Abstract
Hydrogen sulfide (H2S) is a poisonous gas and corrosive with a characteristic rotten egg smell. Exposure to a higher concentration of H2S gas leads to death. Research groups across the globe have been working on developing a gas sensor composed of novel sensing materials to monitor deadly H2S gas for over a decade. Carbon-based materials such as single-wall carbon nanotubes, multi-wall carbon nanotubes, graphene, and graphene derivatives have been incorporated with metal oxides to improve H2S gas sensing properties. Carbon-based composites have unique physicochemical properties which provide the sensor possessing superior sensitivity, selectivity, stability, quick response time, etc. This review highlights the importance of H2S sensors based on rGO/MOx and CNT/MOx, their enhanced sensitivity and selectivity to H2S.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S) is a colorless, corrosive, and flammable gas characteristic of a foul smell at ppm level. H2S gas is produced naturally by degrading organic matter by microorganism and geothermal activities, including petroleum refining and natural gas processing. It is used as a process gas in heavy water plants and chemical laboratories [1,2,3]. People mainly contact H2S through inhaling, diffusing through the respiratory system, and entering the bloodstream. Exposure to H2S gas at a higher concentration (> 100 ppm) for more than 10 min would lead to poisoning and sudden death. H2S can also produce respiratory illness, nausea, vomiting, and nervous problems at very low concentrations [4, 5]. Accordingly, the detection of H2S is important as safety is a concern. Gas chromatography and mass spectrometry are the major analytical tool for H2S analysis at very low concentrations [6, 7]. These methods are required highly sophisticated instruments, complicated sampling preparation, and a time-consuming process. Conventional portable sensors have been deployed in industries and residential places to monitor H2S gas. However, the conventional sensors are not economical and fail to have stability, fast response, and recovery time [8, 9]. Alternatively, several researchers have developed a sensor based on semiconducting nano-oxides (MOx) such as SnO2 [10], ZnO [11], CuO [12], WO3 [13], MoO3 [14], In2O3 [15], and Fe2O3 [16] for monitoring H2S, because the sensors are easy to fabricate, have high stability and high sensitivity, have simple operation and fast dynamics, and do not require complicated step while fabrication [2]. These sensors work based on the chemoresistance method, the easiest transducing scheme. The chemiresistive transducing scheme measures the sensor’s electrical resistance while interacting with analyte gas molecules in the environment. However, the MOx-based sensor also has some drawbacks, including high operating temperature, less sensing response, and long recovery time [17, 18]. Different methods have been explored to enhance the gas-sensing properties of MOx- based sensors [19,20,21,22,23,24,25,26]. Among them, developing carbon derivatives combined with MOx-based composite has been considered an effective approach to improving H2S gas sensitivity. Carbon-based materials such as carbon nanotubes (CNT), graphene, and reduced graphene oxide (rGO) have been incorporated into metal oxides to enhance the gas-sensing properties [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Carbon-based metal oxide composites act as a sensing layer of the sensor to be more stable, selective, quick response recovery in time and work at low operating temperatures, which indirectly reduces the complicated fabrication steps [25, 42]. Carbon-based composite materials have appeared as promising sensing materials for H2S detection, considering the advantage of electronic properties. Carbon–metal oxide composites have been considered interesting materials for gas-sensing applications for the last decade. The objective of the present review is to give an overview of the H2S sensor based on carbon composite materials and the major role played by carbonaceous material in the sensing performance of the sensor. H2S gas sensing mechanism and gas sensing performance of carbon composites are discussed. The gas sensing mechanism of each carbon-based metal oxide nanostructure type has also been addressed. The promising approaches for sensor fabrication for real field application were highlighted.
rGO/ZnO composite sensor for H2S detection
ZnO has been widely studied as a sensing material for H2S gas detection due to its intrinsic electronic properties (band gap–3.37 eV), excellent chemical/thermal stability, and quick response time. For the last three decades, ZnO has been widely used as a sensing material for detecting a wide range of toxic gases, including volatile organic compounds, NH3, CO, and H2S, due to its intrinsic electronic and optical properties [43,44,45]. Normally, ZnO-based sensors work at temperatures ranging from 400 ℃ to 600 ℃ as they have poor electronic conductivity at low temperature/room temperature. ZnO is often combined with a p-type semiconducting metal oxide to make it a p–n junction interface to obtain a highly sensitive sensor to analyte gas [46,47,48]. Similarly, the n-type ZnO had been combined with p-type rGO to enhance the gas sensing response by forming the p-n heterojunction interface [27]. Doping with reduced graphene oxide (rGO) enhances the host material's electronic properties. rGO generates a charge transport carrier at the junction and creates more surface-active sites for analyte gas molecules. At the same time, optimizing rGO % in ZnO is crucial as it determines the sensing performance. Further, the excess amount of rGO on host materials might change the crystal lattice of host materials, eventually reducing the sensitivity. For instance, Shen et al. synthesized mesoporous and nanocrystalline rGO/ZnO through a hydrothermal process for H2S detection [49]. Mesoporous and nano-crystallinity of the materials were confirmed through BET and XRD analysis. The sensor was fabricated by mixing rGO/ZnO with ethyl cellulose and ethanol to make it a rheological paste and drop coated on an alumina ceramic tube and then dried at 60℃. It was reported that rGO/ZnO (5 wt%) exhibited enhanced sensitivity to ZnO. The sensitivity was 55.91 at 160℃ upon exposure to 50 ppm of H2S, which was 12 times higher than pure ZnO. It was also reported that the sensor's selectivity was achieved depending on the analyte gas polarity, molecular weight, and reactivity. According to this report, the selectivity to H2S was attributed to the polar nature and strong reducing characteristics of the target gas than other interfering gas molecules. Dang and his team prepared rGO/ZnO nanofibers for H2S gas detection. The rGO [50] incorporated into ZnO was carried out by internal and external routes, of which the internal junction of rGO/ZnO showed superior gas sensing performance. The optimum working temperature of the sensor was found to be 350 ℃. The sensor had excellent selectivity, and a high response time (1353) to 1 ppm was achieved when doping with 0.1% rGO content.
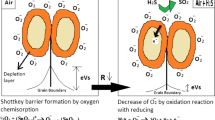
The electron depletion region of ZnO was wider due to the formation of heterojunction between p-type rGO and n-type ZnO. When the sensor composed of rGO/ZnO was exposed to H2S, it reacted with adsorbed oxygen and released electrons back into the conduction band, which increased electron conductivity and contributed to the sensor signal. Further, rGO/ZnO was highly selective to H2S among common interfering gases. The reason is that rGO creates an additional spillover effect on the surface, consequently enhancing the sensor response [51]. However, this justification is inadequate to explain the sensor based on rGO/ZnO, which possesses high sensitivity and selectivity. There should be two additional justifications; first, the bond energy of H2S (363 kJ mol−1) is low and highly reactive to oxygen ions when compared to other reducing gases H2 (386 kJ mol−1) and NH3 (432 kJ mol−1); secondly, the spontaneous sulfuration–desulfuration reaction of ZnO enhances the sensitivity.
Multidimensional (0D, 1D, and 2D) nanostructure has been developed for gas sensor, and energy storage applications as the materials possess a high surface-to-volume ratio, structural defects, active sites, and increased ionic/electronic properties [52, 53]. Tran Viet Cuong and co-workers developed ZnO nanorods (1D) integrated with graphene (2D) film for H2S detection. Photoluminescence yellow emission was attributed to singly ionized oxygen vacancy in ZnO nanorods and the recombination of electron–hole pairs in graphene film. It was reported that the integrated sensor could detect H2S (2 ppm) at room temperature [54]. The operating temperature of most of the ZnO-based sensors was found to be more than 200 ℃. As a result, the sensor would consume more power, which limits real-time application due to risky integration and the risk of gas explosion [55, 56]. To overcome such drawbacks, copper-doped rGO/ZnO nanocomposites have been developed [57]. The morphology, response, and recovery transient of copper-doped ZnO/rGO are shown in Fig. 1.
