Abstract
Spittlebugs from the genus Aeneolamia are important pests of sugarcane. Although the use of the entomopathogenic fungus Metarhizum anisopliae s.l. for control of this pest is becoming more common in Mexico, fundamental information regarding M. anisopliae in sugarcane plantations is practically non-existent. Using phylogenetic analysis, we determined the specific diversity of Metarhizium spp. infecting adult spittlebugs in sugarcane plantations from four Mexican states. We obtained 29 isolates of M. anisopliae s.str. Haplotype network analysis revealed the existence of eight haplotypes. Eight selected isolates, representing the four Mexican states, were grown at different temperatures in vitro; isolates from Oaxaca achieved the greatest growth followed by isolates from Veracruz, San Luis Potosi and Tabasco. No relationship was found between in vitro growth and haplotype diversity. Our results represent a significant contribution to the better understanding of the ecology of Metarhizum spp. in the sugarcane agroecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane is one of the most important crops in the world; during 2012, more than 1,800 million metric tonnes were produced (FAOSTAT 2012). Spittlebugs, including species in the genus Aeneolamia, are serious pests of pastures and sugarcane plantations in neotropical countries (Thompson 2004) such as Brazil, Venezuela, Ecuador and Mexico (Gómez 2007). Among the most important biological control agents of this pest is the entomopathogenic fungus Metarhizium anisopliae sensu lato (Li et al 2010). There is some information reporting the virulence of M. anisopliae isolates against different species of the spittlebug under laboratory (Arango et al 1994, Garcia et al 2012, de la Torres et al 2013) and field (Badilla et al 1996, Matabanchoy Solarte et al 2012) conditions. Furthermore, the use of M. anisopliae isolates for the control of field populations of the spittlebug in countries such as Guatemala, Costa Rica, Panama, Venezuela, Ecuador and Brazil is common (Garcia et al 2012). Brazil is an example of the most extensive use of M. anisopliae for the control of spittlebugs in sugarcane and pastures, with more than 1.5 million ha sprayed with this fungus during 2011 (Vega et al 2012). In Mexico, the use of M. anisopliae for control of this pest is also increasing; unfortunately, information regarding the impact of the treatments on spittlebug field populations is non-existent. In addition, data regarding the origin of the isolates (host), their virulence under laboratory and field conditions, and data describing their biological attributes such as germination, conidial production, and optimal temperature for in vitro growth are seldom published, or only appear in unofficial reports that are difficult or impossible to access.
A greater understanding of the ecology and composition of indigenous populations of entomopathogenic fungi is required and must be considered in the development of these microorganisms as biological control agents in Mexico. For instance, it has been shown that particular habitats can harbour different species of fungi and different genetic groups within those species (Meyling et al 2009, Ormond et al 2010). As a first step, accurate species identification is important based on the revised taxonomic status of M. anisopliae, which now includes cryptic species that cannot be separated morphologically (Bischoff et al 2009). At least two comprehensive studies have been published describing the existence of cryptic species within the M. anisopliae lineage (Bischoff et al 2009, Kepler et al 2014). Previous research reported genetic variation amongst M. anisopliae s.l. isolates (Becerra Velásquez et al 2007, Freed et al 2011), but these did not consider the existence of cryptic speciation. At this respect, to our knowledge, there is no information regarding the identification of Metarhizium species infecting spittlebugs using phylogenetic methods for Mexico or elsewhere.
Therefore, our aims were first to establish the taxonomic status of the Metarhizium isolates infecting spittlebug populations from the genus Aeneolamia spp. collected in five plantations from four of the most important Mexican states producing sugarcane. Secondly, using a reduced number of isolates, but including representatives from all geographical origins, in vitro growth profiles at different temperatures were studied, in order to establish the potential relationships between genetic variation, growth profiles and geographical origin.
Material and Methods
Isolate collection
One hundred and six dead spittlebug adults were collected during August 2012 from the soil of five sugarcane plantations distributed in the states of Veracruz, San Luis Potosi (two plantations), Oaxaca and Tabasco (Table 1). Each dead insect was placed individually into 2-mL Eppendorf tubes; no visible signs of infection were observed in the collected insects. The insects were transported to laboratory and maintained at 5°C prior to processing, but for no longer than 2 weeks.
