Abstract
The particulate pollution problem is very complex due to the diversity of sources, the chemical composition of the effluents, the size of the particles and their evolution (gas-particle conversion). A pressurized solvent extraction method "PSE" for polycyclic aromatic hydrocarbons "PAHs" was developed. Under pressure and at high temperature, it allowed, using a small amount of solvent, a simultaneous extraction of a complex mixture of alkanes and PAHs adsorbed on diesel particulate matter (DPM) in a short time. An experimental central composite design (CCD) was carried out to model the influence of temperature, extraction time and the nature of the solvent (Tetrahydrofuran, Dichloromethane and Chloroform) on the extraction yields in order to identify the influential factors and their possible interaction. Due to the results of this study, the extraction yields of the heaviest PAHs with five or six aromatic rings did not exceed 30%. However, the aromatic solvents alone demonstrated satisfactory desorption of the heavier PAHs (3–4 rings) from the diesel particulate matter within 10 min. Pyridine, with its aromatic and basic character, provides the best performance. Finally, the addition of diethylamine to pyridine, in the proportions 1/6, allowed the quantitative extraction of all the PAHs studied (including the heaviest rings 5–6) and of the alkanes with extraction recoveries of more than 89%. The method developed provides a rapid and reliable method for the identification and quantification of alkanes and PAHs adsorbed from highly refractory matrices such as diesel nanoparticulates.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, pollution has continued to have a negative impact on humans and their environment. Stationary human activities, such as heating and industry, but also mobile activities such as road, river and air traffic are the main contributors to particulate pollution. These pollutants are then widely distributed in all environmental compartments: air, water, sediments, soil and plants, leading to the contamination of aquatic and terrestrial species.
The development of industrial activities and transport has progressively led to an increase in anthropogenic emissions [1,2,3]. The emissions from engines, both gaseous and particulate, once emitted into the atmosphere, pose a threat to human health [4,5,6]. Today diesel particles constitute the largest share of airborne dust, especially in urban areas. Particles emitted by the exhaust pipes of motor vehicles are very small [1, 2, 4] particularly diesel particles which represent more than 80% of all particles emitted by traffic [4, 7]. All recent studies suggest that these particles induce by their size and chemical composition a more noticeable pollution than that associated with industrial combustion processes and traditional residential heating [2, 5, 8].
Diesel particulate physical, chemical and toxic quality is very complex and depends on multiple factors such as the type of engine, the fuel used, additives, the constraints of the engine, its temperature and many others [3, 5, 9]. Polycyclic aromatic hydrocarbons are major organic compounds simultaneously present in the exhaust gas and soot of diesel engines [2, 6, 10]. These pollutants are widely distributed in all environmental compartments and therefore represent a serious problem, but their quantification at low levels in complex matrices is difficult [2, 11, 12]. Some of the PAHs are potential mutagens and carcinogens and are probably the major culprits in lung cancer prevalence [4, 5, 13] although other PAHs derivatives might be responsible for greater carcinogenic activity [1, 10, 13, 14]. For this reason, the US Environmental Protection Agency (US-EPA) and the European Environment Agency now consider 16 of them as priority pollutants [15]. Therefore, there is a need to establish monitoring systems for aromatic hydrocarbons in the environment.
The adsorption of these carbonaceous and aromatic compounds is highly energetic [3, 16]; which proves that these PAHs are therefore very resistant to conventional extraction requiring the development of drastic extraction methods, coupled with very sensitive chromatographic analysis techniques [3, 17,18,19,20]. Usually, the extraction of solid matrices is conducted by soxhlet, ultrasound, microwave-assisted extraction or pressurized solvent extraction (PSE) [10]. Although these methods are currently the most efficient for PAH extraction, they have some inconveniences and in particular a long operating time and the use of large quantities of organic solvents. In addition, some methods, such as microwave extraction, require a high investment and maintenance cost. Pressurized solvent extraction (PSE) is an alternative automated extraction method that offers different extraction parameters. The use of PSE has increased since its adoption as the official US-EPA method for persistent organic pollutants in many environmental solid samples [21]. Also, operating at higher temperature increases the ability of solvent to solubilize the analyte, decreases the viscosity of liquid solvents, thus allowing better penetrability of the solvent into the matrices, whereas the high pressure, in keeping the solvent below its boiling point, ensures the safety of the extraction process. By using high temperatures and pressures, PSE reduces solvent consumption compared to other methods. The number of publications concerning this technique has increased exponentially for the extraction of organic samples such as PAHs, PCBs, dioxins, pharmaceuticals and pesticides [22]. Therefore, PSE is a widely used technique in the treatment of complex environmental matrices, such as sediments [20], airborne particles [23], coal ash [24], soils [22], biological samples [25] and foods [22], …etc.). Despite the toxicity and dangerousness of the solvents used (hexane, dichloromethane, toluene …) and even mixtures of solvents, it remains the most used due to their interesting efficiency. Due to environmental concerns related to the organic solvents used, scientists have tested alternative solvents such as ionic liquids [26] and deep eutectic solvents [27], which have become the better choice than traditional organic solvents for different environmental matrices. However, for diesel particulates, little research has been conducted on the analysis of polycyclic aromatic hydrocarbons and their derivatives.
In this research, a promising technique was developed using pressurized solvent extraction due to its simplicity, efficiency and fast performance with highly sensitive chromatographic analysis (GC/MS) to extract polycyclic aromatic hydrocarbons and heavy alkanes, which are adsorbed onto soot particles resulting from diesel engine combustion. The optimum operating conditions of PSE were investigated using response surface methodology (RSM), using a central composite design to evaluate the influencing factors as well as possible interactions. Also, this research will provide a new extraction approach, using selective and binary solvents (pyridine, diethylamine, toluene…) to obtain higher amounts of soluble organic substances (heavy alkanes, PAHs) from a highly refractory matrix (diesel particles).
