Abstract
A novel photoelectrocatalytic oxidative desulfurization (PEODS) process by using BiVO4@C3N4 composites deposited on fluorine tin oxide glass as a catalyst is designed. Accordingly, g-C3N4 and BiVO4@C3N4 composite with different amounts of BiVO4 (72%, 56%, and 39%) were prepared and used in a biphasic system. Also, photocatalytic oxidative desulfurization (PODS) and electrocatalytic oxidative desulfurization (EODS) systems are studied and compared to PEODS. The BiVO4@C3N472 (72% BiVO4) sample showed the best results compared to other composites in PODS. The visible-light sensitivity of the photocatalysts was investigated. This system was studied under different reaction conditions such as reaction times, temperatures, amounts of the oxidizing agent, extractants, catalyst amounts, and solvents. By optimizing the reaction parameters, DBT removal reached 91%, 54%, and 75% for PEODS, EODS, and PEODS, respectively. The stability of the catalyst was investigated by recycling the catalyst at four successive reactions with the same efficiency. By the addition of radical scavengers, the mechanism of the reaction was studied and the desulfurization decreased to 67%, 72%, and 64% for BQ, EDTA, and isopropanol, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently due to the increasing environmental crisis in the world caused by sulfur-based compounds, governments have decreased the amount of allowed sulfur content in oil products [1,2,3,4]. There are lots of desulfurization techniques to remove sulfur-based compounds. One promising procedure for desulfurizing aliphatic compounds is hydrodesulfurization, but this method does not properly on aromatics compounds [5, 6]. Accordingly, some other desulfurization techniques such as oxidative desulfurization have been developed and scientists are working on different types of catalysts, such as photocatalysts to increase the desulfurization amount [7,8,9]. Photocatalytic oxidative desulfurization (PODS) is an approach that uses photocatalysts to oxidize sulfur-based chemicals to a more polar sulfoxide or sulfone compounds and removes them by liquid extraction method [10,11,12]. However, one of the problems with photocatalysts is charge recombination which can be solved by the addition of electrical bias. Scientists have recently studied the effect of electrical bias on photocatalytic systems, and they showed that these systems have higher efficiency than traditional photocatalytic systems so photoelectrocatalytic (PEC) systems are designed [13]. These systems need two electrodes and the photocatalyst should be one of them [14,15,16]. TiO2-based photocatalysts are one the most studied photocatalysts because of their advantages such as great photocatalytic activity exposed to UV, low cost, and nontoxicity [17,18,19,20]. Zhang et al. [21] achieved 70% desulfurization with TiO2/bamboo charcoal, and H2O2, in their PODS system . Wang et al. [22] used TiO2/SiO2 photocatalyst with UV light at room temperature and reported over 90% sulfur conversion. Goharshadi et al. developed and prepared wood/Ag/Pd and alkaline earth metals doped VO2 nanoparticles photo absorber for solar steam generation of seawater [23, 24]. Recently, researchers are working on Bi-based photocatalysts which exhibit a narrow band gap that causes more activity of these photocatalysts for degradation of organic compounds by absorption of electromagnetic radiation. In addition, the lone pair of electrons at their structure helps them to have hybridized band structures [25,26,27]. This feature reduces the effective mass of holes and electrons and increases the traveling distance for excited carriers, so band gaps of the photocatalysts are decreased which causes higher efficiency in the existence of visible light [28]. Lin et al. [29] achieved 99% thiophene removal by BiVO4-based catalyst, toward visible light and oxidant .
Graphitic carbon nitride (g–C3N4) was described by Berzelius and Liebig for the first time [30]. Since then, researchers are trying to improve the efficiency of g–C3N4 to be used in photocatalytic and electrophoresis systems. Recently, g–C3N4-based photocatalysts are being used for photocatalytic degradation of organic pollutants and to decompose formaldehyde in the air [31,32,33,34]. Recently researchers are developing g-C3N4-based catalysts for photo electrocatalysis. Accordingly, Karimi et al. Prepared an effective photoanode based on FTO/c-TiO2/Bi@g-C3N4 for efficient solar water splitting [35, 36]. But other photocatalysts such as TiO2 and ZnO have better photocatalytic activity than g-C3N4 [37]. BiVO4 composites, due to their sensitivity to visible light, have been studied for different types of chemical reactions [38]. Zhao et al. [39] prepared BiVO4/g-C3N4/NiFe2O4 composite to effectively remove ofloxacin. Wang et al. synthesized a Co3O4-BiVO4/g-C3N4 composite to promote the redox degradation of KN-R [40]. Hu et al. intercalated BiVO4-Bi2WO6 into 2D compressed layered g-C3N4 for boosting catalytic activity to remove RhB and TC [41].
