidia mLˉ1 for MFZ0706 (M. anisopliae).
The estimated LT50s were reduced with the increment of concentrations of fungal concentration (Tables 3 and 4). For example, the LT50s were computable at the concentration of 105 conidia mLˉ1, only for BFZ0409. The two B. bassiana isolates showed rapid mortality of 4.4d-5.0d at the high concentration of 108 conidia mLˉ1. For four tested A. lecanii isolates, the estimates ranged from 5.3d-6.5d, V09 exhibited the slowest mortality with 6.5d at the concentration of 108 conidia mLˉ1. The isolates of A. aleyrodis (AsG0910) and M. anisopliae (MFZ0706) showed intermediate mortality.
The equivalent slopes for concentration effects, the virulence indices (LC50s) and LT50s indicated that two isolates of B. bassiana (BFZ0409 and D1344) were the most virulent and high efficient isolates against CRM, followed by one isolate of A. lecanii (V3450). The others were the intermediate in virulence and efficacy.
Discussion
In the current study, we assessed the potential of eight isolates from four fungal species against CRM on citrus seedlings. Our results proved the effectiveness of all tested isolates against CRM females, but LC50s and LT50s determined by the fitted cumulative relationships varied considerably. All isolates were sufficiently virulent to both stages of CRM, although, all isolates presented significantly different mortality ranges. Adult females were more susceptible to all isolates as compared to nymphs. The reason may be some conidial spores drop off with ecdyses as molting. Therefore, with the time, nymphicidal activities of these isolates varied considerably with the complicated situation for their variation of enzymes and germination potency. All fungal entomopathogens could be applied in the greenhouse and field conditions, through various application methods. These entomopathogens could be spread directly in the target area, as spore coatings, spore bags, spore containing media or indirectly by spreading the fungal-infected insect bodies (Farenhorst and Knols 2010; Pilz et al. 2011; Stafford and Allan 2014).
Numerous entomopathogenic fungi, including B. bassiana, have been assessed for the management of several mites, such as Tetranychus urticae (Bugeme et al. 2014; Ullah and Lim 2015) and T. cinnabarinus (Erler et al. 2013). Tehri et al. (2015) described that B. bassiana reduced more than 60% population of T. urticae on okra in the field conditions. Likewise, the efficiency of B. bassiana was also evaluated against T. urticae at the rate of 1 × 108 conidia mLˉ1 on bean and cucumber, which caused mortality of 50% mite population in the greenhouse (Seyed-Talebi et al. 2014). Moreover, the pathogenicity of B. bassiana was explored against eggs and adults of T. urticae on Okra by Krishna and Bhaskar (2013), which caused mortality only 7 % of both stages. Moreover, B. bassiana was also presented 92% mortality against CRM adults in laboratory bioassays within 5 days at the rate of 1 × 108 conidia mLˉ1 (Alves et al. 2005). Variation in the potency of B. bassiana may be affected by several factors, such as temperature, humidity, experimental conditions, the concentration of used dose as well as plant variety. The pathogenicity of B. bassiana (ARSEF 2860) against P. citri has been documented up to 90% after 20 days of fungal application with a high dose of 1.2 × 1013 conidia haˉ1 in the field conditions (Shi and Feng 2006). But according to our findings, B. bassiana (BFZ0409) caused quick mortality of CRM, and killed half population within 5 days with a dose of 1 × 108 conidia mLˉ1 on citrus seedlings in controlled conditions.
The susceptibility of different mites to various fungal species is much attractive for the management of the mites in a diverse environment. In the current study, we observed that both stages of CRM were significantly susceptible to all tested fungal isolates. Likewise, Aguirre and Krugg (2014) described that adults of CRM were more susceptible to A. lecanii as well as B. bassiana at the concentration of 106 conidia mLˉ1, as both fungal species significantly reduced the adult population of mites by 71% within 2 weeks. Therefore, the results of the current study were in accordance with the findings of Aguirre and Krugg (2014), because two isolates (Vp28 and V3450) of A. lecanii killed more than half populations of nymphs and adults within 9 days by 108 conidia mLˉ1. Similarly, A. lecanii has much potential to inhibit the growth of different mites, like Tetranychus urticae Koch (Amjad et al. 2012) and Dendrolaelaps sp. (Bałazy et al. 2008).