FE-SEM images of a Cu doped ZnO/rGO and b Response transient plot for Cu doped ZnO/rGO nanocomposite sensor upon exposure to 150 ppm H2S gas at 24 ℃ [57]
Cu-doped ZnO nanoparticles were synthesized using a hydrothermal process, and rGO was deposited by the air-spray technique. The Cu-rGO/ZnO nanocomposites and undoped rGO/ZnO film were tested for gas-sensing properties. It was reported that Cu-doped rGO/ZnO nanocomposite exhibited high selectivity and sensitivity to H2S at room temperature. The improved gas sensing properties of Cu-doped rGO/ZnO could be attributed to Cu and rGO both modifying Schottky barrier height and widening charge depleted region, as shown in Fig. 2.
Schematic illustration of the gas-sensing mechanism and band diagram illustration of the Cu-doped ZnO/RGO nanocomposite sensor [57]
A cost-effective, flexible, wearable gas sensor to function at room temperature is of great interest for e-textiles, the Internet of Things, and wearable electronics application. For instance, ZnO-decorated rGO fibers were developed for NO2 and H2S gas sensing applications [58]. It was reported that ZnO/rGO nanofibers exhibited superior gas sensing performance compared to rGO fibers alone. The enhanced sensitivity was attributed to the ZnO/rGO fibers providing more surface area for the adsorption of gas molecules and catalytic sites for gas molecules interaction. It was reported that ZnO/rGO fibers could detect H2S 8 ppm to 100 ppm and exhibited 8 and 24-fold improved sensitivity to NO2 and H2S. Impedance spectroscopy is considered an effective tool for the evaluation of gas sensitivity of sensors, especially, gas sensors made of metal oxide nanohybrids. Impedance data would help to study the electrical properties of grain bulk, grain boundary, and electrode/sensing layers' interface behavior. Several research articles have been published based on impedance analysis of gas sensors made of metal oxide nanohybrids [59,60,61,62,63,64,65,66,67]. For instance, ZnO/rGO hybrid materials were developed for H2S gas detection, where the gas sensitivity was evaluated using impedance spectroscopy [27]. The Nyquist plot is shown in Fig. 3b, indicating the response of the composite upon exposure to different concentrations of H2S. Different rGO concentrations were incorporated into ZnO nano-oxide, and n-ZnO/rGO-5 exhibited better sensitivity and selectivity to H2S. Flower-shaped ZnO nanoparticles anchored on an rGO sheet, the FESEM image of ZnO/rGO-5 nanocomposite is shown in Fig. 3a. Further, the study reported that the sensor could detect 2- 100 ppm of H2S at 90℃. Nyquist plot data revealed that the grain boundary resistance and barrier height decreased as increasing the gas concentration.
FESEM image of a n-ZnO/rGO and b Nyquist impedance plot of ZnO/rGO-5 nanocomposites exposed to air and various concentrations of H2S gas (2 − 100 ppm) at 90 °C [27]
rGO/β-Ga2O3 composite sensor for H2S detection
Another research paper on rGO supported β-Ga2O3 for sensitive, selective detection of H2S, published by Sridhar et al. [68]. The authors used the impedance technique to evaluate the H2S sensing properties of developed composites. They have reported that the sensor composed of rGO/β-Ga2O3 was able to detect 3 ppm of H2S at 100℃. Impedance results revealed that grain boundary resistance of the sensing layer was highly affected by 3 ppm of H2S compared to other common interfering gases. The authors concluded the incorporation of rGO into β-Ga2O3 creates an additional active surface-active site for H2S adsorption and generates great electron transport properties; subsequently, the sensitivity and selectivity of the sensor were increased.
rGO/SnO2 based sensor for H2S detection
Tin oxide (SnO2) is an n-type semiconductor with a wide gap of 3.62 eV and a wide range of industrial applications. Pristine rGO modified with SnO2 has been developed for H2S gas detection and reported [69]. Polyaniline (PANI) has been used for many applications as the material has electrical, optical, and chemosensitive properties to gas detection. The snO2-based sensor is also used for human halitosis diagnosis. Halitosis is a kind of disease with a foul smell in exhaled breath. A foul smell is caused by the breakdown of food particles in the mouth, eventually releasing the foul smell. This affects health as well as social communication. This kind of halitosis could be diagnosed early with a suitable device. Dongzhi Zhang et al. developed SnO2/rGO/PANI ternary nanocomposite to detect H2S in exhaled breath for halitosis diagnosis in the early stage. The composite was screen printed on a flexible polyethene terephthalate substrate [70]. The sensor response towards H2S was 23.9 for 200 ppb. The composites were characterized using FTIR, which confirmed the presence of all peaks of SnO2, PANI, and rGO. Morphologies of SnO2, PANI, rGO, and SnO2/rGO/PANI samples are shown in Fig. 4. Sensor composed of SnO2/rGO/PANI exposed to different concentrations of H2S 50 ppb to 100 ppm and compared with SnO2/PANI and SnO2 (Fig. 5 a, b). It is evident that rGO incorporated SnO2 composite shows enhanced H2S gas sensing response compared to bare SnO2 and SnO2/PANI. According to this study, the sensor response and recovery time were 82 s and 78 s, respectively, towards 2 ppm of H2S, which is very short compared to the SnO2 sensor. The authors also explored the gas sensing mechanism, as shown in Fig. 6.
SEM images of a SnO2, b PANI, c rGO and d in-situ polymerized SnO2/rGO/PANI composite [70]
a Response of the SnO2, SnO2/PANI, SnO2/rGO, and in-situ polymerized SnO2/rGO/PANI sensors toward H2S. b Sensor response as a function of gas concentration for the SnO2, SnO2/PANI, SnO2/rGO, and in-situ polymerized SnO2/rGO/PANI sensors [70]
a Mechanism diagram of the in-situ polymerized SnO2/rGO/PANI nanocomposite toward H2S gas. b Sketch of the interaction between n-type and p-type materials to H2S. c p-n heterojunction in the SnO2/rGO/PANI nanocomposite [70]
Figure 6a explains the mechanism of SnO2/rGO/PANI nanocomposite toward H2S gas as follows, the O2− ions are adsorbed on the sensor surface when it is exposed to atmospheric air; this causes the high resistance and thicker electron depletion layer is formed on sensing layer surface. After H2S exposure, the resistance of the sensing layer is decreased due to the reaction between H2S and oxygen ions on the surface. The high sensitivity was achieved due to the more surface-to-volume ratio of the composite sensor. The small hollow sphere of the composite allows the H2S to diffuse easily, thus increasing the sensitivity. Figure 6b illustrates the inter-reaction between the p-type and n-type semiconductors to H2S. The charge depletion layer is minimized when the sensor is exposed to the target gas, thereby decreasing the film's resistance. Figure 6c illustrates that the charge depletion region is formed between p-type SnO2 and n-type rGO. When the composite sensor was exposed to H2S gas, the electrons of rGO/PANI and holes of SnO2 moved in the opposite direction. As a result, the thickness of the charge depletion layer decreases when the fermi energy level reaches equilibrium. Eventually, the sensor resistance decreased.