In this survey for the first time, a photo electrocatalytic system is designed for oxidative desulfurization of model fuel with BiVO4@C3N4 photocatalyst. The photocatalyst was hydrothermally prepared by the formation of BiVO4 on g-C3N4 support. The g-C3N4 is chosen as catalyst support because it has photocatalytic activity and it can increase the efficiency of BiVO4. Different composites of BiVO4@C3N4 with different loading of BiVO4 were prepared, their efficiencies at PODS were investigated and various parameters of the reaction were optimized. The mechanism of the reaction is studied and the effectiveness of photocatalytic, photoelectrocatalytic, and electrocatalytic systems on DBT removal was compared. Compared to previous research, the main attempt of this survey is to achieve desulfurization higher than 90% at low temperatures and under visible light as a free source of energy.
Experimental
Chemicals and instruments
BiNO3 and NH4VO3 were purchased from Merck. EDTA, ethanol, n-decane, isooctane, HNO3 (68%), benzoquinone, isopropanol, n-hexane, dicyandiamide, dibenzothiophene (DBT), n-heptane, n-octane, Nafion perfluorinated resin solution 5%, acetonitrile and H2O2 were purchased from Aldrich.
The specific surface area and textural properties of samples were determined using the BET method at 77 K recorded on BELSORP-mini two surface area analyzers while the samples were outgassed at 150 ℃. Field Emission Scanning Electron Microscopy (FESEM) pictures were obtained by Philips X130. Investigation on functional groups was carried out with a JASCO-FTIR 680/PLUS instrument. The sensitivity of the catalysts to visible light was tested with chronoamperometry in a three-electrode cell system under irradiation of a 35 W xenon lamp using Origaflex electrochemical working station (OGF500 potentiostat/galvanostat). The optical band gap of the catalysts was investigated on a JASCO V-670 ultraviolet–visible spectrophotometer. The stability of the catalysts toward heat is studied by Q600 TA in a stream of argon. Philips ES 208S Transmission electron microscopy (TEM) was used to obtain TEM images. Takram C40R10 Raman spectrometer with 532 nm laser was used to obtain Raman spectra. The XRD patterns of samples were recorded on Asenware AW-DX300 using Cu Kα radiation (1.5418 Å) and scanning angle from 2θ = 0.5° to 90° were used to record X-ray diffraction (XRD) spectra.
PODS, PEODS, and EODS tests
The light source of the reactor was a LED lamp (40W, 500 nm < λ < 700 nm). To prepare the model fuel, proper amounts of DBT were dissolved in n-heptane. For a typical test extracting solvent(acetonitrile), and H2O2 as an oxidant were added to the reactor. The system used a magnet stirrer too. The initial and final concentrations of DBT were obtained by UV–Vis (Jasco-570) spectrophotometer at 286 nm. The DBT removal percentage was calculated using the following equation:
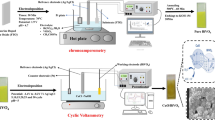
where C0 and C1 are the initial and final concentrations of dibenzothiophene in the model fuel, respectively. Furthermore, electrical bias was added to the previous system to achieve PEODS. The FTO surface with deposited catalysts and Pt electrode were used as an anode, and cathode, respectively. In EODS the whole process was the same as in PEODS but without a lamp. The PEODS system is represented in Scheme 1.