All collected insect cadavers were surface-sterilised by individually immersing them in 5% sodium hypochlorite for 2 min and then rinsing them twice in sterile distilled water. Surface-sterilised cadavers were blot dried with sterile filter paper and incubated individually in 60-mm-diameter Petri dishes on sterile damp filter paper at 25°C in complete darkness for 72 h.
After this time, samples of conidia were taken from sporulating cadavers using a bacterial loop in a laminar flow sterile hood. Samples were placed onto 25 mL of Sabouraud dextrose agar (SDA) in a 90-mm-diameter Petri dish and incubated at 25°C in complete darkness. If no growth was observed on the SDA plates, a second sample of conidia was taken from the same sporulating cadaver, deposited into 25 mL of Sabouraud dextrose broth (SDB) and incubated at 25°C and 250 rpm for 5 days. Any resulting mycelium was washed with sterile distilled water, inoculated into SDA plates and incubated as described above. From each isolate we obtained, a monosporic colony was produced by randomly selecting one colony-forming unit from a SDA plate inoculated with a 1 × 106 conidia mL−1 suspension made from the original isolation. Both the original and monosporic isolates were stored at −80°C in 2-mL cryovials (Nalgene, Thermo Fisher Scientific, Rochester, NY, USA) containing 10% sterile glycerol. Only monosporic isolates were used in subsequent experiments.
From the 106 dead insects processed, 29 produced external sporulation and Metarhizium spp. were successfully isolated (Table 2). The isolates obtained were identified morphologically to genus and species. For this, an undetermined number of conidia from each isolate were collected from a 15-day-old colony grown on SDA plates. The conidia were placed onto a glass slide and covered with a coverslip without any mounting liquid. Preliminary observations showed that immersing conidia in mounting liquid, created a diffuse area surrounding each conidium. This made it very difficult to establish the exact borderline of each conidium for accurate measurements. Without the mounting liquid, the borderline of each conidium was clear and could be photographed and measured accurately. One hundred photos of conidia were taken for each isolate using a Carl Zeiss microscope attached to a digital camera PaxCam 3. From these digital photos, the length and width of approximately 300 conidia per isolate were measured using the software Image Tool for Windows 3.0 (Wilcox et al 2002). The mean conidial size for the isolates was 8.10–4.68 × 4.34–2.09 μm, placing them in M. anisopliae s.l. Metsch. Sorokin (Table 2). Taxonomic placement of all species was made according to Humber (2012).
Phylogenetic placement of isolates
Mycelium production and DNA extraction.
Conidia from each isolate were harvested from 15-day-old colonies and inoculated on to SDA plates with a sterile cellophane sheet on top of the medium. Plates were incubated at 25°C for 14 days in complete darkness. Mycelium was separated from the cellophane sheet using a sterile scalpel, placed into a sterile 20-mL glass container and lyophilised for 24 h. For DNA extraction, 0.2 g of lyophilised mycelium was placed into a 1.5-mL Eppendorf tube and plunged into liquid nitrogen for 5 min. Frozen mycelium was then ground using a pellet pestle rod (Daigger and Company Inc., Vernon Hills, IL, USA), and DNA was extracted using the DNeasy® Plant Minikit following the manufacturer’s instructions with the modifications suggested by Fargues et al (2002). The concentration of DNA was estimated using a NanoDrop (Thermo Fisher Scientific, Inc. Waltham, MA, USA) and stored at 4°C until required.
PCR and sequencing.