Experimental
Solvents and samples
A solution of 21 PAHs and n-alkanes was prepared at a concentration of 100 µg mL−1 for each component diluted in toluene: Anthracene (ANT, 98% purity), Acenaphthene (ACE, 98%), Benzo(e)pyrene (B(e)PYR, 98%), Biphenyl (BIPH, 98%), Benzo(k)fluoranthene (B(k)FLT, 98%), Benzo(a)pyrene (B(a)PYR, 97%), Dibenzo(a,h)anthracene (DB(a,h)ANT, 98%), Heneicosane (HENEI, 99%), Naphthalene (NAPH, 99%), Phenanthrene (PHEN, 96%), Fluorene (FLUO, 99%), Fluoranthene (FLT, 98%), Pyrene (PYR, 98%), Tetracosan (TETRACO, 99%) and Triacontane (TRIACON, 99%) were all supplied by Sigma-Aldrich.
Acenaphthylene (ACTY, 97%), Chrysene (CHRY, 97%) and Indeno(1,2,3,cd)pyrene (I(1,2,3,cd)PYR, 97%) were acquired from Interchim.
Benzo(b)fluoranthene (B(b)FLT, 98%), Benzo(a)anthracene (B(a)ANT, 98%) and Benzo(g,h,i)perylene (B(g,h,i)PER, 98%) were procured from Supelco.
Chloroform, acetonitrile, tetrahydrofuran (THF), diethylamine, pyridine, high purity, were obtained from Accros Organics. Methylene chloride and toluene, HPLC grade, were purchased from Sigma-Aldrich. The sand, washed with sulphuric acid, was supplied by VWR Chemicals.
The diesel particulate matter (DPM) used for extraction by PSE was provided by CERTAM (Centre d'Etude et de Recherche Technologique en Aérothermique et Moteurs—France), samples were collected directly at the outlet of a diesel engine from a cordierite filtration system. The soot particles were stored in a closed bottle after collection for use in the later applications.
GC/MS analysis
All analyses were performed with a Hewlett-Packard HP 5980 Series II gas chromatograph equipped with a non-fractionating injector (purge time: 2.5 min, purge rate: 60 mL min−1). The injector temperature was maintained at 250 °C and the injection volume was 1 μL. The capillary column employed was a DB5-MS column (stationary phase: (5% phenyl)-methylpolysiloxane, dimensions (50 m × 0.25 mm inner diameter, film thickness: 0.25 μm) from J&W Scientific and was operated at a helium flow rate of 0.9 mL min−1.
The chromatograph was coupled to an HP5972 mass spectrometer (Hewlett Packard) with electron impact ionisation at an energy of 70 eV, and the transfer line temperature was maintained at 300 °C. Finally, the solvent delay was controlled to 6 min. The temperature was programmed as follows: 55 °C for 2.5 min, then increased at a rate of 40 °C min−1 to 170 °C, then a second temperature ramp at a rate of 4 °C min−1 to 320 °C, the latter temperature being maintained for 15 min.
Extraction processes
Soxhlet extraction
Diesel particulate matter (20 g) was purified by a Soxhlet device with 100 mL methylene chloride. The boiled solvent was allowed to reflux for 8 h with eight cycles per hour. Three other successive extractions were then conducted under the same conditions, using fresh methylene chloride. The particles were dried by rotary evaporation and ground to homogenize. The cleaned soot was then tested as a blank and analyzed to ensure that all aliphatic and aromatic pollutants were removed. The virgin soot was then stored and used to spike known amounts of PAHs and pure n-alkanes to test the influence of the extraction parameters.
Pressurized solvent extraction (PSE)
Pressurized Solvent Extraction ASE100 (Dionex) extractor was operated at high temperature, maintaining the extracting solvent under pressure (100 bars) to maintain it in a liquid phase. A cellulose filter was placed at the outlet of the extraction cell (V = 10 mL), on which the amount of solid matrix to be extracted was deposited. The cell was placed in the heated chamber. The extraction solvent was injected into the cell to reach the necessary pressure and temperature for extraction, which could take between 5 and 10 min. After the static extraction, the cell was drained. The extract was then transferred to a collection bottle then the cell and the hydraulic system were rinsed by the extraction solvent (purge percentage: 60%). The system was completely purged with a pressurized nitrogen flow (duration: 80 s). All the solutions thus recovered were collected in a single fraction in the collection bottle. This avoided any errors or confusion from one extraction to the next. The extracted volume was reduced by evaporation using a rotary evaporator. In this study, extractions were performed on 100 mg of spiked matrices (virgin soot or sand). The matrices to be extracted were first allowed to remain in contact for 24 h with the spiking solution, i.e. 100 μL of a toluene solution containing the 21 standards, mentioned previously, at the content of 100 μg mL−1.
Data processing
In order to determine the optimal pressure solvent extraction conditions for the 21 analytes studied, a CCD approach coupled with the response surface method (RSM) was performed using jump software, using JMP 55.0 data analysis software (SAS Institute Inc, NC, USA). Statistical significance was checked by the F-test using ANOVA analysis. The accuracy of the fitted polynomial model was assessed by the R2 coefficient. The significant terms of the model were evaluated by the probability value (P-value) at a 95% confidence interval.
Results and discussion
Particulate matter in diesel engine exhaust consists mainly of highly agglomerated solid carbonaceous material and ash, as well as volatile organic compounds and sulphurous, oxygenated … [3, 5, 28]. Diesel particles result from the association of several hundred crystallites randomly oriented with respect to each other. This disordered association is also called a turbostratic structure, which is a semi graphitic structure containing heteroatoms such as oxygen, nitrogen, sulfur or phosphorus [5, 9, 29, 30]. The mechanism of formation of diesel particles during combustion and the chemical structure was shown in Fig. 1.
The mass fraction of each constituent of diesel soot depends on the type of injection system used (direct or indirect), engine regime, vehicle charge, diesel quality, lubricants and additives, atmospheric conditions and driver's conduct [3, 5, 29]. The particles emitted during combustion in a diesel engine are actually complex systems that include diverse compounds [5, 9, 31]:
-
Insoluble grains of soot, generated by combustion;
-
Soluble organic fraction (SOF) composed of aliphatic and aromatic hydrocarbons and oxygen, sulphur and nitrogen derivatives adsorbed or incorporated on the soot;
-
Water-insoluble inorganic fraction: aluminosilicates, Mn, Fe, Cr, Co, Zn, Sn, Pb, …etc.;
-
And finally, a water-soluble inorganic fraction: sulphates, nitrates, …etc.