Catalyst preparation
To prepare g-C3N4, 2 g of dicyandiamide were kept in a furnace under 500 °C for 5 h. For the synthesis of BiVO4@C3N4, 250 mg of g-C3N4, was dispersed in 10 mL of deionized water under ultrasonic propagation. To this mixture was added a solution containing 234 mg NH4VO3 (2 mmol) which dissolved in 20 mL HNO3 (2 M) followed by the addition of 970 mg Bi(NO3)3·5H2O (2 mmol) and this suspension was stirred for 20 min and followed by sonication for 1 h at 25 °C. After setting pH at 7 with NH4OH solution, the mixture was kept at 180 °C for 24 h in an autoclave for aging. Then the sample was filtered, washed with ethanol and DI water (three times), and dried in an oven at 80 °C. The samples were denoted as BiVO4@C3N472. Furthermore, two more catalyst samples were synthesized in the same way by using 1 and 0.5 mmol of BiVO4 named BiVO4@C3N439, and BiVO4@C3N456, respectively.
The photocatalysts were deposited on FTO by the drop-casting process. For this purpose, a catalyst suspension was initially prepared by dissolving 1 mL water 0.5 mL Nafion, and 20 mg of each prepared sample. Then 0.1 mL of suspension was dropped on an FTO surface (1 × 2 cm2), and FTO was kept in the oven to remove the solvent.
Results and discussion
Catalyst characterization
Chronoamperometry
After finding the best catalyst for the desulfurization process, the sensitivity of the photocatalysts to visible light was investigated by chronoamperometry under on–off light cycles (Applied potential is 1000 mV versus Ag/AgCl). This part of the survey aims to investigate the photocurrent density (PCD) of the photocatalyst which is determined by the amount of separation between electrons and holes [42]. Figure 1 shows the chronoamperometry of catalysts. The catalysts showed a reaction to on–off light cycles, so it can be concluded that the catalysts are visible light active. In addition, the BiVO4@C3N472 catalyst showed the highest current density in response to the light which is due to the higher efficiency of this catalyst toward visible light compared to pristine C3N4. DRS analysis of catalysts and UV analysis of the PODS process showed the same results.
Optical studies
The Diffuse Reflectance Spectroscopy (DRS) of g-C3N4 and BiVO4@C3N472 photocatalysts are shown in Fig. 3. According to Fig. 2, g-C3N4 shows an absorption band below 300 nm, the region that belongs to UV light. So, this catalyst is not visible light active. On the other hand, the BiVO4@C3N472 sample showed absorption at higher wavelengths (> 400 nm), indicating the synergy between g-C3N4 and BiVO4 and the sample can be active under visible light irradiation. According to the Tauc plot of the catalysts (Inset Fig. 3), BiVO4@C3N472 has a lower optical bandgap than pristine g-C3N4. There are two reasons for these observations. First, the interaction between BiVO4 and g-C3N4 could decrease the electron density in the conductive band of g-C3N4 as well as the valence band of BiVO4, helping the electrons of the VB to move into the CB of g-C3N4, so, the electrons of CB can be transported into the surface of BiVO4. The second reason is the conjugated structure of g-C3N4 that decreased the optical band gap of the composite compared to pristine BiVO4 [43, 44]. According to the Tauc plots, the optical band gap for C3N4 and BiVO4@C3N472 is 2.8 and 2.5 eV, respectively.
TGA and N 2 adsorption–desorption analysis
According to the TGA profile of the catalyst samples which are shown in Fig. 3, the weight of the g-C3N4 sample gradually decreased from 100 to 550 °C followed by a rapid weight loss at about 600 °C. On the other hand, at the TGA profile of the BiVO4@C3N472 sample, weight loss started at about 400 °C and continued to 550 °C. At higher temperatures, there was no significant change in BiVO4@C3N472 sample weight. By comparing pure g-C3N4 and BiVO4@C3N472 samples, it is clear that pure g-C3N4 was stable below 600 °C but C3N4 content in the composite sample was stable below 500 °C, so it can be concluded that the addition of BiVO4 into g-C3N4 structure changed its structure and decreased its resistance against heat.
Further investigation on the physicochemical properties of the selected catalyst was carried out by a nitrogen adsorption–desorption experiment. According to the results shown in Fig. 4, the samples due to their nonporous character, exhibited type 3 isotherm. Other textural specifications such as the total pore volume, BET-specific surface area, and pore size distribution, are presented in Table 1. The total pore volume of the g-C3N4 and BiVO4@C3N472 is 0.06 m3/g and their BET surface area is 9.46 and 11.54 m2/g respectively.