SF6 gas has been used in gas-insulated switchgear due to its excellent insulating properties. However, SF6 release H2S and SO2 when it reacts with a trace amount of H2O and O2 molecules. Consequently, SF6 damage the insulation of equipment. Further, the toxicity of H2S is well-known, and to detect decomposed products (H2S, SO2), rGO/SnO2 sensor was developed and reported [71]. The rGO/SnO2 sensor responded to 100 ppm H2S at 125 ℃. The sensor's selectivity towards H2S and SOF2 upon exposure to typical SF6 decomposition components. The kinetic model and equilibrium of the rGO/SnO2-based sensor for enhanced H2S detection at low operating temperatures were reported [72]. Song et al. (2017) established a kinetic model to correlate sensitivity, response time, activation energy Ea and response rate constant k. Langmuir and Freundlich isotherm models were taken to fit the response curve at a different temperature, and response equilibrium parameters were extracted. According to this report, rGO-modified SnO2 exhibited a great promoting reaction between H2S and the surface of rGO/SnO2 due to less activation energy (Ea—19.01 kJ mol−1) than SnO2 (Ea–20.09 kJ mol−1) alone. This quantitative model would empower an exciting thought about gas sensing mechanism and enable the researchers to develop sensors operable at low temperatures for flexible electronic devices. Though the rGO/SnO2 has exhibited great sensitivity to analyte gas, the pristine SnO2 suffers from poor selectivity. The metal sensitizer (Pt, Pd, Au, Mn) has been incorporated into the rGO/SnO2 matrix to improve the selectivity of the SnO2 sensor. The selectivity of the SnO2 sensor sensitized with the sensitizer was attributed due to the sensitizer increasing the chemisorption site, catalytic activity with the formation of Schottky barrier on the surface, and mainly interact with adsorbed oxygen molecules, thereby imparting highly selective response to target gases [59, 60, 73, 74]. For example, rGO/SnO2 nanohybrids sensitized with Pt and Pd were developed and reported for selective detection of H2S, NO2, and H2. The maximum response of 124 and 49 was obtained towards 2 and 1.6% of NO2 and H2, respectively. The lowest detection limits were 0.2%, 0.4, and 1 ppm for H2, NO2, and H2S, respectively [75]. This study concluded that the rGO/SnO2 nanohybrids showed excellent response kinetics to H2S gas than NO2 and H2 gas.
rGO/WO3 composite for H2S detection
WO3 is an n-type semiconducting material, and it has been widely used as a sensing layer for various gas (H2, NH3, H2S, CH3COCH3, NO2) detection due to its advantages with wide band gap energy (2.4–2.8 eV), chemical stability, electronic property, and low cost [76,77,78,79,80,81,82]. WO3 has shortcomings including, poor selectivity, long response/recovery time, and poor sensitivity at low temperatures because the WO3 has a wide band gap and high resistance at room temperature. To overcome these shortcomings, doping with other oxides and functionalization with transition metal ions were employed [83,84,85].
Functionalization of WO3 with reduced graphene oxide was developed for H2S detection [86]. The rGO/WO3 nanosheet composite was synthesized through hydrothermal and post-calcination treatment. The composite was mixed with terpineol to form like paste and then coated on an alumina tube and annealed at 400 ℃. Morphology of (a) GO and (b) rGO/h-WO3 composites, (c) HR-TEM image of rGO/h-WO3 composites shown in Fig. 7. The H2S gas sensing property of the sensor is shown in Fig. 8. The sensor response was reported to be 10.8, with a detection range of 10 ppb–40 ppm to 1 ppm of H2S at 330 ℃. The article reported that the improved gas sensing properties were due to rGO effectively enhancing the electron's transportation/acceptance and improving the gas transportation channel in the nanostructure. The dopant materials could improve the sensing performance; however, the operating temperature was found to be still in the range of 100 ℃–400 ℃. This limits the use of WO3 in the gas sensor for flexible and portable electronic devices.
TEM images of a GO and b rGO/h-WO3composites, c HRTEM image of rGO/h-WO3 composites [86]
H2S gas sensing property of the sensors at different working temperatures and Responses of the sensors to various gases (the concentration of all gases was 40 ppm) [86]
H2S sensor based on Fe2O3/graphene
Ferric oxide (Fe2O3) is an industrially important material as the material has a wide range of applications in the rubber industry, anti-corrosion coating, and biomedical industry [87,88,89,90]. Regardless of these applications, it was discovered that ferric oxide could detect H2S based on chemiluminescence. Zaixing Jiang and his team reported papers like Fe2O3/graphene nanosheets for H2S sensing applications [91]. The device exhibited a selective response to 10 ppm H2S at 190 based on chemiluminescence intensity. It was reported that the Fe2O3/graphene paper provides a larger contact area to react with analyte gas and less resistance to flow. Enhanced gas sensing properties are generally achieved by loading the proper amount of rGO metal oxides. Nguyen Van Hoang et al. developed rGO/α-Fe2O3 nanofiber for enhanced H2S sensing. The α-Fe2O3/rGO nanofiber was prepared by on-chip electrospinning in which optimized rGO content was loaded with a precursor solution of PVA and Fe(NO3)3.9H2O. According to this report, the sensor response was 1.5 at 350 ℃ to 1 ppm H2S gas, which is attributed to its porous nature, and large surface area of nanofibers loaded with 1.0 wt % of rGO. High selectivity and long-term stability were achieved due to forming a potential barrier at the heterojunction between rGO and α-Fe2O3. The sensor had long-term stability, i.e., 9 cycles to 1 ppm H2S at 350 ℃. Spinel zinc ferrite is an n-type semiconducting metal oxide, in which the octahedral site was occupied by trivalent Fe3+, and divalent Zn2+ cations occupied the tetrahedral site. Zinc ferrite has been used in lithium-ion batteries as an anode material to enhance the electrochemical properties. Recently, the material also has been utilized for H2S gas sensing applications. Nguyen and his team developed rGO-loaded ZnFe2O4 nanofibers for H2S detection [92].
Simple on-chip electrospinning processes were carried out to prepare rGO/ZnFe2O4 nanofibers. They reported that the rGO-loaded ZnFe2O4 nanofibers showed n-type sensing behavior, i.e., the material's resistance decreased while introducing H2S gas. rGO/ZnFe2O4 exhibited maximum sensor response while incorporating 1.0 wt% of rGO, the response to 1 ppm of H2S was about 147 at 350 ℃, which was 1.5 times higher than pure ZnFe2O4 nanofibers. The reported results of the selectivity and stability of the sensor are shown in Fig. 9. The highest response was attributed to heterojunction formation between rGO/ ZnFe2O4 and potential barrier at grain boundaries. Further, this study reported the 1.0 wt% of rGO-loaded ZnFe2O4 samples annealed at 600 ℃ exhibited enhanced response to 1 ppm H2S (operating temperature—350 ℃) due to the inverse effect of nanograins size and crystallinity concerning annealing temperature [93, 94].