In this research, the target PAHs were selected not only for their abundance or toxicity (PAHs from the list proposed by the International Agency for Research on Cancer “IARC” or the American Environmental Protection Agency “US-EPA”) [8, 15, 32,33,34] but also for their capacity to be tracers of traffic pollution sources, for example benzo(e)pyrene, characteristic of diesel engine emissions [35, 36]. In addition to PAHs, linear heavy alkanes were added, which are also likely to be present in diesel car emissions [2, 3, 37]. Figure 2 shows the 16 priority PAHs:
Biphenyl, chrysene and benzo(e) pyrene and three heavy alkanes (Heneicosane, Tetracosan and Triacontane) were added to this mixture containing the 16 primary PAHs.
Initially, our objective was to develop an efficient analysis method in order to evaluate and ultimately optimise the technique for extracting these pollutants from the soot emitted during combustion in diesel engines. Considering the diversity and complexity of the compounds adsorbed on these diesel particles, trace analysis requires a sensitive and efficient analytical technique. This is why the GC/MS was used (see Fig. 3). From the total ionic current (TIC), and for a complete mass scan between 29 and 650 amu, it is then possible to consult the corresponding mass spectrum at each point of the chromatogram and to compare it with the spectra of the NIST Mass Spectral Library, for possible identification. In addition, it is also possible to select one or more particular ions by time range: this is the SIM mode for Single Ion Monitoring. This method has the advantage of increasing the sensitivity of the detection, as the background noise is greatly reduced. The gain in sensitivity is obvious in SIM mode: instead of scanning a very wide range of masses, the detector focuses on at most ten masses in different time ranges.
The analysis of the complex test mixture was carried out in two stages, i.e. using two analysis programmes: one for PAHs and the other for aliphatic hydrocarbons. Indeed, since alkanes can be very numerous (not only in diversity but also in quantity) on the surface of soot, they risked masking the identification of PAHs during a simultaneous analysis. Table 1 presents the limits of detection and quantification obtained for each of the compounds in the test mixture when analysed by GC/MS in SIM mode.
The detection limits, established in SIM mode were between 0.15 and 2.5 μg L−1 for PAHs and 30 and 40 μg L−1 for heavy alkanes respectively. For this GC/MS analytical approach, a reproducibility study was carried out for both retention times and quantification. It showed that the fidelity at the retention time level is quite excellent and the precision is between 0.03 and 0.09%. If this analytical method was sufficiently sensitive and robust, it was also suggested that the method of extracting PAHs and heavy alkanes from the soot collected in the exhaust of a diesel engine could be optimized.
Preliminary extraction process
Using PSE, the solvent is heated more rapidly than with conventional heating, allowing samples to be extracted in minutes, compared to several hours with traditional methods [17, 20]. Therefore, this study first evaluated two different extraction processes using virgin soot and sand.
For this, a mixture of 21 components was spiked on 100 mg of the solid matrix (see experimental section) and extracted using hexane (the most commonly used). The extraction was carried out at 100 bars and 100 °C for 20 min. As shown by the results presented in Table 2 a quantitative recovery of heavy alkanes was achieved in both matrices (virgin soot and sand). In contrast recovery efficiencies were not quantitative for the heavier PAHs (2 – 30%) when extracted from diesel particulate matter.
On the other hand, there is clear evidence that recovery efficiency increases quantitatively for PAHs extracted from sand, including the heavier ones, for example from 2% (soot extraction) to 87% (sand extraction) for DB(ah)ANT. These results demonstrate the strong interactions between PAHs and diesel particles. Therefore, it was necessary to optimize the extraction efficiency of alkanes and polyaromatics adsorbed on diesel soot.
Optimization of PSE extraction using conventional solvents
The response surface methodology (RSM) is based on four important phases: experimental design, model development, model validation and condition optimisation. Experimental designs such as Central Composite Designs (CCD) are useful for RSM as they do not require an excessive number of experimental tests [38].
In this optimization, a chemometric approach using a central composite design was chosen not only to identify the interaction between the experimental factors, but also to determine the optimal extraction conditions [39, 40]. The Modelling of pressurized extraction of PAHs permitted initially to identify which factors and interactions between the factors had a significant effect on the experimental response.
However, as the volume of solvent (10 mL) and the pressure (100 bar) are fixed parameters due to the type of equipment used, it was decided to study the influence of the following three factors: temperature (T), extraction time (t) and the nature of the extraction solvent (Slv). It is important to note that the number of static cycles and the rinse volume did not appear to be influential during the preliminary tests.
This design requires 23 trials at two reduced levels (−1 and + 1), six trials at the centre of the experimental domain (0) to evaluate the variance (for a uniform precision design), and six trials at the −α and + α levels in order to obtain a quadratic modelling of the responses. In addition to the parameters to be studied, it was necessary to define the limits of the experimental domain, considering that α = 1.68 with k = 3 factors. Following the experiments, a mathematical modelling of the Yj (%) response (extraction efficiency) was performed for each of the compounds studied, according to equation:
bo being the average of the response, Xk representing the factors studied, Xk Xm their second-order interactions, Xk Xm Xn their third-order interactions, Xk2 the squared factors and bk, m, n… terms are the mean effects of the factors and their interactions and were calculated by the JMP software.
The three solvents were chosen according to their position in L.R. Snyder's triangle of selectivity [41, 42] and considered to develop a single type of interaction force with the solute to be extracted:
-
1.
dipolar solvents, such as dichloromethane,
-
2.
proton-donating solvents like chloroform and finally
-
3.
proton-accepting solvents, such as Tetrahydrofuran “THF”.
In the end, the following solvents were used for the experimental design: chloroform, THF and dichloromethane, encrypted at levels −1, 0 and + 1 respectively. The range of the other factors was determined by preliminary experiences, mentioned above, and according to technical constraints. Table 3 reports the correspondence between reduced values and actual experimental values for the three factors studied.