SEM, EDX, elemental mapping, and, TEM
To investigate the morphologies and microstructures of catalysts, SEM and TEM images of the catalysts were taken and analyzed. As shown in Fig. 5a, b both catalysts exhibit well-defined ultrathin 2D sheet-like morphology. Also, the existence of BiVO4 on the C3N4 is proved by looking at Fig. 6b. The dark spots in the TEM image of BiVO4@C3N472 in Fig. 5c, can be attributed to the BiVO4 nanoparticles formed on the g-C3N4. The study on the purity of the catalysts was achieved by EDX images of g-C3N4 and BiVO4@C3N472. According to the results, Bi, V, and O elements stand for BiVO4, C, and N for g-C3N4. So, it is clear that there is no impurity in the structure of the catalysts. Elemental mapping of C3N4, and BiVO4@C3N472, confirmed the existence of BiVO4 nanoparticles on the different sites of the C3N4 (See supporting Information Fig. S1 and S2).
XRD, FTIR, and Raman spectroscopy
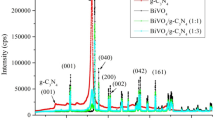
The XRD patterns of catalysts are presented in Fig. 6. The g-C3N4 pattern contains the main peak that appears at 2θ = 28° which are corresponded to the (002) plane in the g-C3N4 structure [45]. The XRD pattern of BiVO4@C3N472 has peaks at 2θ = 18.6, 29.4, 35.1, 39.9, 44.1, 47.4, and 48.6 degrees which are corresponded to (101), (112), (200), (211), (015), (220) and (123) planes of BiVO4, respectively. These peaks are attributed to the monoclinic structure of BiVO4 [46] and the peak at 2θ = 25º belongs to the (002) plane of C3N4. The monoclinic structure of BiVO4 has the highest sensitivity to visible light among the different structures of BiVO4. Other structures are tetragonal zircon and tetragonal scheelite [47, 48].
Figure S3 shows the FTIR spectra of g-C3N4 and BiVO4@C3N472. In the g-C3N4 spectrum, the breathing vibration mode of triazine appeared at 806 cm−1. Two other structural vibrations of C–N and C = N are detected at 1238 and 1361 cm−1 which these signals can be seen in the BiVO4@C3N472 spectrum. In addition, VO bending vibration mode in the BiVO4 structure appeared at 742 cm−1 [49]. Figure 7 shows the Raman spectra of g-C3N4 and BiVO4@C3N472 samples. The spectrum of g-C3N4 contains six broad bands around 1720, 1599, 1475, 1259, 544, and 461 cm−1 which are typical vibrational bands of g-C3N4 [50]. In the spectrum of BiVO4@C3N472, only the signals at 1851, 1614, and 1265 cm−1 can be detected by a small shift. One other peak at 839 cm−1 is assigned to the VO vibrational modes of the BiVO4 structure [51].
Catalyst effect
To investigate the efficiency of catalysts in the desulfurization process, three reactors were filled with model fuel, acetonitrile, and H2O2. Then 10 mg BiVO4@C3N439, BiVO4@C3N456, and BiVO4@C3N472 catalysts were added to each one and the reactions were carried out at room temperature. According to the results, desulfurization reached 46, 51, and 58% for BiVO4@C3N439, BiVO4@C3N456, and BiVO4@C3N472, respectively (See Fig. 8a). So, it can be seen that BiVO4@C3N472 was the most efficient catalyst and further reactions were carried out by using this sample. Furthermore, two other tests were done with pristine g-C3N4 and without a catalyst which the DBT removal was 39 and 28%, respectively. According to the DRS and chronoamperometry results, the BiVO4@C3N472 catalyst has a lower bandgap than other ones, higher absorption of visible light, and the highest current density. On the other hand, the effect of catalyst amount was studied and according to the results (Fig. 1b), the highest desulfurization happened with 40 mg (10,000 ppm) of this sample. According to the results, it can be concluded that by increasing the catalyst concentration to 10,000 ppm the ratio between active sites of the catalyst toward DBT molecules is increased to higher desulfurization percentage occurred. But by increasing catalyst concentration higher than 10,000 ppm, there was no significant change due to the existence of excess catalyst that did not affect DBT removal.