Selectivity to various gases at 350 °C a and stability at 1 ppm H2S gas at 350 °C b of the sensors based on the 1 wt.% RGO-loaded ZnFe2O4 NFs calcined at 600 °C [87]
rGO/MoO3 based sensor for H2S detection
Molybdenum trioxide (MoO3) is an important n-type semiconducting material. It has been widely investigated and applied to gas sensors, catalysts, and energy storage due to its structural flexibility and polymorphism [95,96,97]. Recently, research on MoO3 nanostructure for gas sensor applications has increased exponentially as it has unique properties, including high electron mobility and a high surface-to-volume ratio compared to their bulk counterparts [98,99,100,101]. However, the conductivity of MoO3 is very low at room temperature, and it requires a high operating temperature which hinders its use in sensor applications. Research has been carried out to improve the gas sensing property of MoO3 by doping with Fe2+, in which the sensor exhibited the highest response of 184.1–100 ppm H2S at 270 ◦C [102].
rGO has been incorporated into MoO3 for H2S sensing application, which still lowered the operating temperature. Bai et al. developed MoO3 nanorods on the rGO nanosheets via the microwave hydrothermal method [103]. The sensing response of rGO/MoO3 hybrids is presented in Fig. 10. The rGO/MoO3 hybrids exhibited an H2S sensing response of 59 to 40 ppm H2S at 110 ℃, and the response and recovery were found to be 9 s and 17 s, respectively. MalekAlaie et al. prepared MoO3 decorated with rGO and studied H2S gas sensing properties. The sensor was fabricated by spin coating rGO/MoO3 on an alumina substrate. The highest response was obtained at 160 ℃ for the 3wt% MoO3–rGO and was highly selective to H2S gas than common interfering gases [104].
Sensing responses of MoO3/rGO hybrids with different graphene content to 40 ppm H2S and Response of -MoO3/5 wt% rGO hybrid-based sensor to different gases [103]
rGO/CuO based sensor for H2S detection
Copper oxide (CuO) is a p-type semiconducting oxide, having a band gap energy of 1.35 eV. Most often, it has been incorporated with other n-type semiconducting metal oxides for H2S gas sensing studies [82, 105]. It is well known that CuO is converted into metallic CuS when in contact with H2S gas even under ambient conditions. However, the desulfuration of CuO is poor at room temperature and it requires a high operating temperature. CuO combined with n-type material, forming heterostructure at the interface, can modulate carrier transport channel, and facilitate decomposition of analyte gas molecules. CuO nanoparticles combined with rGO have been prepared via a microwave-assisted method and studied for H2S gas sensing properties [106]. The obtained CuO@rGO composite was dispersed in aqueous/water for sensor measurement and dropped on interdigitated Au electrode to form a resistive film. The response profile of the sensor exposed to H2S gas is presented in Fig. 11.
a Response profiles to H2S gases and b plots of the response versus [H2S] operating at 100 °C: (A) CuO, (B) 6-CuO@rGO, (C) 8-CuO@rGO, and (D) 10-CuO@rGO. The insert is the repeated response of the 8-CuO@rGO sensor to H2S gas at 100 °C [106]
The authors demonstrated the gas sensing mechanism of the sensor exposed to H2S gas in Fig. 12. According to this study, the composite composed of rGO/CuO is a p-type semiconductor with holes as the main carrier [107]. Adsorbed oxide ions (O−, O2−, O2−) are generated on rGO/CuO surfaces in ambient air. Thus, less resistance is formed on the surface. The conductivity is increased when the sensor is exposed to H2S by reacting with oxide ions (Fig. 12).
A schematic demonstration of the CuO@rGO sensor to H2S gas: a the adsorption and reaction process and b, c the qualitative evolution diagram of the two energy bands [106]
Research on Cu2O has been explored for gas sensor application, but the gas sensitivity is limited, and the operating temperature was typical > 150 ℃ , as closely packed Cu2O on interdigitated electrode (IDE) allows low electrons to flow. Sensor working at high temperature does give high sensing property, but it is difficult during device (inbuilt with heater) fabrication and explosive risks. Functionalization with graphene could realize the sensor operable at room temperature. Lisha Zhou et al. developed Cu2O/graphene sheets for enhanced H2S sensing properties. Au/Cr IDE was prepared through photolithography and sputtering. Cu2O/Graphene was drop cast on IDE and dried to evaporate the solvent. I-V characteristics showed that the Cu2O conductivity was poor and higher for Cu2O/Graphene composite. The composite exhibited Schottky contacts rather than ohmic behavior as the composite covered uniformly on the IDE substrate. The resistance of the composite was increased with increasing H2S gas concentration (5 ppb to 100 ppb) at room temperature. It was reported the high sensitivity was attributed to the nano-size effect and interfacial bonding between graphene and Cu2O.
CuO is transformed to CuS when injecting H2S, the desulfuration can be as follows
It was reported that the work function for CuO and CuS is as follows 3.61 eV and 4.9 eV, respectively [107]. Due to this work function difference, the electrons flow from CuO to CuS at the CuO/CuS heterointerfaces, establishing a potential barrier. The potential barrier will be minimized when the grain boundary region is converted to CuS. The CuS conversion back to CuO is achieved during the recovery period.
H2S sensing characteristics of various rGO/MOx-based sensor is provided in Table 1.
H2S sensor based on Carbon nanotube/Metal oxide composites
The principal requirement for the highly sensitive gas sensor is the structure must be a high surface-to-volume ratio. Porous thick and thin film structures have been designed to obtain highly sensitive sensors. Carbon nanotube (CNT) is a class of advanced materials having a wide range of applications due to their excellent physicochemical properties. It is generally categorized into two types: conducting and semiconducting nanotubes. Further, these CNT can be either single-walled or multi-walled nanotubes. Carbon nanotubes are highly reactive gaseous molecules adsorbed on the surface by charge conversion at RT. Nathan et al. reported CNT-modified electrodes for H2S detection using an electrochemical method [110]. It was reported the electrode had a low potential response towards H2S. Glassy carbon-modified electrodes had a linear range of 1.25–112.5 µM with a detection limit of 0.3 µM (9 ppb). Further, it was reported the electrode was more stable and selective to sulfide, enabling the development of a sensor for real-time application. Jun Fan et al. developed CuO/SnO2 doped with acidified CNT to detect H2S at low concentrations [111]. It was reported the developed sensor exhibited excellent sensitivity to H2S ranging from 0.1 to 0.5 ppm at 40℃, and response and recovery time was found to be 8.3 s and 11.5 s, respectively. The formation of p-n heterojunction between CuO/SnO2 and CNT introduced nanochannel and played a major role. Hyun Young Jung et al. reported highly effective detection of H2S by CNT functionalized with 2,2,6,6–tetramethylpiperidine- 1- oxyl (TEMPO). The device exhibited high H2S sensitivity of 420% at 60% humidity. Navaratnarajah and his co-worker reported Ru-doped SWCNT for H2S and SO2 detection [112]. Spin-polarized DFT simulations were used to determine the encapsulation and adsorption behavior of H2S and SO2 gas molecules by Ru-doped CNT. Ru doping on CNT slightly enhanced the adsorption efficacy. However, the obtained results should be confirmed by practical experiments. H2S sensing characteristics of various rGO/MOx-based sensor is provided in Table 2.
Asad et al. developed copper-decorated SWCNT for highly sensitive and selective detection of H2S [118]. Copper-decorated SWCNT was prepared by the chemical reduction process. The sample was spin-coated on Al patterned flexible substrate and annealed in a vacuum oven at 80 °C for 30 min. The sensor response/recovery was reported to be ~ 10 s and ~ 15 s, respectively, to 5 ppm H2S gas.
Mohsen Asad et al. reported hybrid nanomaterials based on CuO/SWCNT for wireless H2S sensing applications [121]. CuO with different morphologies was synthesized by hydrothermal method, and SWCNT was functionalized by mixing 20 mg into CuO, stirred at 100 ℃ for 30 min. As a prepared sample, CuO/SWCNT was spin-coated on Au IDE structured electrode to obtain a thin film for gas sensing studies. The study summarized that a fabricated wireless sensor could detect 100 ppb H2S gas. The SWCNT formed as an effective charge carrier channel also reported that it caused quick response 7 s to 1 ppm H2S. The reported gas-sensing mechanism of the sensor is presented in Fig. 13. It was reported the sensing mechanism (Fig. 13) of CuO-SWCNT gas sensors to H2S follows, at ambient air, oxygen adsorbed on CuO surface forming as 3O2− by extracting electron from CuO conduction band. When H2S gas molecules interact with oxygen ions, the trapped electrons are released and go towards CNTs due to the high electron affinity of the SWCNTs, as results decrease in the conductivity of p-type SWCNTs. At higher concentrations of gas, in addition to H2S oxidation, a chemical reaction between H2S and CuO causes the formation of the CuS layer (inset Figure Eq. 2).