Statistical analysis and validation of the proposed model
The central composite CCD design was used to model and evaluate the interaction effect. The extraction efficiency (R%) was considered as the analytical response of our model for all studied PAHs and alkanes. Furthermore, to adjust the experimental data obtained to the analytical response and to identify the most important effects and the different interactions of the variables, the experimental results were fitted to a quadratic model, suggested by the fit summary software (ANOVA). However, the adequacy of the adjusted model had to be verified.
These statistical calculations of the model were justified by the values of three indicators: The high Fisher value (F between 35.76 and 105.96) above the critical F, the probability P (P ≤ 0.001) and the coefficient of determination R2 above 0.9485 for all compounds, indicating that the proposed model is significant for all PAHs and alkanes studied. As this value is higher than 0.85, which is required for good model fit [38, 43], then it can be concluded that satisfactory results have been obtained. The very low F value of the lack of fit of 1.03–1.78 implied that the lack of fit is not significant compared to the pure error in the model. In addition, the very satisfactory values of the predicted and fitted R2 illustrated the high accuracy of the model with a small difference, well below 0.2 [43]. This shows that the proposed model has a good predictive ability as well as a good fit.
Analysis of CCD design (3D surface plots)
Statistical significance was checked by the F-test in the same program. The accuracy of the fitted polynomial model was assessed through the R2 coefficient [44]. Furthermore, for the reliability of the model, it is essential to check whether the model has described the process correctly by determining which coefficients can be neglected. The significance of these terms (linear, quadratic and with interactions) was identified by comparing the P-values (< 0.05), which are the variance of a measure from the mean, according to the ratio of the group variance due to error [38, 45]. The significant terms of the model were evaluated by the probability value (P-value) at a 95% confidence interval. The influence of the various factors studied on the extraction of PAHs, and heavy alkanes by PSE is shown in Table 4, which recapitulates the estimated effects at a confidence level of more than 95%. The design used includes 20 experiments where relative standard deviations (RSDs) in the range of 2–9% were found for alkanes and light PAHs. However, they were higher for heavy PAHs (7–14%).
In this table, only some compounds representative of all the constituents of the sample mixture (alkanes, light Haps, semi-volatile PAHs, heavy PAHs) were selected to illustrate the results obtained by this model. Unsurprisingly, the three principal parameters directly influenced extraction yields. However, the nature of the solvent (S) and the extraction time (t) did not have the same impact at all depending on the class of compounds studied. For example, if the increase in extraction time seemed favourable for semi-volatile and heavy PAHs, that was not the case for volatile PAHs, where the estimated effects are negative. As for the temperature (T), this parameter appeared at first view to evolve in a homogeneous way, independent of the class of compounds studied, with an increase in temperature resulting in an increase in the extraction efficiency. However, this trend was found to be minimal for heavy PAHs at very high temperatures. In order to explain and interpret the effects of the factors studied correctly, it seemed essential to take into account their double or triple interactions.
According to the second-order polynomial model derived for each compound, three-dimensional response surface plots were constructed to assess the effect of the factors and their interactions on the response surface. Figure 4 shows the most relevant fitted surface plots for PAHs and alkanes, in which the response was plotted as a function of any two independent variables, as long as the other variables were kept at their central level. Therefore, response surfaces were plotted for some representative compounds of the studied alkanes and PAHs (volatile, semi-volatile and heavy HAPs (see Fig. 4).
Summarizing the main points of this set of figures, it was concluded that:
-
1.
Dichloromethane appears to be the most suitable solvent for the extraction of volatile PAHs, semi-volatile PAHs and heavy alkanes (Fig. 4a, b).
-
2.
Systematically a high temperature favors the extraction of these different classes of products although the temperature should be chosen at the + 1 level (150 °C) and not at the + α level (167 °C) because at very high temperatures the extraction efficiency of heavy PAHs (Fig. 4c) and heavy alkanes (Fig. 4d) decreases.
-
3.
However, the situation is not as straightforward in terms of extraction time. The extraction of heavy alkanes and volatile PAHs, i.e. the least polar compounds of all the products in the studied test mixture, is favored by short times, whereas the extraction of semi-volatile PAHs is higher when its duration is increased. It is therefore necessary to consider a compromise in terms of extraction time, which should be neither too long nor too short.
-
4.
For the extraction of non-volatile PAHs, THF is both optimal and robust, with the optimum for the pair of temperature–time operating parameters being in the middle of the range investigated (Fig. 4c). Unfortunately, at the optimum in this domain, the extraction efficiency of this class of products remains very low (about 25% with the best solvent tested).
Optimization using the desirability function
Therefore, finding the optimal desirable parameters to use the appropriate solvent at its maximum capacity for the extraction of the compounds under study can be very useful for the optimisation of this CCD-generated model. There are several optimisation techniques; the use of desirability functions has advantages such as efficiency, economy and objectivity in the optimisation of multiple response procedures [38, 46].
The operational conditions based on RSM optimization were obtained after ensuring that the fitted model provided good predictability. Each of these separately modelled responses was treated by a desirability function, rather than combining several elementary responses into a more complex objective function [44, 47, 48]. The overall optimum extraction conditions, identified by the maximum desirability, were an extraction temperature of 147 °C and an extraction time of 9.7 min (10 min) and as solvent dichlomethane. The experiments were repeated under the optimal conditions and the predicted extraction yield values under these conditions were obtained experimentally in three repetitions, resulting in a relative error of less than 8.5%, indicating the reliability of the model.
Thus, the results of this first part of the PSE optimization are relatively positive, quantitative extractions were obtained for volatile (80–90%) and semi-volatile PAHs (75–85%), but also for n-alkanes (90–100%) except for the extraction of heavy PAHs, with relatively low yields being obtained for this class of compounds (less than 20% with the best solvent tested).
Use of aromatic and heterocyclic solvents
Following this first study and in view of the unsatisfactory recovery yields for heavy PAHs adsorbed on diesel particles (from four to six cycles), it was proceeded to tests on other aromatic solvents, in particular those with a basic character which showed interesting potential in the context of the optimization of PAH extraction by Soxhlet. The results were presented in Fig. 5.