Optimization of PODS
To optimize the PODS process, the effect of different parameters on DBT removal was investigated. In the first step, the concentration of DBT was chosen because, at high concentrations of DBT, the possibility of interaction between each DBT molecule and active sites of the catalyst decreases dramatically. Accordingly, six reactions with DBT concentrations in the range of 50–1000 ppm were tested. According to the results (Fig. 9), the reaction with a 100 ppm sample showed the highest DBT removal. The results show that increasing DBT concentration from 50 to 100 ppm increased DBT removal that is due to the possibility of interaction between active sites of the catalyst, oxidant, and DBT molecules. So, at low concentrations of DBT, the possibility of interaction between DBT molecules and oxidant is low, because the reaction occurs at the interface between polar and nonpolar media of the reactor. At higher concentrations of DBT ratio between the ratio between oxidant and DBT molecules is decreased, because the amount of oxidant is not changed. So DBT removal is decreased at higher concentrations of DBT.
Temperature is another parameter that was investigated. Higher temperature means a higher velocity of DBT molecule so higher possibility of collision to active sites of the catalyst. But in PODS, light is the main stimulant of reaction so it can decrease the importance of temperature. This part of the study was accomplished from 10 to 60 °C. As can be seen from Fig. 10, after 25 °C DBT removal exhibited a small change that can be the result of photocatalytic activity rather than thermal activity mechanism in this PODS process. So, 25 °C was selected as the optimized temperature and considered in further experiments.
As mentioned before higher concentrations of DBT with the same amount of catalyst and oxidants decreased the efficiency of PODS so it is important to find out how the system works in different ratios between oxidant and DBT. Moreover, the molar ratio between H2O2 and DBT is a vital variant for economic reasons and environmental challenges. According to Fig. 11, the best ratio between H2O2 and DBT is 10 to 1, which caused to highest DBT removal. As is expected increasing the H2O2 amount gives higher DBT removal that is due to the higher possibility of the interaction between oxidant and DBT molecules. However, H2O2 can produce water in the reaction, which disturbs the reaction’s progress. So, after the 10 to 1 ratio, there is no significant change in DBT removal.
Since some reactions were performed with different fuel types, the effect of fuel types on DBT conversion, was the other parameter that was investigated. According to Fig. 12, the reaction with n-heptane had the highest DBT removal. The importance of this part of the survey is due to the diversity of hydrocarbons that contain sulfur compounds and each one can have a different interaction with chemicals such as DBT. The results show that lighter hydrocarbons have higher DBT conversion which can be due to the better interaction between DBT and oxidant in lighter hydrocarbon systems.
The key factor of the PODS is the extraction of the oxidated sulfur content because DBT and its sulfone are more soluble in nonpolar and polar solvents, respectively. Figure 13a, b shows extractant type and fuel-to-extractant ratio on DBT removal, respectively. Accordingly, acetonitrile as the extractant solvent showed the best results. Besides, the interaction between acetonitrile and H2O2 can activate H2O2 molecules in the reaction [52]. Some researchers tried to know if there is any relationship between solvent and the percentage of DBT removal. Lu et al. [53] found that for DBT removal in different media, solvents having amide groups such as NMP and DMF have more activity than acetonitrile and in their survey, protic solvents such as methanol and H2O exhibited low efficiency. On the other hand, Ramirez et al. [54] worked on some other solvents and found that the trend is DMF > γ-butyrolactone > 2-ethoxyethanol > acetonitrile .
Figure 14 represents the effect of reaction time on DBT removal. In this part, many reactions with optimized parameters were performed at different times. According to the results, increasing times causes higher DBT removal up to 65 min. After that, there is no change in DBT removal and it seems that the reaction cannot go further. This can be due to the low concentration of DBT at the end of the reaction lowering the possibility of the interaction between DBT and oxidant.
For PEODS tests, catalysts should be deposited on a conductive film. For this purpose, the Fluorine-doped Tin Oxide (FTO) had been chosen as the electrode and BiVO4@C3N472 was deposited by the drop-casting process and a series of experiments were carried out to optimize the PODS system with a new catalyst. To do so, nH2O2/nDBT and the time of the reaction were optimized as important parameters. According to the results represented in Fig. 15, and Fig. 16, respectively. The best nH2O2/nDBT of the reaction is 10 (See Fig. 15).