Schematic of the sensing mechanism of fabricated CuO-SWCNT gas sensors were exposed to H2S. [121]
Soyeon Moon et al. developed the Co3O4-SWCNT composite by arc discharge method for H2S detection [117]. The sensor structure was fabricated on an alumina substrate patterned with a gold electrode. The porous nature of the composite was observed through Sem and XRD analysis. The response profile of the sensor is shown in Fig. 14. For gas sensing studies, Co30–SWCNT composite film exhibited the highest response to 100 ppm H2S at 250℃. The composite film resistance decreased when exposed to H2S (5–150 ppm), indicating the film behaved p-type semiconductor. Thus, the CNT in the film played a transport path and had not contributed to gas sensing. Functionalizing CNT with some chemical reactants could improve conductivity and compensate for drawbacks like less response and lack of sensitivity. To improve the CNT sensor performance, functionalized multiwall carbon nanotube (MWCNTs-COOH) has been prepared and evaluated for H2S sensing performance. For example, Nosrat Izadi et al. investigated the effect of functionalized MWCNTs on H2S sensing properties. The carbon nanotubes were synthesized by chemical vapor deposition and functionalized with carboxyl, amide groups, Pt, and Mo nanoparticles. Mo/CNT and Pt/CNT-COOH-based sensors exhibited the highest response than CNT-COOH-based sensors. The highest response is attributed to discrete band gap states induced by metallic nanoclusters on the CNT surface, generating additional charge transfer between CNT and gas molecules to enhance gas sensitivity. Carboxylated MWCNTs were developed and used as counter and working electrodes while H2S sensing [114]. Oxidation of H2S on the working electrode produces a current which is directly proportional to gas concentration depending on the electrode characteristics. Cyclic voltammetry with a scan rate of 0.1 V/s was conducted for MWCNT and carboxylated MWCNT electrodes to examine the H2S (500 ppm) sensitivity before and after exposure. It was concluded the carboxylated MWCNT exhibited a slightly higher response than raw MWCNT. To improve the sensing characteristics of MWCNT, Jae Hoon Bang et al. developed MWCNT decorated CuO/Cu2O nanoparticles for selective sensing of H2S and reported [115].
a The gas selectivity of the Co30-400 sensor to H2S was derived from a comparison with the responses to typical reducing gases of NH3, CH4, and H2. b Comparison of sensing responses of Co30-400 and Co5-400 with sensors of thin film Co3O4 and nanowires of Co3O4–SWCNT [117]
A different layer of Cu was sputtered on MWCNT and annealed at 500 ℃. The developed MWCNT decorated CuO/Cu2O formed a p-p heterojunction. According to their study, the sensor response was 1244% to 1 ppm H2S, and response and recovery time was 219 s and 77 s, respectively. Decorating carbon nanotubes with Mn, Co, Mg, and Zn can improve magnetic, optical, and electrochemical properties, consequently enhancing the H2S gas sensitivity. Hajihashemi et al. synthesized NiFe2O4–MWCNT by sol–gel method and reported the H2S gas sensing behavior of the prepared material. It was reported that crystallite size of the composite material found to be 23.93 nm. The sensing film was prepared by spin coating method and exhibited appropriate response to 100 ppm of H2S gas. Superior sensitivity was achieved due to incorporation of carbon nanotube which act as substrate and transferred temperature to NiFe2O4. Further, surface morphology and surface-to-volume ratio of the composite play a role in adsorption of more H2S on the surface of materials [119].
Conclusions and future perspectives
In this article, we critically reviewed a significant achievement in the field of H2S sensors made of carbonaceous materials and doped metal oxides. The general approach to enhancing the H2S sensing behavior of the MOx sensor is to incorporate optimized carbonaceous materials (CNT, graphene, and rGO). Incorporating those carbonaceous materials enhances gas sensitivity, decreasing the sensor operating temperature and reducing sensor response time, leading to composites contending the sensor made of metal oxides alone. For over a decade, extensive research has been conducted based on rGO/MOx for H2S detection. However, the H2S detection based on carbonaceous materials reported in this article is basic research and needs a lot of investigation such as sensor behavior at different humidity, temperature, etc., are required to build a sensor for monitoring H2S to be deployable in the field, and indeed the researchers are working extensively to develop that type of sensor. Most research articles explain the sensing mechanism but need to explain the chemical nature of sensing materials and the selectivity mechanism. In future work on developing an H2S gas sensor-based carbonaceous material for real-time monitoring, one should note that the sensor performance is based on humidity, temperature, and interfering gases. The selectivity of carbonaceous/metal oxide-based sensors could be achieved by the functionalization of new molecules which are specifically interacting with the target gas. The effect on humidity could be stabilized by coating with appropriate hydrophobic materials. The carbonaceous materials (CNT, graphene, rGO)/MOx-based sensors still need to be evaluated under different volatile organic compounds.
Data availability
The datasets supporting the conclusions of this article are included within the article.
References
K. Vikrant, V. Kumar, Y.S. Ok, K.-H. Kim, A. Deep, Metal-organic framework (MOF)-based advanced sensing platforms for the detection of hydrogen sulfide. TrAC, Trends Anal. Chem. 105, 263–281 (2018)
A. Mirzaei, S.S. Kim, H.W. Kim, Resistance-based H2S gas sensors using metal oxide nanostructures: a review of recent advances. J. Hazard Mater. 357, 314–331 (2018)
K.S. Yoo, S.D. Han, H.G. Moon, S.-J. Yoon, C.-Y. Kang, Highly sensitive H2S sensor based on the metal-catalyzed SnO2 nanocolumns fabricated by glancing angle deposition. Sensors 15, 15468–15477 (2015)
C.S. Rout, M. Hegde, C.N.R. Rao, H2S sensors based on tungsten oxide nanostructures. Sens. Actuators B Chem. 128, 488–493 (2008)
S. Navale et al., CuxO nanostructure-based gas sensors for H2S detection: an overview. Chemosensors 9(6), 127 (2021)
V. Vitvitsky, R. Banerjee, Chapter Seven - H2S analysis in biological samples using gas chromatography with sulfur chemiluminescence detection. in methods in enzymology (eds. Cadenas, E. & Packer, L.) vol. 554 p. 111–123 (Academic Press, 2015).