Among the aromatic solvents tested, pyridine, which is both aromatic and basic, always gave the best extraction rates of PAHs adsorbed on diesels particles (see Fig. 5). Extraction capacity of the three heavy PAHs went from 5 to 15% with dichloromethane, or 20–30% with toluene, to 40–50% with pyridine which is therefore a powerful electron donor π that might have the ability to interfere with π–π* interactions between π-soot surface receptors and PAHs (π-electron donors) [5, 49,50,51]. This is a very interesting result although the extraction yields of this type of compound are far from being quantitative.
Use of binary aromatic solvents
In view of these results, it appears that the aromatic character of the extractant solvent is certainly necessary, but not sufficient. Indeed, pyridine's aromatic character is less pronounced than that of toluene, if we refer to their resonance energy: 94.9 kJ mol−1 for pyridine and close to 156.7 kJ mol−1 for toluene. Besides aromaticity the basic character of the solvent has to be considered. As pyridine is more basic than toluene, an attempt was made to use binary mixtures to identify possible synergistic effects between pyridine and another basic solvent to increase the extraction efficiency of very heavy PAHs (addition of diethylamine).
As shown in Fig. 6, the addition of diethylamine to pyridine in a (1/6) proportion improves the extraction yields of the heavy PAHs, which are the most difficult PAHs to extract, from 40% with pyridine alone to close to 87%. It should be noted that with the binary pyridine diethylamine mixture for all proportions we have achieved recoveries at around 100%. Under these optimal conditions for the extraction of all PAHs, two replicate experiments were attempted to determine the influence of the number of static cycles on extraction yields. Extraction with two consecutive cycles of 5 min each did not in fact bring any improvement in extraction rates. Therefore, a single static cycle of 10 min was assumed.
Following all these results, it appears that the pressurised solvent extraction method “PSE” proved to be quantitative for light and heavy PAHs and heavy alkanes. Following this assessment, it was decided to test this technique in the context of the extraction of naturally polluted diesel particles collected during engine bench tests.
Extraction of PAHs from natural soot from a diesel engine
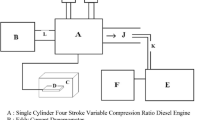
The diesel particulate used provided by CERTAM, were collected directly at the outlet of a diesel engine, from a cordierite filtration system, operating with a four-cylinder direct injection engine (power: 60 kW, torque 270 Nm) operating at low engine speeds (under 2000 rpm). The experimental installation for the collection of soot from a diesel engine is shown in the following Fig. 7:
The analysis of the extract in TIC mode revealed a preponderant presence of alkanes (linear and ramified) and a priori very few PAHs or nitrated PAHs, as shown in Fig. 8.
It should be noted that the background noise of the TIC mode analysis is very high and is mainly due to the presence of ramified alkanes. The extract was then analyzed in SIM mode in order to try to visualise, by category, only PAHs on one hand and heavy alkanes on the other. As shown in Fig. 9, heavy alkanes with 17 to 32 carbons are clearly adsorbed on this natural soot, ranging from 1.6 µg g−1 for C17, the least abundant, to 33.8 µg g−1 for C24, the most abundant.
A number of other similar studies have been reported where the most frequent PAHs were found to be naphthalene, a volatile compound that exists in both the gas and particulate phases, with the presence of alkylated PAHs. Fluoranthene, chrysene, pyrene and phenanthrene were found to be present [52,53,54]. Geller et al. correlated the emission factors of particulate matter components from diesel vehicle emissions with operating conditions: fuel type and vehicle operational and environmental conditions that lead to large uncertainties in PAHs and alkane constituents remain poorly understood. PAHs concentrations in vehicle engine exhaust are much lower in low-load cycles than in high-load cycles, because three- and four-cycle PAHs are present in the gas phase in low-load cycles, but in the particulate phase in high-load cycles. In a typical transient cycle, naphthalene, phenanthrene, anthracene and pyrene in diesel vehicles [55].
In fact, the distribution of heavy alkanes adsorbed on diesel particles is a Gaussian distribution [2, 56,57,58], with a maximum concentration for alkanes with a carbon number between C24 and C26. These results coincide with those found during the analysis of alkanes emitted during incomplete combustion of petroleum derivatives, for which the authors noted a majority concentration of C27 alkane on the collected soot [57]. On the other hand, other studies have found that the most abundant alkane is C17 adsorbed on P2.5 particles [59].
With the analysis of PAHs in SIM mode, there are very few PAHs, mainly naphthalene, biphenyl with contents of a few µg per gram (see Table 5).
Conclusion
Following the optimization of the PSE extraction of all PAHs and alkanes from diesel particles using a central composite design, it appears necessary to work at a temperature of 147 °C and a time of 10 min. The use of non-aromatic solvents has shown a very low capacity to extract PAHs containing five to six condensed aromatic rings from diesel particles although the extraction conditions have been optimized. From the results obtained it can be concluded that the binary mixture of diethylamine/pyridine solvents in the proportions (1/6) is very effective in extracting alkanes and polycyclic aromatic hydrocarbons (volatile, semi-volatile and even heavy PAHs) adsorbed on the diesel particles, compared to the low rates obtained using the non-aromatic solvents tested.
In the case of naturally polluted soot, significant amounts of linear alkanes from C17 to C32 were consistently isolated, as well as some levels of ramified saturated hydrocarbons, but few PAHs: naphthalene (2.19 µg g−1), biphenyl (0.81 µg g−1), fluorene (0.21 µg g−1), phenanthrene (0.62 µg g−1) and anthracene (0.43 µg g−1). The pressurized solvent extraction method developed in this study allowed a quantitative and reproducible recovery of PAHs adsorbed on diesel particles. Moreover, it uses much less solvent than conventional extraction methods with a very short time.
Highlights
-
A GC/MS method was optimized for the analysis of PAHs and alkanes.
-
A Pressurised solvent extraction “PSE” method has been developed which is fast, efficient and cost effective.
-
An experimental design metrology permitted the evaluation of the influential parameters and thus the optimal conditions of extraction by PSE.
-
Quantification of PAHs and alkanes adsorbed on soot Diesel was considered.
Data availability
All data generated or analyzed during this study are included in this published article.