Optimization of PEODS process
To investigate of electrocatalytic behavior of the catalyst, electrical bias was added to PODS and the PODS system was converted to PEODS. Accordingly, the deposited catalyst on FTO was used as an anode and the cathode of the reactor was platinum. Figure 17 shows the DBT removal percentages with different electrical biases, and the best result was obtained at 3.5 V. Moreover, the effect of n H2O2/n DBT and the time of the reaction on the desulfurization process was investigated. In the optimized situation, reaction time and n H2O2/nDBT were 55 min and 10:1, respectively. The results are presented in Fig. 18 and Fig. 19. Electrical bias is studied and applied to this system because it can prevent the charge recombination process and increase DBT conversion [55].
PEODS, PODS, and EDS comparison
To compare PEODS, PODS, and EDS efficiencies, three experiments were conducted under the same conditions. A closer look at Fig. 20 indicates PEODS has the highest efficiency by 91% DBT conversion. So, it can be concluded that electrical bias helped the PODS system to achieve higher DBT conversion.
Reaction mechanism and catalyst stability
To study the mechanism of the designed desulfurization system, three more reactions by the addition of radical scavengers were performed. The results are represented in Fig. 21. It is clear that by adding isopropyl alcohol, benzoquinone (BQ), and EDTA, DBT conversion decreased to 64%, 67%, and 72%, respectively. The existence of hydroxyl radicals can be investigated by the addition of isopropyl alcohol [56] while, EDTA can be effective on holes [57] and BQ can absorb superoxide radicals [58]. The results prove that in the designed desulfurization system, the reaction goes through the formation of radicals such as hydroxyl and hydroperoxyl. The suggested mechanism is represented in Scheme 2. After exposure of the catalyst to light, electrons move from the VB of the catalyst to its CB, and positive and negative charge is formed on the VB and CB of the catalyst, respectively. Then by the interaction between VB and CB of the catalyst H2O2, hydroperoxyl and hydroxyl radicals are formed at VB and CB of the catalyst, respectively. After that by dehydration, the sulfoxide derivative of DBT is formed. By repeating this process sulfoxide is converted to sulfone.
Reusability is one of the most important aspects of catalysts. In comparison between heterogenous and homogenous catalysts, heterogenous are more reusable due to the facile separation of heterogeneous catalysts. Regeneration and reusability of the BiVO4@C3N472 catalyst were investigated in the desulfurization reaction. The recycling of the catalyst at each reaction was accomplished by washing it with ethanol and water after a cycle. The results of the effectivity of the catalyst at each reaction are shown in Fig. 22. And it can be concluded that this catalyst can be recycled four times.
Conclusion
PEODS, PODS, and EDS systems were used and compared for DBT conversion in model fuel. H2O2 and BiVO4@C3N472 composite were used as oxidizing agents and catalysts respectively. By Addition of electrical bias to PODS and optimizing the reaction, DBT removal reached 91%. DRS and chronoamperometry proved visible light activity of The BiVO4@C3N472 composite. Recyclability tests of the catalyst showed that it can be recycled 4 times. Addition of radical scavengers lowered DBT removal and it was found that radicals such as hydroxyl and hydroperoxyl have an important role in the reaction mechanism. The results of this survey can be the basis for future studies of scientists to develop this technology on an industrial scale. There are lots of advantages to this desulfurization system such as low reaction temperature, consumption of sunlight as free energy, and low charge recombination due to electrical bias, compared to other methods.