H. Ibrahim, A. Serag, M.A. Farag, Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J Adv Res 27, 137–153 (2021)
A. Mirzaei, S.G. Leonardi, G. Neri, Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: a review. Ceram Int 42, 15119–15141 (2016)
P.J. Sabourin, W.E. Bechtold, R.F. Henderson, A high pressure liquid chromatographic method for the separation and quantitation of water-soluble radiolabeled benzene metabolites. Anal. Biochem. 170, 316–327 (1988)
G. Sarala Devi, S. Manorama, V.J. Rao, High sensitivity and selectivity of an SnO2 sensor to H2S at around 100 °C. Sens. Actuators B Chem. 28, 31–37 (1995)
S.D. Shinde, G.E. Patil, D.D. Kajale, V.B. Gaikwad, G.H. Jain, Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloys Compd. 528, 109–114 (2012)
D. Li et al., Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit. Int J Hydrogen Energy 44, 3985–3992 (2019)
H.-M. Lin, C.-M. Hsu, H.-Y. Yang, P.-Y. Lee, C.-C. Yang, Nanocrystalline WO3-based H2S sensors. Sens. Actuators B Chem. 22, 63–68 (1994)
L. Zhang et al., Self-assembly gridding α-MoO3 nanobelts for highly toxic H2S gas sensors. Sens Actuators B Chem 237, 350–357 (2016)
J. Xu, X. Wang, J. Shen, Hydrothermal synthesis of In2O3 for detecting H2S in air. Sens. Actuators B Chem. 115, 642–646 (2006)
Z. Li et al., A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard Mater. 300, 167–174 (2015)
Akhtar, F. S. E.-S. B. K. E.-A. M. A. E.-K. 2019 Metal Oxide Gas Sensors by Nanostructures. in Gas Sensors Ch. 1 (IntechOpen). https://doi.org/10.5772/intechopen.88858
N. Tao, Challenges and promises of metal oxide nanosensors. ACS Sens. 4, 780 (2019)
C. Su et al., Controllable synthesis of heterostructured CuO–NiO nanotubes and their synergistic effect for glycol gas sensing. Sens. Actuators B Chem. 304, 127347 (2020)
Z. Yang et al., Construction, application and verification of a novel formaldehyde gas sensor system based on Ni-doped SnO2 nanoparticles. IEEE Sens J 21, 11023–11030 (2021)
X. Chen et al., Two-dimensional Cd-doped porous Co3O4 nanosheets for enhanced room-temperature NO2 sensing performance. Sens. Actuators B Chem. 305, 127393 (2020)
L. Xu et al., A novel CoxNi1-xP/fs-Si self-supporting electrodes manufactured via femtosecond laser for highly efficient hydrogen evolution reaction. Surf. Interfaces 32, 102173 (2022)
C. Yang et al., Indium element - induced oxygen vacancies and polycrystalline structure enabled SnO2 nanofibers for highly sensitive detection of NOx. Sens. Actuators B Chem. 362, 131754 (2022)
C. Yang et al., High selectivity of Ag-doped Fe2O3 hollow nanofibers in H2S detection at room operating temperature. Sens. Actuators B Chem. 341, 129919 (2021)
C. Zhang, S. Zhang, Y. Yang, H. Yu, X. Dong, Highly sensitive H2S sensors based on metal-organic framework driven γ-Fe2O3 on reduced graphene oxide composites at room temperature. Sens. Actuators B Chem. 325, 128804 (2020)
S. Zhang et al., Femtosecond laser micro-nano processing for boosting bubble releasing of gas evolution reactions. Nano. Res. 15, 1672–1679 (2022)
V. Balasubramani, S. Sureshkumar, T.S. Rao, T.M. Sridhar, Impedance spectroscopy-based reduced graphene oxide-incorporated ZnO composite sensor for H2S investigations. ACS Omega 4, 9976–9982 (2019)
S. Mallakpour, E. Khadem, Carbon nanotube–metal oxide nanocomposites: fabrication, properties and applications. Chem. Eng. J. 302, 344–367 (2016)
R. Leghrib, A. Felten, J.J. Pireaux, E. Llobet, Gas sensors based on doped-CNT/SnO2 composites for NO2 detection at room temperature. Thin Sol. Films 520, 966–970 (2011)
D. Jung, M. Han, G.S. Lee, Room-temperature gas sensor using carbon nanotube with cobalt oxides. Sens. Actuators B Chem. 204, 596–601 (2014)
H. Liu et al., Synthesis and gas sensing characteristic based on metal oxide modification multi wall carbon nanotube composites. Appl. Surf. Sci. 258, 1991–1994 (2012)
A. Wisitsoraat, A. Tuantranont, C. Thanachayanont, V. Patthanasettakul, P. Singjai, Electron beam evaporated carbon nanotube dispersed SnO2 thin film gas sensor. J. Electroceram. 17, 45–49 (2006)
D. Punetha, S.K. Pandey, Sensitivity enhancement of ammonia gas sensor based on hydrothermally synthesized rGO/WO3 nanocomposites. IEEE Sens. J. 20, 1738–1745 (2020)
D. Guo et al., Reduced-graphene-oxide/metal-oxide p-n heterojunction aerogels as efficient 3D sensing frameworks for phenol detection. Carbon N Y 99, 571–578 (2016)
V.S. Bhati et al., Improved sensitivity with low limit of detection of a hydrogen gas sensor based on rGO-loaded Ni-doped ZnO nanostructures. ACS Appl. Mater. Interfaces 10, 11116–11124 (2018)
P.-G. Su, L.-Y. Yang, NH3 gas sensor based on Pd/SnO2/RGO ternary composite operated at room-temperature. Sens. Actuators B Chem. 223, 202–208 (2016)
Z. Yuan, Y. Liu, J. Zhang, F. Meng, H. Zhang, Rose-like MoO3/MoS2/rGO low-temperature ammonia sensors based on multigas detection methods. IEEE Trans. Instrum. Meas. 70, 1–9 (2021)
X. Li et al., Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators B Chem. 230, 330–336 (2016)
T. Salehi, A. Taherizadeh, A. Bahrami, A. Allafchian, V. Ghafarinia, Toward a highly functional hybrid ZnO nanofiber–rGO gas sensor. Adv. Eng. Mater. 22, 2000005 (2020)
O. Ogbeide et al., Inkjet-printed rGO/binary metal oxide sensor for predictive gas sensing in a mixed environment. Adv Funct Mater n/a 32(25), 2113348 (2022)
P. Hao, Z. Lin, P. Song, Z. Yang, Q. Wang, rGO-wrapped porous LaFeO3 microspheres for high-performance triethylamine gas sensors. Ceram Int 46, 9363–9369 (2020)
S. Moon et al., Co3O4-SWCNT composites for H2S gas sensor application. Sens. Actuators B Chem. 222, 166–172 (2015)
S. Sureshkumar, B. Venkatachalapathy, T.M. Sridhar, Enhanced Hsub2/subS gas sensing properties of Mn doped ZnO nanoparticlesan impedance spectroscopic investigation. Mater. Res. Express 6, 75009 (2019)
S. Sureshkumar, B. Jothimani, T.M. Sridhar, A. Santhosh, B. Venkatachalapathy, Synthesis of hexagonal ZnO-PQ7 nano disks conjugated with folic acid to image MCF – 7 cancer cells. J. Fluoresc. 27, 21–29 (2017)
S. Lakshmipurada, K. Reddy, S. Sundaravel, Synthesis and characterization of activated carbon/ZnO nanocomposite from water hyacinth for heavy metals adsorption and its antimicrobial activity. Asian J. Chem. 33, 551–556 (2021)
D. Li et al., Preparation and gas-sensing performances of ZnO/CuO rough nanotubular arrays for low-working temperature H2S detection. Sens. Actuators B Chem. 254, 834–841 (2018)