Partial sequences of the nuclear gene elongation factor 1-α (EF1-α) (Bischoff et al 2006, 2009) and the ITS region of the rDNA were obtained. A region of the elongation factor 1-α was amplified using primers 983F and 2218R (Rehner & Buckley 2005). PCR amplifications were made in a total volume of 30 μL, which included 6 μL of 5X PCR buffer provided by the manufacturer, 1.2 mM of each dNTP, 1.4 μM each of opposing amplification primers, 3.5 mM of MgCl2 and 0.3 μL of Taq polymerase (Promega, Madison, WI, USA). PCR was performed using a touchdown procedure (Don et al 1991) and the thermal conditions reported by Rehner & Buckley (2005). Reactions were made in a MyCycler thermal cycler (Bio-Rad Laboratories Inc., Hercules, CA, USA). PCR products were visualised on 1.5% agarose gels in 1X TAE. GelPilot® 100 bp Plus and 1 kb Plus (QIAGEN®, GmbH, Hilden, Germany) size markers were used. The gels were stained with ethidium bromide (0.1 μg mL−1) and photographed. All PCR products were purified using a QIAquick PCR Purification Kit (QIAGEN®, GmbH, Hilden, Germany) following the manufacturer’s instructions, and samples were sent to Macrogen Inc. (Geumchen-gu, Seoul, Korea) for direct sequencing.
The ITS region of isolates was amplified using the universal primer set ITS5 and ITS4 (White et al 1990). PCR amplifications were made in 0.2-mL Eppendorf tubes in a total reaction volume of 25 μL. Each reaction contained 1.4 μM of each primer, 0.12 mM of each dNTP (QIAGEN), 1 μL (5 units) of Taq polymerase (New England Biolabs Inc., Ipswich, MA, USA) in 10× PCR reaction buffer and 30 ng of DNA. Thermal cycling conditions were one cycle of denaturation at 95°C for 3 min followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 45°C for 1 min, extension at 72°C for 1.5 min, and with a final extension at 72°C for 5 min. PCR products were visualised and sequenced as described previously.
Data analysis.
All sequence traces were edited and assembled using BioEdit (Hall 1999). Multiple sequence alignments were made using the Clustal W programme (Thompson et al 1994). Maximum parsimony (MP) analyses were done using the Molecular Evolutionary Genetic Analysis (MEGA) software ver. 5.0 for Windows (Tamura et al 2011) using the close-neighbour-interchange algorithm. The robustness of branches was estimated by bootstrap analysis with 1000 repeated samplings of the data (Felsenstein 1985). Sequences from related species within the genus Metarhizium were retrieved from GenBank and used for comparison.
As no significant topological conflicts between consensus trees generated for each loci data were observed (data not shown), sequence information data sets from two nuclear loci (EF1-α and ITS) were combined and analysed together as described previously. Genetic differences among haplotypes were detected in a maximum parsimony network (Templeton et al 1992) using TCS v. 1.21 (Clement et al 2000).
Effect of temperature on the in vitro growth of fungal isolates
For this experiment, eight M. anisopliae sensu stricto isolates were randomly selected but represented all sample locations. The effect of temperature on the growth of each isolate was assessed by measuring the area of growth achieved by colonies when incubated at 20, 25, 30 and 35°C. The procedure for each isolate was the same. Sterile SDA plates were inoculated with 100 μL of a 1 × 107 conidia mL−1 conidial suspension. After 48 h, a 10-mm-diameter plug was taken from the growing edge, inverted and placed in the centre of a Petri dish containing 15 mL of solidified SDA. Each dish was sealed with Parafilm “M” (Pechiney Plastic Packaging Inc., Menasha, WI, USA) and incubated at 20, 25, 30 or 35°C. After 48 h, and thereafter every 24 h, the area of each colony was measured for 11 days (13 days in total), unless the colony had reached the edge of the plate within this time. For the measurements, a digitised image was created for each colony, which was then processed using the software GIMP ver. 2.8 and the area of growth quantified using Image Tool version 3.0 software (Wilcox et al 2002). Five replicates of each isolate were measured, and the whole experiment done on two occasions (ten replicates per isolate × temperature combination overall).
Data analysis.
The statistical package GenStat ver. 8 was used for analysis (Payne et al 2005). Successive colony measurements over time were analysed using an ante-dependence analysis (Kenward 1987) via the GenStat procedures ANTORDER and ANTTEST. The order of ante-dependence structure was estimated, and then, the treatment effects were estimated adjusting for the observed order of ante-dependence. The results from the ante-dependence analysis indicated those times at which treatment effects occurred as well as the overall treatment effect. A factorial treatment structure (isolates × temperature) was incorporated into the analysis to investigate main effects of both treatments and whether an interaction existed between them. In a separate analysis, isolate comparisons were partitioned to compare geographical origins.