References
G.M. Ceratti da Costa, D.D. Alves, L.M. Cansi, J. Hansen, F. Brochier, D.M. de Quevedo, D.M.M. Osorio, Polycyclic aromatic hydrocarbons (PAH) in atmospheric particles (PM2.5 and PM2.5–10): integrated evaluation of the environmental scenario in urban areas. Water Air Soil Pollut. 232(1), 30 (2021). https://doi.org/10.1007/s11270-020-04967-3
S. Rana, M.R. Saxena, R.K. Maurya, A review on morphology, nanostructure, chemical composition, and number concentration of diesel particulate emissions. Environ. Sci. Pollut. Res. 29, 15432–15489 (2022). https://doi.org/10.1007/s11356-021-15999-5
M. Matti Maricq, Chemical characterization of particulate emissions from dieselengines: a review. J. Aerosol Sci. 38, 1079–1118 (2007). https://doi.org/10.1016/j.jaerosci.2007.08.001
B. Ambade, A. Kumar, L.K. Sahu, Characterization and health risk assessment of particulate bound polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor atmosphere of Central East India. Environ. Sci. Pollut. Res. Int. 28, 56269–56280 (2021). https://doi.org/10.1007/s11356-021-14606-x
H. Burtscher, Physical characterization of particulate emissions from diesel engines: a review. J. Aerosol. Sci. 36(7), 896–932 (2005). https://doi.org/10.1016/j.jaerosci.2004.12.001
A.T. Lawal, Polycyclic aromatic hydrocarbons: a review. Cogent. Environ. Sci. 3(1), 1–89 (2017). https://doi.org/10.1080/23311843.2017.1339841
J.C. Fussell, M. Franklin, D.C. Green, M. Gustafsson, R.M. Harrison, W. Hicks, F.G. Kelly, F. Kishta, M.R. Miller, I.S. Mudway, F. Oroumiyeh, L. Selley, M. Wang, Y. Zhu, A review of road traffic-derived non-exhaust particles: emissions, physicochemical characteristics, health risks, and mitigation measures. Environ. Sci. Technol. 56(11), 6813–6835 (2022). https://doi.org/10.1021/acs.est.2c01072
X. Wang, Y. Wang, Y. Bai, P. Wang, Y. Zhao, An overview of physical and chemical features of diesel exhaust particles. J. Energy Inst. 92(6), 1864–1888 (2019). https://doi.org/10.1016/j.joei.2018.11.006
D. Uy, M. Ford, D. Jayne, A. O’Neill, L. Haack, J. Hangas, M. Jagner, A. Sammut, A. Gangopadhyay, Characterization of gasoline soot and comparison to diesel soot: morphology, chemistry and wear. Tribol. Ind. 80, 198–209 (2014). https://doi.org/10.1016/j.triboint.2014.06.009
M. Nowakowski, I. Rykowska, R. Wolski, P. Andrzejewski, Polycyclic aromatic hydrocarbons (PAHs) and their derivatives (O-PAHs, N-PAHs, OH-PAHs): determination in suspended particulate matter (SPM)—a review. Environ. Process 9(1), 1–27 (2022). https://doi.org/10.1007/s40710-021-00555-7
L. Huang, S.V. Bohac, S.M. Chernyak, S.A. Batterman, Composition and Integrity of PAHs, nitro-PAHs, hopanes, and steranes in diesel exhaust particulate matter. Water Air Soil Pollut. 224(8), 1630 (2013). https://doi.org/10.1007/s11270-013-1630-1
N.A. Ismail, N. Kasmuri, N. Hamzah, Microbial Bioremediation techniques for polycyclic aromatic hydrocarbon (PAHs)—a review. Water Air Soil Pollut. 233, 124 (2022). https://doi.org/10.1007/s11270-022-05598-6
T. De Kok, H. Driece, J. Hogervorst, J. Briede, Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies. Mutat. Res. 613(2–3), 103–122 (2006). https://doi.org/10.1016/j.mrrev.2006.07.001
I. Abbas, G. Badran, A. Verdin, F. Ledoux, M. Roumié, D. Courcot, G. Garçon, Polycyclic aromatic hydrocarbon derivatives in airborne particulate matter: sources, analysis and toxicity. Environ. Chem. Lett. 16(2), 439–475 (2018). https://doi.org/10.1007/s10311-017-0697-0
N. Pichler, F.M. de Souza, V.F. Dos Santos, C.C. Martins, Polycyclic aromatic hydrocarbons (PAHs) in sediments of the amazon coast: evidence for localized sources in contrast to massive regional biomass burning. Environ. Pollut. 268, 115958 (2021). https://doi.org/10.1016/j.envpol.2020.115958
F. Viteri, D. Pezo, Á. Millera, R. Bilbao, M.U. Alzueta, Joint quantification of PAH and oxy-PAH from standard reference materials (urban dust and diesel particulate matter) and diesel soot surrogate by GC-MS. J. Environ. Anal. Chem. 101(12), 1649–1661 (2021). https://doi.org/10.1080/03067319.2019.1691177
S.B. Hawthorne, C.B. Grabanski, E. Martin, D.J. Miller, Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: recovery, selectivity and effects on sample matrix. J. Chromatogr. A 892, 421–433 (2000). https://doi.org/10.1016/s0021-9673(00)00091-1
J.S. Nikolic, V.P. Stankov Jovanovic, M.V. Dimitrijevic, D.J. Cvetkovic, L.P. Stanojevic, L.B. Nikolic, V.D. Mitic, Dispersive solid-phase extraction clean up combined with Soxhlet extraction for the determination of 16 PAHs in soil samples by GC-MS. J. Environ. Anal. Chem. 97(2), 112–123 (2017). https://doi.org/10.1080/03067319.2017.1290801
M. Galmichea, O. Delhommea, Y.N. François, M. Milleta, Environmental analysis of polar and non-polar polycyclic aromatic compounds in airborne particulate matter, settled dust and soot: Instrumental analysis and occurrence. Trends Anal. Chem. 134, 116146 (2020). https://doi.org/10.1016/j.trac.2020.116146