H. Wang, Y. Luo, B. Liu, L. Gao, G. Duan, CuO nanoparticle loaded ZnO hierarchical heterostructure to boost H2S sensing with fast recovery. Sens. Actuators B Chem. 338, 129806 (2021)
S. Park et al., Enhanced H2S gas sensing performance of networked CuO-ZnO composite nanoparticle sensor. Mater. Res. Bull 82, 130–135 (2016)
Y. Shen et al., Preparation and sensing properties of mesoporous ZnO–rGO composites based on in situ hydrothermal synthesis. J. Mater. Sci.: Mater. Electron. 27, 12660–12668 (2016)
T.K. Dang et al., Extraordinary H2S gas sensing performance of ZnO/rGO external and internal heterojunctions. J Alloys Compd 879, 160457 (2021)
S. Singkammo et al., Electrolytically exfoliated graphene-loaded flame-made Ni-doped SnO2 composite film for acetone sensing. ACS Appl. Mater. Interfaces 7, 3077–3092 (2015)
D. Fu, C. Zhu, X. Zhang, C. Li, Y. Chen, Two-dimensional net-like SnO2/ZnO heteronanostructures for high-performance H2S gas sensor. J. Mater. Chem. A Mater. 4, 1390–1398 (2016)
F.-L. Meng, Z. Guo, X.-J. Huang, Graphene-based hybrids for chemiresistive gas sensors. TrAC, Trends Anal. Chem. 68, 37–47 (2015)
T.V. Cuong et al., Solution-processed ZnO-chemically converted graphene gas sensor. Mater Lett 64, 2479–2482 (2010)
Z. Li et al., Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 6, 470–506 (2019)
D. Sun, Y. Luo, M. Debliquy, C. Zhang, Graphene-enhanced metal oxide gas sensors at room temperature: a review. Beilstein J. Nanotechnol. 9, 2832–2844 (2018)
P.S. Shewale, K.S. Yun, Synthesis and characterization of Cu-doped ZnO/RGO nanocomposites for room-temperature H2S gas sensor. J Alloys Compd 837, 155527 (2020)
A.D. Ugale et al., ZnO decorated flexible and strong graphene fibers for sensing NO2 and H2S at room temperature. Sens. Actuators B Chem. 308, 127690 (2020)
S.S. Kumar, B. Venkatachalapathy, L. Sujatha, T.S. Rao, T.M. Sridhar, Influence of H2S concentration on Mn sensitized tin dioxide nanoparticles based sensor—an impedance spectroscopic investigation. Sens. Lett. 14, 949–954 (2016)
S. Sureshkumar, B. Venkatachalapathy, T.M. Sridhar, Enhanced H2S gas sensing properties of Mn doped ZnO nanoparticles—an impedance spectroscopic investigation. Mater Res Express 6(7), 075009 (2019)
F. Schipani et al., Electrical characterization of semiconductor oxide-based gas sensors using impedance spectroscopy: a review. Rev Adv Sci Eng 5, 86–105 (2016)
D.K. Bandgar et al., Novel route for fabrication of nanostructured α-Fe2O3 gas sensor. Mater. Sci. Semicond. Process 17, 67–73 (2014)
V.A. Moshnikov et al., Porous silicon with embedded metal oxides for gas sensing applications. J Non Cryst Solids 358, 590–595 (2012)
J.M. Rheaume, A.P. Pisano, A review of recent progress in sensing of gas concentration by impedance change. Ionics (Kiel) 17, 99–108 (2011)
S. Luo, G. Fu, H. Chen, Z. Liu, Q. Hong, Gas-sensing properties and complex impedance analysis of Ce-added WO3 nanoparticles to VOC gases. Sol. State Electron 51, 913–919 (2007)
M. Miyayama, K. Hikita, G. Uozumi, H. Yanagida, A.c. impedance analysis of gas-sensing property in CuO/ZnO heterocontacts. Sens. Actuators B Chem. 25, 383–387 (1995)
N. Al-Hardan, M.J. Abdullah, A.A. Aziz, Impedance spectroscopy of undoped and Cr-doped ZnO gas sensors under different oxygen concentrations. Appl. Surf. Sci. 257, 8993–8997 (2011)
V. Balasubramani et al., Highly sensitive and selective H2S gas sensor fabricated with β-Ga2O3 /rGO. ECS J. Sol. State Sci. Technol. 9, 055009 (2020)
S. Cui et al., Indium-doped SnO2 nanoparticle–graphene nanohybrids: simple one-pot synthesis and their selective detection of NO2. J. Mater. Chem. A 1, 4462–4467 (2013)
D. Zhang, Z. Wu, X. Zong, Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 289, 32–41 (2019)
J. Chu et al., Highly selective detection of sulfur hexafluoride decomposition components H2S and SOF2 employing sensors based on tin oxide modified reduced graphene oxide. Carbon N Y 135, 95–103 (2018)
Z. Song et al., Enhanced H2S gas sensing properties based on SnO2 quantum wire/reduced graphene oxide nanocomposites: equilibrium and kinetics modeling. Sens. Actuators B Chem 249, 632–638 (2017)
N.S. Ramgir, Y.K. Hwang, S.H. Jhung, I.S. Mulla, J.-S. Chang, Effect of Pt concentration on the physicochemical properties and CO sensing activity of mesostructured SnO2. Sens. Actuators B Chem. 114, 275–282 (2006)
N.S.A. Eom et al., Ultrasensitive detection of low-ppm H2S gases based on palladium-doped porous silicon sensors. RSC Adv 8, 29995–30001 (2018)
B. Bhangare et al., Role of sensitizers in imparting the selective response of SnO2/RGO based nanohybrids towards H2S, NO2 and H2. Mater. Sci. Semicond. Process 105, 104726 (2020)
J. Shi et al., WO3 nanocrystals: synthesis and application in highly sensitive detection of acetone. Sens Actuators B Chem 156, 820–824 (2011)
L.F. Reyes et al., Gas sensor response of pure and activated WO3 nanoparticle films made by advanced reactive gas deposition. Sens. Actuators B Chem. 117, 128–134 (2006)
Y.-S. Shim et al., Highly sensitive and selective H2 and NO2 gas sensors based on surface-decorated WO3 nanoigloos. Sens. Actuators B Chem. 198, 294–301 (2014)
W. Yan, M. Hu, P. Zeng, S. Ma, M. Li, Room temperature NO2-sensing properties of WO3 nanoparticles/porous silicon. Appl. Surf. Sci. 292, 551–555 (2014)
Z. Hua, M. Yuasa, T. Kida, N. Yamazoe, K. Shimanoe, H2 sensing mechanism of Pd-loaded WO3 nanoparticle gas sensors. Chem. Lett. 43, 1435–1437 (2014)
H. Zhou, D.-Y. Xu, H.-Q. Zuo, W. Liu, S. Lin, Preparation of flower-like Cu-WO3 nanostructures and their acetone gas sensing performance. J. Chem. 2015, 382087 (2015)
M. He et al., Highly sensitive and selective H2S gas sensors based on flower-like WO3/CuO composites operating at low/room temperature. J. Alloys Compd. 788, 36–43 (2019)
M. Punginsang et al., Selective H2S gas sensors based on ohmic hetero-interface of Au-functionalized WO3 nanowires. Appl. Surf. Sci. 571, 151262 (2022)
D. Feng et al., Highly sensitive and selective NiO/WO3 composite nanoparticles in detecting H2S biomarker of halitosis. ACS Sens. 6, 733–741 (2021)
T.T.N. Hoa et al., Highly selective H2S gas sensor based on WO3-coated SnO2 nanowires. Mater. Today Commun. 26, 102094 (2021)
J. Shi et al., Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 230, 736–745 (2016)
M. Shahrousvand et al., Flexible magnetic polyurethane/Fe2O3 nanoparticles as organic-inorganic nanocomposites for biomedical applications: properties and cell behavior. Mater. Sci. Eng., C 74, 556–567 (2017)