Similarly, the lethal potential of M. anisopliae was also appealing against several insect pests, as well as mite pests. M. anisopliae is much effective against several pest mites, like Brevipalpus phoenicis (Magalhães et al. 2005), Mononychellus tanajoa (Barreto et al. 2004), T. urticae (Bugeme et al. 2015), T. evansi (Maniania et al. 2016), T. truncates and T. turkestani (Shi et al. 2008a, 2008b). Likewise, it was observed in our current work that P. citri had faced 63% and 44% mortality of adult and nymphs, respectively, by M. anisopliae at 108 conidia mLˉ1 within 9 days of exposure, which shows that our findings are in accordance with the reports of García and Krugg (2015), who described that M. anisopliae caused up to 83% mortality of CRM in lab conditions under the fungal exposure for 2 weeks. Moreover, Aschersonia aleyrodis also has a significant potential to control various insect pest, like whitefly (Zhang et al. 2017). However, there is one report by Tamai et al. (2002) who described that A. aleyrodis had presented minute potential to inhibit the growth of T. urticae. Beyond all fungal explorations against CRM, A. aleyrodis still uncovered for its pathogenicity. We observed in the current research that A. aleyrodis (AsG0910) caused 70% and 52% mortality of adults and nymphs of CRM within a week, respectively, after application of 108 conidia mLˉ1.
Conclusion
In conclusion, two isolates of B. bassiana (BFZ0409, D1344) and one isolate of A. lecanii (V3450) were highly virulent against both stages of CRM at the lowest doses, and therefore, these isolates could be recommended as promising candidates for the management of CRM. Whereas, other five isolates were less effective against both stages of CRM. Thus, employing these isolates into integrated management of mites could assist synthetic acaricides in the citrus orchards and avoid some predator mites susceptible to mycosis. However, the potency of these isolates is still needed to be evaluated in field conditions, especially, the compatibilities with some phytoseiid predators before field application.
References
Aguirre EPA, Krugg JHW (2014) Efecto de Lecanicillium lecanii y Beauveria bassiana sobre el ácaro Panonychus citri en condiciones de laboratorio. Rebiol 34:42–50
Al-Azzazy MM (2016) Population fluctuation and control of the citrus rust mite, Phyllocoptruta olievora (Ashmead)(Arachnida: Prostigmata: Eriophyidae). J Agric Vet Sci 267:1–12
Alves SB, Rossi LS, Lopes RB, Tamai MA, Pereira RM (2002) Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and Tetranychus urticae (Acari: Tetranychidae). J Invertebr Pathol 81:70–77
Alves SB, Tamai MA, Rossi LS, Castiglioni E (2005) Beauveria bassiana pathogenicity to the citrus rust mite Phyllocoptruta oleivora. Exp Appl Acarol 37:117–122
Amjad M, Bashir MH, Afzal M, Sabri MA, Javed N (2012) Synergistic effect of some entomopathogenic fungi and synthetic pesticides, against two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Pak J Zool 44:977–984
Bałazy S, Miętkiewski R, Tkaczuk C, Wegensteiner R, Wrzosek M (2008) Diversity of acaropathogenic fungi in Poland and other European countries. In “Diseases of Mites and Ticks”, pp. 53-70. Springer
Barreto RS, Marques EJ, Gondim MGC Jr, Oliveira JVD (2004) Selection of Beauveria bassiana (Bals.) Vuill. And Metarhizium anisopliae (Metsch.) Sorok. For the control of the mite Mononychellus tanajoa (Bondar). Sci Agric 61:659–664
Bugeme DM, Knapp M, Boga HI, Ekesi S, Maniania NK (2014) Susceptibility of developmental stages of Tetranychus urticae (Acari: Tetranychidae) to infection by Beauveria bassiana and Metarhizium anisopliae (Hypocreales: Clavicipitaceae). Int J Trop Insect Sci 34:190–196