References
G.M. Dhar, B. Srinivas, M. Rana, M. Kumar, S. Maity, Catal. Today 86, 45 (2003)
A. Najafi Chermahini, A. Moslemi, G. Mohammadnezhad, Appl. Organomet. Chem. 33, 4877 (2019)
Z.S. Aghbolagh, M.R.K. Khorrami, M.S. Rahmatyan, J. Iranian Chem. Soc. 19, 219 (2022)
N. Jamali, N. Ramezani, M.H. Mousazadeh, Phys. Chem. Res. 9, 637 (2021)
Z. Long, C. Yang, G. Zeng, L. Peng, C. Dai, H. He, Fuel 130, 19 (2014)
Z. Khalilian, A. Najafi Chermahini, M.M. Momeni, J. Najafi Sarpiri, M. Motalebian, J. Porous Mater. 28, 629 (2021)
F.S. Mjalli, O.U. Ahmed, T. Al-Wahaibi, Y. Al-Wahaibi, I.M. AlNashef, Rev. Chem. Eng. 30, 337 (2014)
I. Babich, J. Moulijn, Fuel 82, 607 (2003)
A. Najafi Chermahini, M. Rafiee, S. Shaybanizadeh, J. Porous Mater. 26, 1691 (2019)
X. Lu, X. Li, J. Qian, N. Miao, C. Yao, Z. Chen, J. Alloys Compd. 661, 363 (2016)
B. Moeinifard, A.N. Chermahini, J. Environ. Chem. Eng. 9, 105430 (2021)
A. Moslemi, A.N. Chermahini, J.N. Sarpiri, S. Rezaei, M. Barati, J. Taiwan Inst. Chem. Eng. 97, 237 (2019)
K. Vinodgopal, S. Hotchandani, P.V. Kamat, J. Phys. Chem. 97, 9040 (1993)
R.J. Candal, W.A. Zeltner, M.A. Anderson, Environ. Sci. Technol. 34, 3443 (2000)
J. Highfield, Molecules 20, 6739 (2015)
G.G. Bessegato, T.T. Guaraldo, J.F. de Brito, M.F. Brugnera, M.V.B. Zanoni, Electrocatalysis 6, 415 (2015)
W. Zhu, Y. Xu, H. Li, B. Dai, H. Xu, C. Wang, Y. Chao, H. Liu, Korean J. Chem Eng. 31, 211 (2014)
O. Al-Madanat, Y. AlSalka, W. Ramadan, D.W. Bahnemann, Catalysts 11, 317 (2021)
J. He, P. Wu, L. Chen, H. Lim, M. Hua, L. Lu, Y. Wei, Y. Chao, S. Zhou, W. Zhu, Chem. Eng. J. 416, 129022 (2021)
L. Wang, Y. Ma, D. Xie, M. Zhang, N. Zuo, N. Mominou, C. Jing, Microporous Mesoporous Mater. 323, 111258 (2021)
J. Zhang, D. Zhao, J. Wang, L. Yang, J. Mater. Sci. 44, 3112 (2009)
G. Miao, F. Ye, L. Wu, X. Ren, J. Xiao, Z. Li, H. Wang, J. Hazard. Mater. 300, 426 (2015)
M. Aziznezhad, E.K. Goharshadi, R. Mehrkhah, M.M. Ghafurian, Mater. Res. Bull. 149, 111705 (2022)
K. Goharshadi, S.A. Sajjadi, E.K. Goharshadi, R. Mehrkhah, Mater. Res. Bull. 154, 111916 (2022)
X. Wang, J. Ran, M. Tao, Y. He, Y. Zhang, X. Li, H. Huang, Mater. Sci. Semicond. Process. 41, 317 (2016)
A. Sleight, H.-Y. Chen, A. Ferretti, D. Cox, Mater. Res. Bulletin 14, 1571 (1979)
J.K. Cooper, S. Gul, F.M. Toma, L. Chen, P.-A. Glans, J. Guo, J.W. Ager, J. Yano, I.D. Sharp, Chem. Mater. 26, 5365 (2014)
X. Lin, F. Huang, W. Wang, Z. Shan, J. Shi, Dyes Pigments 78, 39 (2008)
F. Lin, D. Wang, Z. Jiang, Y. Ma, J. Li, R. Li, C. Li, Energy Environ. Sci. 5, 6400 (2012)
K. Maeda, ACS Catalysis. 3(7), 1486–503 (2013)
H. Yan, H. Yang, J. Alloy. Compd. 509, L26 (2011)
J. Fu, J. Yu, C. Jiang, B. Cheng, Adv. Energy Mater. 8, 1701503 (2018)
X. Liu, R. Ma, L. Zhuang, B. Hu, J. Chen, X. Liu, X. Wang, Crit Rev. Environ. Sci. Technol. 51, 751 (2021)