Results
Twenty-nine Metarhizium isolates were obtained from 106 dead adults of Aeneolamia spp. collected from five locations (sugar plantations) distributed in four Mexican states (Tables 1 and 2).
Phylogenetic placement of the Metarhizium isolates
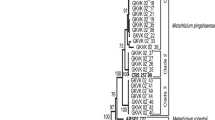
GenBank accession numbers for sequence data generated are listed in Table 2 for all the Metarhizium isolates collected. After alignment and trimming, the EF1-α and ITS sequence data set consisted of 886-889 and 519-525 aligned positions, respectively. Phylogenetic analysis combining both data sets grouped all isolates as M. anisopliae s.str. (Fig 1).
Genetic variation amongst M. anisopliae isolates
Haplotype network analysis showed the existence of eight haplotypes amongst the 29 isolates (Table 2 and Fig 2). The most common haplotype was H7 with 11 isolates and included representatives from all locations except Santa Rosalia, Tabasco (Table 2). The second most common haplotype was H8 with nine isolates, again with representatives from all locations except Santa Rosalia, Tabasco, followed by haplotypes H1, H2 and H6, each with two isolates, and by haplotypes H3, H4 and H5 with one isolate each (Fig 2 and Table 2).
The most parsimonious haplotype network for Metarhizium anisopliae isolates. Haplotypes are connected with a 95% confidence limit. Each line in the network represents a single mutational change. Small circles indicate missing haplotypes. Numbers of samples per haplotype are shown in parentheses. Colours indicate geographical origin within each haplotype.
Effect of temperature on the in vitro growth of fungal isolates
In the first comparison (isolate × temperature) of M. anisopliae isolates, the order of ante-dependence was estimated to be seven; tests of treatment terms were therefore based on this order of ante-dependence structure. Significant differences were found in the overall growth profiles of the eight isolates (χ 277 = 404.77, p < 0.001). Also, significant differences were found in growth at all observation times (days) for the eight isolates (p < 0.001). There was a significant interaction between temperature and isolate on the overall growth of the isolates (χ 2231 = 738.30, p < 0.001). This interaction was present at all observation times (p < 0.001). When the isolate comparison was partitioned to compare geographical origins, the order of ante-dependence structure was seven, and further comparison was based on this structure. Overall, the growth profiles differed for the M. anisopliae isolates from different geographical origins (χ 233 = 195.12, p < 0.001), and this difference was consistent at all observation times (p < 0.001). M. anisopliae isolates from Oaxaca achieved the greatest colony size at 20, 25 and 30°C, followed by the isolates from Veracruz, San Luis Potosi and Tabasco (Fig 3). The overall difference between the four geographic origins of M. anisopliae isolates varied with temperature (χ 299 = 319.40, p < 0.001), and an interaction was present at all observation times (p = 0.048 to p < 0.001). Isolates from Oaxaca achieved their greatest colony size at 25°C (Fig 3b), whereas isolates from Tabasco obtained the smallest colony size overall (Fig 3b). Although isolates from Tabasco achieved the greatest colony size at 30°C (Fig 3c), this size was still small compared to the growth achieved by other isolates at the other temperatures (Fig 3c). Differences amongst growth profiles were less evident at 20°C (Fig 3a) and practically non-existent at 35°C (Fig 3d), where the colony size of all isolates was never greater than 1 cm2.
Cumulative growth of eight Metarhizium anisopliae isolates grouped into four geographical origins are expressed in square centimetres. Three isolates were from San Luis Potosi, two isolates each were from Oaxaca and Veracruz and one isolate was from Tabasco (Table 2). Oaxaca ( ), San Luis Potosi (
), San Luis Potosi ( ), Veracruz (
), Veracruz ( ) and Tabasco (
) and Tabasco ( ). Growth profiles represent mean values of all isolates at a 20°C, b 25°C, c 30°C and d 35°C.