Bugeme DM, Knapp M, Ekesi S, Chabi-Olaye A, Boga HI, Maniania NK (2015) Efficacy of Metarhizium anisopliae in controlling the two-spotted spider mite Tetranychus urticae on common bean in screenhouse and field experiments. Insect Sci 22:121–128
Chandler D, Davidson G, Pell JK, Ball BV, Shaw K, Sunderland KD (2000) Fungal biocontrol of Acari. Biocont Sci Technol 10:357–384
Christensen ER, Chen C-Y (1985) A general noninteractive multiple toxicity model including probit, logit, and Weibull transformations. Biometrics 41:711–725
Dhooria MS (2016) “Fundamentals of applied acarology,” springer, Singapore pp 470
Erler F, Ates AO, Bahar Y (2013) Evaluation of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae, for the control of carmine spider mite, Tetranychus cinnabarinus (Boisduval) under greenhouse conditions. Egypt J Biol Pest Cont 23:233–240
Farenhorst M, Knols BGJ (2010) A novel method for standardized application of fungal spore coatings for mosquito exposure bioassays. Malar J 9:27
Feng M-G, Liu C-L, Xu J-H, Xu Q (1998) Modeling and biological implication of time–dose–mortality data for the entomophthoralean fungus, Zoophthora anhuiensis, on the green peach aphid Myzus persicae. J Invertebr Pathol 72:246–251
García ZBS, Krugg WJH (2015) Efecto de Isaria fumosorosea y Metarhizium anisopliae sobre Panonychus citri, en condiciones de laboratorio. M.Sc Thesis, Facultad de Ciencias Biologicas, Universidad Nacional de Trujillo, Peru. pp. 47
Gerson U, Cohen E (1989) Resurgences of spider mites (Acari: Tetranychidae) induced by synthetic pyrethroids. Exp Appl Acarol 6:29–46
Goettel MS, Inglis GD (1997) Fungi: hyphomycetes. In; manual of techniques in insect pathology. Academic Press, London 5:213–251
Haiyuan K (1996) The occurence and control of important agricultural mites in China [J]. Pestic 35:6–11
Idrees A, Qasim M, Ali H, Qadir ZA, Idrees A, Bashir MH, Qinge J (2016) Acaricidal potential of some botanicals against the stored grain mites, Rhizoglyphus tritici. J Entomol Zool Stud 4:611–617
Jamieson LE, Charles JG, Stevens PS, McKenna CE, Bawden R (2005) Natural enemies of citrus red mite (Panonychus citri) in citrus orchards. New Zealand Plant Protec 58:299–305
Kennett CE, McMurtry JA, Beardsley JW (1999) Biological control in subtropical and tropical crops. In “Handbook of Biological Control”, pp. 713-742. Elsevier
Kepler RM, Luangsa-Ard JJ, Hywel-Jones NL, Quandt CA, Sung G-H, Rehner SA, Aime MC, Henkel TW, Sanjuan T, Zare R (2017) A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 8:335–353
Kranz J, Schmutterer H, Koch W (1977) Diseases, pests, and weeds in tropical crops. Paul Parey, Berlin, p 666
Krishna AR, Bhaskar H (2013) Ovicidal and adulticidal effect of acaropathogenic fungi, neem oil and new acaricide molecules on Tetranycus urticae Koch. Entomon 38:177–182
Li L-S (1990) Recent progress in the study of agricultural mites (J). Sci Agric Sin 23:22–30
Li L, Zhu W, Hu G (1990) A preliminary study of the binomics of citrus red mites , Panonychus citri, McGregor (J). Acta Phytophyl Sin 7:17–26
Magalhães BP, Rodrigues JCV, Boucias DG, Childers CC (2005) Pathogenicity of Metarhizium anisopliae var. acridum to the false spider mite Brevipalpus phoenicis (Acari: Tenuipalpidae). Fla Entomol 88:195–199
Maniania NK, Ekesi S, Kungu MM, Salifu D, Srinivasan R (2016) The effect of combined application of the entomopathogenic fungus Metarhizium anisopliae and the release of predatory mite Phytoseiulus longipes for the control of the spider mite Tetranychus evansi on tomato. Crop Prot 90:49–53