B. Shentu, T. Gan, Z. Weng, Modification of Fe2O3 and its effect on the heat-resistance of silicone rubber. J Appl Polym Sci 113(5), 3202–3206 (2009)
E. Gerasimova, The effect of Fe2O3 on the mechanical properties of the polymer modified cement containing fly ash. Procedia. Eng. 150, 1553–1557 (2016)
M.B. Hegde, K.N. Shetty Mohana, S.R. Nayak, A.M. Madhusudhana, Solution combustion synthesis of rGO-Fe2O3 hybrid nanofiller for linseed oil based eco-friendly anticorrosion coating. Colloids. Surf. A Physicochem. Eng. Asp 633, 127863 (2022)
Z. Jiang et al., A high efficiency H2S gas sensor material: paper like Fe2O3/graphene nanosheets and structural alignment dependency of device efficiency. J. Mater. Chem. A Mater. 2, 6714–6717 (2014)
N. van Hoang et al., Excellent detection of H2S gas at ppb concentrations using ZnFe2O4 nanofibers loaded with reduced graphene oxide. Sens. Actuators B Chem. 282, 876–884 (2019)
S.-W. Choi, J.Y. Park, S.S. Kim, Growth behavior and sensing properties of nanograins in CuO nanofibers. Chem. Eng. J. 172, 550–556 (2011)
A. Katoch, G.-J. Sun, S.-W. Choi, J.-H. Byun, S.S. Kim, Competitive influence of grain size and crystallinity on gas sensing performances of ZnO nanofibers. Sens Actuators B Chem 185, 411–416 (2013)
T. Tao, Q. Chen, H. Hu, Y. Chen, MoO3 nanoparticles distributed uniformly in carbon matrix for supercapacitor applications. Mater. Lett. 66, 102–105 (2012)
A. Manivel et al., Synthesis of MoO3 nanoparticles for azo dye degradation by catalytic ozonation. Mater Res Bull 62, 184–191 (2015)
A. Ganguly, R. George, Synthesis, characterization and gas sensitivity of MoO3 nanoparticles. Bull. Mater. Sci. 30, 183–185 (2007)
S.-K. Shen et al., Sensing mechanism of Ag/α-MoO3 nanobelts for H2S gas sensor. Rare Met. 40, 1545–1553 (2021)
J. Zhang et al., Enhanced trimethylamine sensing performance of single-crystal MoO3 nanobelts decorated with Au nanoparticles. J. Alloys Compd. 685, 1024–1033 (2016)
S. Zhang, Y. Zheng, P. Song, J. Sun, Q. Wang, Enhanced trimethylamine gas-sensing performance of CeO2 nanoparticles-decorated MoO3 nanorods. J. Mater. Sci.: Mater. Electron. 33, 3453–3464 (2022)
S. Sau, S. Chakraborty, T. Das, M. Pal, Ethanol sensing properties of nanocrystalline α-MoO3. Front Mater. 6, 285 (2019)
Q.-Y. Ouyang et al., Facile synthesis and enhanced H2S sensing performances of Fe-doped α-MoO3 micro-structures. Sens. Actuators B Chem. 169, 17–25 (2012)
S. Bai, C. Chen, R. Luo, A. Chen, D. Li, Synthesis of MoO3/reduced graphene oxide hybrids and mechanism of enhancing H2S sensing performances. Sens. Actuators B Chem. 216, 113–120 (2015)
M. Malekalaie, M. Jahangiri, A.M. Rashidi, A. Haghighiasl, N. Izadi, Selective hydrogen sulfide (H2S) sensors based on molybdenum trioxide (MoO3) nanoparticle decorated reduced graphene oxide. Mater. Sci. Semicond. Process 38, 93–100 (2015)
H. Wang, Y. Luo, B. Liu, L. Gao, G. Duan, CuO nanoparticle loaded ZnO hierarchical heterostructure to boost H2S sensing with fast recovery. Sens. Actuators B Chem. 338, 129806 (2021)
L. Yin et al., Microwave-assisted preparation of hierarchical CuO@rGO nanostructures and their enhanced low-temperature H2S-sensing performance. Appl Surf Sci 476, 107–114 (2019)
H. Meng, W. Yang, K. Ding, L. Feng, Y. Guan, Cu2O nanorods modified by reduced graphene oxide for NH 3 sensing at room temperature. J. Mater. Chem. A Mater. 3, 1174–1181 (2015)
J.H. Kim et al., Enhancement of H2S sensing performance of p-CuO nanofibers by loading p-reduced graphene oxide nanosheets. Sens. Actuators B Chem. 281, 453–461 (2019)
N. van Hoang et al., Enhanced H2S gas-sensing performance of α-Fe2O3 nanofibers by optimizing process conditions and loading with reduced graphene oxide. J. Alloys Compd. 826, 154169 (2020)
N.S. Lawrence, R.P. Deo, J. Wang, Electrochemical determination of hydrogen sulfide at carbon nanotube modified electrodes. Anal. Chim. Acta. 517, 131–137 (2004)
J. Fan et al., Carbon nanotubes-CuO/SnO2 based gas sensor for detecting H2S in low concentration. Nanotechnology 30(47), 475501 (2019)
N. Kuganathan, A. Chroneos, Ru-doped single walled carbon nanotubes as sensors for SO2 and H2S detection. Chemosensors 9, 120 (2021)
Y. Zhao, J. Zhang, Y. Wang, Z. Chen, A highly sensitive and room temperature CNTs/SnO2/CuO sensor for H2S gas sensing applications. Nanoscale Res. Lett. 15(1), 1–8 (2020)
N. Parsafar, V. Ghafouri, A. Banaei, Electrochemical Sensing of H2S Gas in Air by Carboxylated Multi-walled Carbon Nanotubes. J. Chem. Chem. Eng. Res. 38, 53–62 (2019)
J.H. Bang et al., Decoration of multi-walled carbon nanotubes with CuO/Cu2O nanoparticles for selective sensing of H2S gas. Sens. Actuators B Chem. 344, 130176 (2021)
S. Mubeen et al., Sensitive detection of H2S using gold nanoparticle decorated single-walled carbon nanotubes. Anal. Chem. 82, 250–257 (2010)
S. Moon et al., Co3O4-SWCNT composites for H2S gas sensor application. Sens. Actuators B Chem. 222, 166–172 (2016)
M. Asad, M.H. Sheikhi, M. Pourfath, M. Moradi, High sensitive and selective flexible H2S gas sensors based on Cu nanoparticle decorated SWCNTs. Sens. Actuators B Chem. 210, 1–8 (2015)
R. Hajihashemi, A.M. Rashidi, M. Alaie, R. Mohammadzadeh, N. Izadi, The study of structural properties of carbon nanotubes decorated with NiFe2O4 nanoparticles and application of nano-composite thin film as H2S gas sensor. Mater. Sci. Eng. C 44, 417–421 (2014)
M. Asad, M.H. Sheikhi, Surface acoustic wave based H2S gas sensors incorporating sensitive layers of single wall carbon nanotubes decorated with Cu nanoparticles. Sens. Actuators B Chem. 198, 134–141 (2014)
M. Asad, M.H. Sheikhi, Highly sensitive wireless H2S gas sensors at room temperature based on CuO-SWCNT hybrid nanomaterials. Sens. Actuators B Chem. 231, 474–483 (2016)
H.Y. Jung et al., High-performance H2S detection by redox reactions in semiconducting carbon nanotube-based devices. Analyst 138, 7206–7211 (2013)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors are declaring that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sureshkumar, S., Rajakumari, S. & Manonmani, R. Recent advances in the development of carbon/metal oxides nanohybrids for enhanced H2S detection: a review. J IRAN CHEM SOC 20, 1007–1022 (2023). https://doi.org/10.1007/s13738-023-02742-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02742-9