). Growth profiles represent mean values of all isolates at a 20°C, b 25°C, c 30°C and d 35°C.
Discussion
There are some reports indicating the pathogenicity of M. anisopliae s.l. isolates against spittlebug (de la Torres et al 2013); however, despite the importance of this pest in sugarcane plantations (Peck et al 2001) and the necessity to assess indigenous isolates before introducing biological control agents (Meyling & Eilenberg 2007), there are no studies reporting the occurrence and diversity of fungal species in sugarcane. Phylogenetically, all isolates collected were M. anisopliae s.str. We cannot be sure that this fungal species was the only one present in sugarcane plantations or whether other Metarhizium species might be present in this agroecosystem but not infecting the spittlebug. It would be important to investigate the presence of other potential Metarhizium species in soil, roots and leaves in sugarcane plantations. We also found two isolates for each of the species Isaria farinosa and Isaria fumosorosea (data not shown). These were not included in the molecular study, but warrant further investigation, particularly in relation to their interactions with the spittlebug.
Genetic variation was found amongst the M. anisopliae s.str. isolates as confirmed by the haplotype network (Fig 2); however, geographical distance between locations seems not to be an important factor driving genetic variation. It is always desirable to analyse as many samples as possible; however, not all the dead insects collected showed sporulation, which suggested that they may have died from other causes than fungal infection, thus reducing the number of potential isolates. However, we believe that our results are still valid and represent an important step forward in the study of the ecology of Metarhizium in this important agroecosystem. The genetic variation found amongst isolates collected in Paso Nuevo (Veracruz) could be related to the fact that they have their own small factories that produce M. anisopliae; however, there are no consistent and/or strict procedures in place to maintain and to produce large quantities of M. anisopliae conidia. For instance, field applications are made using multisporic isolates and sometimes, when isolates fail to grow while being mass-produced in vitro, it is common to obtain a different isolate from field-infected insects to substitute the failing isolate. In addition, fungi with asexual reproduction such as M. anisopliae are haploid, which means they express all their genes and, therefore, are exposed to mutations leading to the presence of genetically different nuclei in the cytoplasm of the hyphae. Interestingly, this may result in the ratio of nuclear types within each isolate varying according to environmental conditions, eventually allowing the isolate to alter the nuclear ratio in response to prevailing environmental conditions (Deacon 1997), and as a consequence, genetically diverse isolates may be obtained from the same location. In addition, a further source of genetic diversity in fungal populations is recombination events, such as the exchange of nuclei during hyphal fusion events (anastomoses), which can be followed by nuclear recombination (parasexual cycle) (Burdon & Silk 1997). However, this event has been demonstrated only in the laboratory, as reported for Beauveria bassiana (Castrillo et al 2004), and its impact in field fungal populations remains unclear (Burdon & Silk 1997, Read et al 2010).
Temperature is one of the most important abiotic factors affecting the biology and ecology of entomopathogenic fungi (Tanada & Kaya 1993, Inglis et al 2001). Therefore, it is important to evaluate in vitro growth profiles of entomopathogenic fungi as a clue to the field behaviour of the fungi for a better understanding of the influence of abiotic factors on the diversity and distribution of fungal species. Overall, our results were similar to those previously reported, with optimal growth occurring between 20 and 25°C for M. anisopliae s.l. (Ekesi et al 1999, Dimbi et al 2004). When isolates of M. anisopliae were grouped by geographical origin and compared, isolates from Oaxaca normally achieved the greatest growth at all temperatures (Fig 3), and isolates from Tabasco consistently achieved the smallest colony size at all temperatures. We consider that it is now very important to assess the ability of the isolates collected to infect spittlebug adults at different temperatures and to determine whether there is any potential relationship between in vitro growth and virulence.
We were not able to find a relationship between in vitro growth of the isolates and genetic variation. We accept that if we had used a larger number of isolates from each location, some significant trends could have been observed. However, our samplings were done during 1 month and focused only in isolates infecting the spittlebug; therefore, we consider the number of isolates obtained to be significant and important in underpinning our understanding of the organisation and ecology of Metarhizium in this agroecosystem. It is also likely that different results regarding genetic variation could be obtained using a multigene approach (Kepler et al 2014) or a different method such as mass spectrometry (Kepler et al 2014).