Martins CC, Alves LFA, Mamprim AP, Souza LPA (2016) Selection and characterization of Beauveria spp. isolates to control the broad mite Polyphagotarsonemus latus (banks, 1904)(Acari: Tarsonemidae). Braz J Biol 76:629–637
Meng H, Wang K, Jiang X, Yi M (2000) Breeding and biochemical mechanism of Panonychus citri’s resistance to pyridaben. Chinese J Pestic Sci 39:26–28
Nowierski RM, Zeng Z, Jaronski S, Delgado F, Swearingen W (1996) Analysis and modeling of time–dose–mortality of Melanoplus sanguinipes, Locusta migratoria migratorioides, and Schistocerca gregaria (Orthoptera: Acrididae) from Beauveria, Metarhizium, and Paecilomyces isolates from Madagascar. J Invertebr Pathol 67:236–252
Paz Z, Gerson U, Sztejnberg A (2007) Assaying three new fungi against citrus mites in the laboratory, and a field trial. Biocontrol 52:855–862
Pilz C, Enkerli J, Wegensteiner R, Keller S (2011) Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J Appl Entomol 135:393–403
Poinar G Jr, Poinar R (1998) Parasites and pathogens of mites. Annu Rev Entomol 43:449–469
Preisler HK, Robertson JL (1989) Analysis of time-dose-mortality data. J Econ Entomol 82:1534–1542
Puspitarini RD, Rauf A, Sosromarsono S, Santoso T, Santoso S (2011) Abundance of citrus red mite Panonychus citri (McGregor) (Acari: Tetranychidae ), other mites and its natural enemies at several citrus plantation locations. J Agric Food Technol 1:212–217
Qasim M, Lin Y, Dash CK, Bamisile BS, Ravindran K, Islam SU, Ali H, Wang F, Wang L (2018) Temperature-dependent development of Asian citrus psyllid on various hosts, and mortality by two strains of Isaria. Microb Pathog 119:109–118
Roberts DW, Leger RJS (2004) Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol 54:1–70
Robertson JL, Preisler HK (1992) Pesticide bioassays with arthropods. CRC, Boca Raton
Ruppert EE, Fox RS, Barnes RD (2004) “Invertebrate zoology: a functional evolutionary approach”
Seyed-Talebi F-S, Kheradmand K, Talaei-Hassanloui R, Talebi-Jahromi K (2014) Synergistic effect of Beauveria bassiana and spirodiclofen on the two-spotted spider mite (Tetranychus urticae). Phytoparasit. 42:405–412
Shaw KE, Davidson G, Clark SJ, Ball BV, Pell JK, Chandler D, Sunderland KD (2002) Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol Control 24:266–276
Shi W-B, Feng M-G (2004) Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol Control 30:165–173
Shi W-B, Feng M-G (2006) Field efficacy of application of Beauveria bassiana formulation and low rate pyridaben for sustainable control of citrus red mite Panonychus citri (Acari: Tetranychidae) in orchards. Biol Control 39:210–217
Shi WB, Zhang LL, Feng MG (2008a) Field trials of four formulations of Beauveria bassiana and Metarhizium anisopliae for control of cotton spider mites (Acari: Tetranychidae) in the Tarim Basin of China. Biol Control 45:48–55
Shi W-B, Zhang L, Feng M-G (2008b) Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to three hypocrealean fungi as biocontrol agents. Biol Control 46:495–501
Stafford KC, Allan SA (2014) Field applications of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae F52 (Hypocreales: Clavicipitaceae) for the control of Ixodes scapularis (Acari: Ixodidae). J Med Entomol 47:1107–1115
Sztejnberg A, Paz Z, Boekhout T, Gafni A, Gerson U (2004) A new fungus with dual biocontrol capabilities: reducing the numbers of phytophagous mites and powdery mildew disease damage. Crop Prot 23:1125–1129
Tamai MA, Alves SB, de Almeida JEM, Faion M (2002) Avaliação de fungos entomopatogênicos para o controle de Tetranychus urticae Koch (Acari: Tetranychidae). Arq. Inst. Biol. São Paulo 69:77–84