G. Gbeddy, P. Egodawatta, A. Goonetilleke, G. Ayoko, A. Jayarathne, L. Chen, S. Russell, Optimized simultaneous pressurized fluid extraction and in-cell clean-up, and analysis of polycyclic aromatic hydrocarbons (PAHs), and nitro-, carbonyl-, hydroxy-PAHs in solid particles. Anal. Chim. Acta 1125, 19–28 (2020). https://doi.org/10.1016/j.aca.2020.05.021
P. Vazquez-Roig, Y. Picó, Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 71, 55–64 (2015). https://doi.org/10.1016/j.trac.2015.04.014
V. Andreu, Y. Picó, Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 118, 709–721 (2019). https://doi.org/10.1016/j.trac.2019.06.038
I. Kim, S. Lee, S.D. Kim, Determination of toxic organic pollutants in fine particulate matter using selective pressurized liquid extraction and gas chromatography–tandem mass spectrometry. J. Chromatogr. A 1590, 39–46 (2019). https://doi.org/10.1016/j.chroma.2019.01.009
J.S.S. Pinto, L.M. Assis, M.A. Andrade, F.M. Lanças, Pressurized liquid extraction of Brazilian coal followed by the extracts characterization by gas chromatography coupled to mass spectrometry. J. Chromatogr. Sci. 56(8), 761–769 (2018). https://doi.org/10.1093/chromsci/bmy051
P.M. Santos, M. del Nogal Sánchez, J.L.P. Pavón, B.M. Cordero, Determination of polycyclic aromatic hydrocarbons in human biological samples: a critical review. TrAC Trends Anal. Chem. 113, 194–209 (2019). https://doi.org/10.1016/j.trac.2019.02.010
A. Damokhi, S. Yousefinejad, R. Yarmohammadi, S. Jafari, Ionic liquids in biological monitoring for exposure assessments. J. Mol. Liq. 344, 117732 (2021). https://doi.org/10.1016/j.molliq.2021.117732
Z. Gholami, M.H. Marhamatizadeh, S. Yousefinejad, M. Rashedinia, S.M. Mazloomi, Vortex-assisted dispersive liquid-liquid microextraction based on hydrophobic deep eutectic solvent for the simultaneous identification of eight synthetic dyes in jellies and drinks using HPLC-PDA. Microchem. J. 170, 106671 (2021). https://doi.org/10.1016/j.microc.2021.106671
H.A. Michelsen, M.B. Colket, P.E. Bengtsson, A. D’Anna, P. Desgroux, B.S. Haynes, J. Houston Miller, G.J. Nathan, H. Pitsch, H. Wang, A review of terminology used to describe soot formation and evolution under combustion and pyrolytic conditions. ACS Nano 14(10), 12470–12490 (2020). https://doi.org/10.1021/acsnano.0c06226
S. Mohankumar, P. Senthilkumar, Particulate matter formation and its control methodologies for diesel engine: a comprehensive review. Renew. Sustain. Energy Rev. 80, 1227–1238 (2017). https://doi.org/10.1016/j.rser.2017.05.133
Y. Guo, Z. Ristovski, E. Graham, S. Stevanovic, P. Verma, M. Jafari, B. Miljevic, R. Brown, The correlation between diesel soot chemical structure and reactivity. Carbon 161, 736–749 (2020). https://doi.org/10.1016/j.carbon.2020.01.061
S. Rana, M.R. Saxena, R.K. Maurya, A review on morphology, nanostructure, chemical composition, and number concentration of diesel particulate emissions. Environ. Sci. Pollut. Res. 29(11), 15432–15489 (2022). https://doi.org/10.1016/B978-0-323-90875-7.00020-4
Z. Zelinkova, T. Wenzl, The occurrence of 16 EPA PAHs in food—a review. Polycycl. Aromat. Comp. 35(2–4), 248–284 (2015). https://doi.org/10.1080/10406638.2014.918550
L.H. Keith, The source of U.S. EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Compd. 35(2–4), 147–160 (2014). https://doi.org/10.1080/10406638.2014.892886
Z. Zelinkova, T. Wenzl, The occurrence of 16 EPA PAHs in food—a review. Polycycl. Aromat. Compd. 35(2–4), 248–284 (2015). https://doi.org/10.1080/10406638.2014.918550
K. Shibata, K. Enya, N. Ishikawa, K. Sakamoto, EC/OC and PAHs emissions from a modern diesel engine with DPF regeneration fueled by 10% RME biodiesel. Aerosol. Air Qual. Res. 19, 1765–1774 (2019). https://doi.org/10.4209/aaqr.2018.12.0476
S. Arias, F. Molina, R. Palacio, D. López, J.R. Agudelo, Assessment of carbonyl and PAH emissions in an automotive diesel engine fueled with butanol and renewable diesel fuel blends. Fuel 316, 123290 (2022). https://doi.org/10.1016/j.fuel.2022.123290
Y. Su, F. Xie, W. Hong, X. Li, T. Hu, Experimental study of particulate emission characteristics from a gasoline direct injection engine during starting process. Int. J. Automot. Technol. 20(2), 411–421 (2019). https://doi.org/10.1007/s12239-019-0040-9
Z. Kalantar, S. Ghanavati Nasab, Modeling and optimizing Cd (II) ions adsorption onto Corn Silk/Zeolite-Y composite from industrial effluents applying response surface methodology: Isotherm, kinetic, and reusability studies. J. Iran. Chem. Soc. 19(10), 4209–4221 (2022). https://doi.org/10.1007/s13738-022-02594-9
M. Asadi, S. Shahabuddin, A. Mollahosseini, R. Saidur, Electrospun magnetic zeolite/polyacrylonitrile nanofibers for extraction of PAHs from waste water: optimized with central composite design. J. Inorg. Organomet. Polym. 29, 1057–1066 (2019). https://doi.org/10.1007/s10904-018-1027-0
A. Dizaj Khalili, A. Ghaemi, Utilization of response surface methodology, kinetic and thermodynamic studies on cadmium adsorption from aqueous solution by steel slag. J. Iran. Chem. Soc. 18, 3031–3045 (2021). https://doi.org/10.1007/s13738-021-02248-2
E. Lesellier, Σpider diagram: a universal and versatile approach for system comparison and classification: Application to solvent properties. J. Chromatogr. A 1389, 49–64 (2015). https://doi.org/10.1016/j.chroma.2015.02.017