In summary, we only found M. anisopliae s.str. infecting adults of Aeneolamia in Mexican sugarcane plantations. The M. anisopliae isolates were genetically diverse but without any obvious geographic structure. More detailed studies of the ecology of the genus Metarhizium within this agroecosystem are needed to elucidate the possible role of this fungal genus in insect population dynamics, particularly the presence of other Metarhizium species in soil, adjunct vegetation or infecting other insects at the same niches.
References
Arango GL, Torres C, Lapointe SL (1994) Pathogencity of three strains of Metarhizium anisopliae to eggs and nymphs of Aeneolamia varia (Fabricius) (Homoptera: Cercopidae). Rev Colomb Entomol 20:43–46
Badilla F, Toledo JC, Barreno C (1996) Pathogencity of Metarhizium anisopliae on Aeneolamia albofasciata and Prosapia spp. (Homoptera: Cercopidae) adult insects in sugarcane in Escuintla, Guatemala. Manejo Integrado de Plagas 42:39–44
Becerra Velásquez V, Paredes Cárcamo M, Rojo Meriño C, France Iglesias A, Franco Durán J (2007) Intraspecific differentiation of Chilean isolates of the entomopathogenic fungus Metarhizium anisopliae var. anisopliae as revealed by RAPD, SSR and ITS markers. Genet Mol Biol 30:89–99
Bischoff JF, Rehner SA, Humber RA (2006) Metarhizium frigidum sp. nov.: a cryptic species of M. anisopliae and a member of the M. flavoviridae complex. Mycologia 98:737–745
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101:512–530
Burdon JJ, Silk J (1997) Sources and patterns of diversity in plant-pathogenic fungi. Phytopathology 87:664–669
Castrillo LA, Griggs MH, Vandenberg JD (2004) Vegetative compatibility groups in indigenous and mass-released strains of the entomopathogenic fungus Beauveria bassiana: likelihood of recombination in the field. J Invertebr Pathol 86:26–37
Clement M, Posada D, Crandall K (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660
de la Torres CM, Cortez MH, Ortiz GCF, Capello GS, de la Cruz PA (2013) Caracterización de aislamientos nativos de Metarhizium anisopliae y su patogenicidad hacia Aeneolamia postica, en Tabasco, México. Rev Colomb Entomol 39:40–46
Deacon JW (1997) Modern Mycology, 3rd. ed. Blackwell Science Ltd. Cambridge, p 303
Dimbi S, Maniana NK, Lux SA, Mueke JM (2004) Effect of constant temperatures on germination, radial growth and virulence of Metarhizium anisopliae to three species of African tephritid fruit flies. BioControl 49:83–94
Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS (1991) ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acid Res 19:4008
Ekesi S, Maniania NK, Ampong-Nyarko K (1999) Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci Tech 9:177–185
FAOSTAT (2012) Final 2012 data and preliminary 2013 data for 5 major commodity aggregates. http://faostat.fao.org/site/339/default.aspx/ Accessed 31 Jul 2014.