Tang Q-Y, Feng M-G (2007) DPS data processing system: experimental design, statistical analysis and data mining. Science Press, Beijing, p 972
Tehri K, Gulati R, Geroh M, Dhankhar SK (2015) Dry weather: a crucial constraint in the field efficacy of entomopathogenic fungus Beauveria bassiana against Tetranychus urticae Koch (Acari: Tetranychidae). J Entomol Zool Stud 3:287–291
Ullah MS, Lim UT (2015) Laboratory bioassay of Beauveria bassiana against Tetranychus urticae (Acari: Tetranychidae) on leaf discs and potted bean plants. Exp Appl Acarol 65:307–318
Van der Geest LP, Elliot SL, Breeuwer JAJ, Beerling EAM (2000) Diseases of mites. Exp Appl Acarol 24:497–560
Vechia JFD, Ferreira MC, Andrade DJ (2018) Interaction of spirodiclofen with insecticides for the control of Brevipalpus yothersi in citrus. Pest Manag Sci 74:2438–2443
Wang L, Huang J, You M, Liu B (2004) Time-dose-mortality modelling and virulence indices for six strains of Verticillium lecanii against sweetpotato whitefly, Bemisia tabaci (Gennadius). J Appl Entomol 128:494–500
Wekesa VW, Maniania NK, Knapp M, Boga HI (2005) Pathogenicity of Beauveria bassiana and Metarhizium anisopliae to the tobacco spider mite Tetranychus evansi. Exp Appl Acarol 36:41–50
Wekesa VW, Knapp M, Maniania NK, Boga HI (2006) Effects of Beauveria bassiana and Metarhizium anisopliae on mortality, fecundity and egg fertility of Tetranychus evansi. J Appl Entomol 130:155–159
Wraight SP, Carruthers RI, Jaronski ST, Bradley CA, Garza CJ, Galaini-Wraight S (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Control 17:203–217
Yang H-Z, Hu J-H, Qing L, Li H-J, Liu H-Q, Yao T-S, Chun R, Lei H-D (2009) Acaricidal activity of Boenninghausenia sessilicarpa against Panonychus citri. Agric Sci China 8:1097–1102
Ye S-D, Dun Y-H, Feng M-G (2005) Time and concentration dependent interactions of Beauveria bassiana with sublethal rates of imidacloprid against the aphid pests Macrosiphoniella sanborni and Myzus persicae. Ann Appl Biol 146:459–468
Yu YJ (2001) Zhejiang standards for production of safe vegetables and control of pesticide application. Integrated Pest Management in Relation to Safe Agricultural Products. China Agricultural Press, Beijing, pp 11–16
Zhang C, Ali S, Musa PD, Wang XM, Qiu BL (2017) Evaluation of the pathogenicity of Aschersonia aleyrodis on Bemisia tabaci in the laboratory and greenhouse. Biocont Sci Technol 27:210–221
Zhou L, Yue B, Zou F (1999) Life table studies of Eotetranychus kankitus (Acari: Tetranychidae) at different temperatures. Syst Appl Acarol 4:69–74
Acknowledgements
We thank Miss Qiuping Jiang and Mr. Zhisheng Liang for help in the experiment. This research was supported jointly by research grants funded by the National Key Projects of R & D of China (2018YFD0201502); Key projects of Science and Technology of Fujian Province (2016 N0005); and Research Fund for the International Collaborative Program (KXGH17004), grant (CXZX2017211), (CXZX2018101), (CXZX2019008S) and (KF2015068) from FAFU. We also acknowledge the support of a grant RCAMS/KKU/08/20 under Research Center for Advanced Materials Science (RCAMS) at King Khalid University, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest, and are agree to proceed the article in the International J. Tropical Insect Science.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qasim, M., Ronliang, J., Islam, W. et al. Comparative pathogenicity of four entomopathogenic fungal species against nymphs and adults of citrus red mite on the citrus plantation. Int J Trop Insect Sci 41, 737–749 (2021). https://doi.org/10.1007/s42690-020-00263-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00263-z