M. Zarei, H. Ahmadzadeh, E.K. Goharshadi, A. Farzaneh, Analytica Chimica Acta 887, 245 (2015)
M. Karimi-Nazarabad, E.K. Goharshadi, H.-S. Sajjadizadeh, J. Phys. Chem. C 126(19), 8327–8336 (2022)
M. Karimi-Nazarabad, E.K. Goharshadi, J. Electroanal. Chem. 904, 115933 (2022)
S.K. Choi, S. Kim, S.K. Lim, H. Park, J. Phys. Chem. C 114, 16475 (2010)
A. Kudo, K. Omori, H. Kato, J. Amer. Chem. Soc. 121, 11459 (1999)
G. Zhao, J. Ding, F. Zhou, X. Chen, L. Wei, Q. Gao, K. Wang, Q. Zhao, Chem. Eng. J. 405, 126704 (2021)
Y. Wang, D. Yu, W. Wang, P. Gao, S. Zhong, L. Zhang, Q. Zhao, B. Liu, Sep. Purif. Technol. 239, 116562 (2020)
H. Hu, W. Kong, J. Wang, C. Liu, Q. Cai, Y. Kong, S. Zhou, Z. Yang, Appl. Surf. Sci. 557, 149796 (2021)
D. Tekin, H. Kiziltas, H. Ungan, J. Mol. Liq. 306, 112905 (2020)
S. Mosleh, M. Rahimi, M. Ghaedi, K. Dashtian, S. Hajati, RSC Adv. 6, 17204 (2016)
S.N.S. Nasir, N.A. Mohamed, M.A. Tukimon, M.F.M. Noh, N.A. Arzaee, M.A.M. Teridi, Phys. B Condens. Matter 604, 412719 (2021)
Y.-J. Bai, B. Lü, Z.-G. Liu, L. Li, D.-L. Cui, X.-G. Xu, Q.-L. Wang, J. Cryst. Growth 247, 505 (2003)
J. Liu, H. Wang, S. Wang, H. Yan, Mater. Sci. Eng. B 104, 36 (2003)
A. Kudo, Int. J. Hydrogen Energy 31, 197 (2006)
A.R. Lim, S.H. Choh, M.S. Jang, J. Phys. Condens. Matter 7, 7309 (1995)
G. Miao, D. Huan, X. Ren, X. Li, Z. Li, J. Xiao, Appl. Catal. B Environ. 192, 72 (2016)
J. Jiang, L. Ou-yang, L. Zhu, A. Zheng, J. Zou, X. Yi, H. Tang, Carbon 80, 213 (2014)
S. Thalluri, C. Martinez-Suarez, A. Virga, N. Russo, G. Saracco, Inter. J. Chem. Eng. Appl. 4, 305 (2013)
Y. Tian, Y. Yao, Y. Zhi, L. Yan, S. Lu, Energy Fuels 29, 618 (2015)
J.L. García-Gutiérrez, G.A. Fuentes, M.E. Hernandez-Teran, P. Garcia, F. Murrieta-Guevara, F. Jiménez-Cruz, Appl. Catal. A Gen. 334, 366 (2008)
L. Ramirez-Verduzco, E. Torres-Garcia, R. Gomez-Quintana, V. Gonzalez-Pena, F. Murrieta-Guevara, Catal. Today 98, 289 (2004)
X. Li, H. Liu, P. Yue, Y. Sun, Environ. Sci. Technol. 34, 4401 (2000)
L.M. Frias Batista, V.K. Meader, K. Romero, K. Kunzler, F. Kabir, A. Bullock, K.M. Tibbetts, J. Phys. Chem. B. 123, 7204 (2019)
A. Galińska, J. Walendziewski, Energy Fuels 19, 1143 (2005)
J.T. Schneider, D.S. Firak, R.R. Ribeiro, P. Peralta-Zamora, Phys. Chem. Chem. Phys. 22, 15723 (2020)
Acknowledgements
We gratefully thank the Isfahan University of Technology for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raeisi, A., Najafi Chermahini, A. & Momeni, M.M. Fabrication of g-C3N4 decorated BiVO4 composites and their application in photoelectrocatalytic and electrocatalytic oxidative desulfurization of dibenzothiophene under visible light irradiation. J IRAN CHEM SOC 20, 1371–1381 (2023). https://doi.org/10.1007/s13738-023-02762-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02762-5