Fargues J, Bon MC, Manguin S, Couteaudier Y (2002) Genetic variability among Paecilomyces fumosoroseus isolates from various geographical and host insect origins based on the rDNA-ITS regions. Mycol Res 106:1066–1074
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Freed S, Jin FL, Ren SX (2011) Determination of genetic variability among the isolates of Metarhizium anisopliae var. anisopliae form different geographical origins. World J Microbiol Biotechnol 27:359–370
Garcia DA, Bustillo AE, Castro V, Arenas JB (2012) Selection of entomopathogenic fungi to control spittlebugs (Hemiptera: Cercopidae) of sugar cane in Colombia. Rev Colomb Entomol 38:252–259
Gómez LA (2007) Manejo del salivazo Aeneolamia varia en cultivos de caña de azúcar en el valle del río Cauca. Cenicaña (Colombia). Carta Trimestral 29:10–17
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Humber RA (2012) Identification of entomopathogenic fungi. In: Lacey LA (ed) Manual of techniques in invertebrate pathology, 2nd edn. Academic Press, Inc, California, pp 151–187
Inglis GD, Goettel MS, Strasser H, Butt TM (2001) Use of hyphomycetous fungi for managing insect pests. In: Butt TM, Jackson CW, Magan N (eds) Fungi as biocontrol agents: progress problems and potential. CAB International, Wallingford, pp 23–70
Kenward MG (1987) A method for comparing profiles of repeated measurements. Appl Stat 36:296–308
Kepler RM, Humber RA, Bischoff JF (2014) Clarification of generic and species boundaries for Metarhizium and related fungi through multigene phylogenetics. Mycologia 106:811–829
Li Z, Alves SB, Roberts DW, Fan M, Jr Delalibera I, Tang J, Lopes RB, Faria M, Rangel DEN (2010) Biological control of insects in Brazil and China: history, current programs and reasons for their successes using entomopathogenic fungi. Biocontrol Sci Tech 20:117–136
Matabanchoy Solarte JA, Bustillo Pardey AE, Castro Valderrama U, Mesa Cobo NC, Moreno Gil CA (2012) Eficacia de Metarhizium anisopliae para controlar Aeneolamia varia (Hemiptera: Cercopidae), en caña de azúcar. Rev Colomb Entomol 38:177–181
Meyling NV, Eilenberg J (2007) Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol Control 43:145–155
Meyling NV, Lübeck M, Buckley EP, Eilenberg J, Rehner SA (2009) Community composition, host range and genetic structure of the fungal entomopathogen Beauveria in adjoining agricultural and seminatural habitats. Mol Ecol 18:1282–1293
Ormond EL, Thomas APM, Pugh PJA, Pell JK, Roy HE (2010) A fungal pathogen in time and space: the population dynamics of Beauveria bassiana in a conifer forest. FEMS Microbiol Ecol 74:146–154
Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2005) GenStat for Windows (8th Edition) Introduction. VSN International, Hemel Hempstead
Peck D, Castro U, Lopez F, Morales AR, Rodriguez J (2001) First record of the sugar cane and forage grass pest, Prosapia simulans (Homoptera; Cercopidae) from South America. Fla Entomol 84:402–409
Read ND, Fleißner A, Roca MG, Glass NL (2010) Hyphal fusion. In: Borkovich KA, Ebbole DJ (eds) Cellular and molecular biology of filamentous fungi. American Society for Microbiology Press, Washington DC, pp 260–273
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tanada Y, Kaya H (1993) Insect pathology. Academic, San Diego, p 666
Templeton AR, Crandall KA, Sing CF (1992) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132:619–633
Thompson V (2004) Associative nitrogen fixation, C4 photosynthesis, and the evolution of spittlebugs (Hemiptera: Cercopidae) as major pests of neotropical sugarcane and forage grasses. Bull Entomol Res 94:189–200
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vega FE, Meyling NV, Luangsa-ard JJ, Blackwell M (2012) Fungal entomopathogens. In: Vega FE, Kaya HK (eds) Inset pathology, 2nd edn. Elsevier Inc, London, pp 171–220
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Wilcox D, Dove B, McDavid D, Grenn D (2002) UTHSCSA Image Tool for Windows v. 3.0. The University of Texas Health Science Center, San Antonio. http://maxrad6.uthscsa.edu
Acknowledgments
CHD received a scholarship from CONACYT, Mexico. This research was funded by the project ‘Diseño de un programa contemporáneo de manejo integrado de mosca pinta en caña de azúcar’, granted by Fundación Produce Veracruz-COFUPRO-SAGARPA, Mexico. Grant No. 2009-056.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Fernando L Cônsoli – ESALQ/USP
Rights and permissions
About this article
Cite this article
Hernández-Domínguez, C., Guzmán-Franco, A.W., Carrillo-Benítez, M.G. et al. Specific Diversity of Metarhizium Isolates Infecting Aeneolamia spp. (Hemiptera: Cercopidae) in Sugarcane Plantations. Neotrop Entomol 45, 80–87 (2016). https://doi.org/10.1007/s13744-015-0337-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-015-0337-y