A. Guidea, C. Sârbu, Fuzzy characterization and classification of solvents according to their polarity and selectivity. A comparison with the Snyder approach. J. Liq. Chromatogr. Relat. 43(9–10), 336–343 (2020). https://doi.org/10.1080/10826076.2020.1725550
S. Abbaszadeh, S. Yousefinejad, S. Jafari, E. Soleimani, In-syringe ionic liquid-dispersive liquid–liquid microextraction coupled with HPLC for the determination of trans, trans-muconic acid in human urine sample. J. Sep. Sci. 44(16), 3126–3136 (2021). https://doi.org/10.1002/jssc.202100044
M.A. Fouad, S.A. Elsabour, E.F. Elkady, H.M. Elshazly, Design of experiment (DOE), multiple response optimization and utilizing the desirability function in the simultaneous HPLC separation of five antihypertensive drugs. J. Iran. Chem. Soc. 19(1), 269–282 (2022). https://doi.org/10.1007/s13738-021-02316-7
M.A. Hassan, A.A. Amooey, S. Ghasemi, A. Azizzadeh, Response surface methodology optimized adsorptive removal of the lead (II) ion from aqueous solution using reduced graphene oxide/zeolitic imidazolate framework-67. J. Iran. Chem. Soc. 20(1), 57–68 (2023). https://doi.org/10.1007/s13738-022-02643-3
M.A. Bezerra, S.L.C. Ferreira, C.G. Novaes, A.M.P. Dos Santos, G.S. Valasques, U.M.F. da Mata Cerqueira, J.P. dos Santos Alves, Simultaneous optimization of multiple responses and its application in analytical chemistry–a review. Talanta 194, 941–959 (2019). https://doi.org/10.1016/j.talanta.2018.10.088
L.V. Candioti, M.M. De Zan, M.S. Cámara, H.C. Goicoechea, Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 124, 123–138 (2014). https://doi.org/10.1016/j.talanta.2014.01.034
D. Ramutshatsha-Makhwedzha, P.G. Ndungu, P.N. Nomngongo, Adsorptive removal of major and trace metal ions from synthetic saline and real seawater samples onto magnetic zeolite nanocomposite: application of multicomponent fixed-bed column adsorption. J. Iran. Chem. Soc. 19(7), 2949–2961 (2022). https://doi.org/10.1007/s13738-022-02506-x
R. Abrantes, J.V. Assunçao, C.R. Pesquero, Emission of polycyclic aromatic hydrocarbons from light-duty diesel vehicles exhaust. Atmos. Environ. 38(11), 1631–1640 (2004). https://doi.org/10.1016/j.atmosenv.2003.11.012
S.M. Correa, G. Arbilla, Aromatic hydrocarbons emissions in diesel and biodiesel exhaust. Atmos. Environ. 40(35), 6821–6826 (2006). https://doi.org/10.1016/j.atmosenv.2006.05.068
J.J. Pignatello, Dynamic interactions of natural organic matter and organic compounds. J. Soils Sediments 12(8), 1241–1256 (2012). https://doi.org/10.1007/s11368-012-0490-4
C.A. Alves, A.M.P. Vicente, A.I. Calvo, D. Baumgardner, F. Amato, X. Querol, M. Gustafsson, Physical and chemical properties of non-exhaust particles generated from wear between pavements and tyres. Atmos. Environ. 224, 117252 (2020). https://doi.org/10.1016/j.atmosenv.2019.117252
R. Li, Y. Han, L. Wang, Y. Shang, Y. Chen, Differences in oxidative potential of black carbon from three combustion emission sources in China. J. Environ. Manage 240, 57–65 (2019). https://doi.org/10.1016/j.jenvman.2019.03.070
Y. Liu, C.K. Chan, The oxidative potential of fresh and aged elemental carbon-containing airborne particles: a review. Environ. Sci. Process Impacts 24, 525–546 (2022). https://doi.org/10.1039/D1EM00497B
M.D. Geller, L. Ntziachristos, A. Mamakos, Z. Samaras, D.A. Schmitz, J.R. Froines, C. Sioutas, Physicochemical and redox characteristics of particulate matter (PM) emitted from gasoline and diesel passenger cars. Atmos. Environ. 40(36), 6988–7004 (2006). https://doi.org/10.1016/j.atmosenv.2006.06.018
Z.A. Mansurov, soot formation in combustion processes-review. Combust. Explos. Shock Waves 41(6), 727–744 (2005). https://doi.org/10.1007/s10573-005-0083-2
S. Caumo, R.E. Bruns, P.C. Vasconcellos, Variation of the distribution of atmospheric n-alkanes emitted by different fuels’ combustion. Atmosphere 11(6), 643 (2020). https://doi.org/10.3390/atmos11060643
W. Li, Y. Peng, Z. Bai, Distributions and sources of n-alkanes in PM2.5 at urban, industrial and coastal sites in Tianjin China. J. Environ. Sci. 22(10), 1551–1557 (2010). https://doi.org/10.1016/S1001-0742(09)60288-6
R. Lyu, Z. Shi, M.S. Alam, X. Wu, D. Liu, T.V. Vu, C. Stark, R.P. Fu, Y. Fenga, R.M. Harrisonb, Alkanes and aliphatic carbonyl compounds in wintertime PM2.5 in Beijing, China. Atmos. Environ. 202, 244–255 (2019). https://doi.org/10.1016/j.atmosenv.2019.01.023
Acknowledgements
We wish to thank CERTAM (centre d’Etudes et de Recherche Technologique en Aérothermique et Moteur, France) for the supply of diesel particulate matter.
Funding
No research grant or external funding was utilized in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oukebdane, K. Pressurized solvent extraction for the determination of alkanes and polycyclic aromatic hydrocarbons by GC/MS in diesel engine emissions: optimization by response surface methodology. J IRAN CHEM SOC 20, 1857–1871 (2023). https://doi.org/10.1007/s13738-023-02802-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02802-0