Abstract
The rise of technology and the human population cause a variety of toxic chemicals and gases to form, which endanger all living things. Monitoring these harmful gas emissions is essential for keeping us safe in daily life. In order to detect harmful gases and vapours for environmental control, industrial monitoring, medical diagnosis, and domestic safety, gas sensors that are portable, flexible, and extremely sensitive are widely utilised. The development of materials that can react to lower gas concentrations in a remarkably short period of time has proven difficult. Nanomaterials have the potential to be developed into very efficient sensing technology due to the outstanding gas–solid interaction they show and the high surface-to-volume ratio. The currently available commercialized sensors are built on metal oxides, which typically operate at high temperatures and create extra challenges in the desorption of chemisorbed gas molecules. The interest in carbon nanomaterials (CNMs) among scientists has significantly increased during the past few years. They are highly intriguing for forming the next-generation of miniature, low-power, ubiquitous sensors due to their distinctive electrical, optical, and mechanical features. In particular, over the past few decades, the discovery of CNMs, such as carbon black (CB), carbon nanohorns (CNHs), carbon nano-onions (CNOs), nanodiamond (ND), carbon quantum dots (CQDs) carbon nanotubes (CNTs), graphene etc., has accelerated the study of gas sensors. More research has been done on CNM nanocomposites containing metal, metallic nanoparticles, metal oxides, and polymers to boost their selectivity, which shows better sensing capabilities even at room temperature. In this chapter, I will discuss the most recent developments in electrical gas sensors for hazardous gases employing CNMs and their hybrid/composites materials. Several papers including experimental and theoretical data will be reviewed and debated. For the discussions, the key findings for CB, CNHs, CNOs, ND, CQDs, CNTs, graphene with particular focus on the composites/hybrids of CNT and graphene with metal oxides, polymers, metals, etc. that exhibit sensing properties in many sectors will be taken into consideration. Finally, a future prognosis will be discussed, together with its highlighted difficulties and potential.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Gas sensors
- Carbon nanomaterials
- Carbon black
- Carbon nanohorns
- Carbon nano-onions
- Nanodiamond
- Carbon nanotubes
- Graphene
- Composite/hybrid
1 Introduction
The modern way of life has a substantial negative influence on the environment due to the continuous emission of toxic gases that are invisible to the naked eye. Air pollutants including NO2, COx, and CH4 are mostly to blame for the damaging atmospheric changes resulting from environmental changes that have increased Earth's temperature [1,2,3]. Along with polluting gases, the environment also contains a number of additional hazardous gases, including CH4, NH3, H2, and H2S, which can explode when mixed in a specific ratio with air. A number of volatile organic compounds (VOCs), such as acetone, ethanol, toluene, and triethylamine (TEA), are harmful to human health in addition to these toxic gases. Thus, sensing these gases is crucial for environmental analysis, medical diagnosis, agriculture, public safety and security objectives etc. Consequently, there is a considerable need for gas sensors that are light-weight, portable, versatile, and affordable. There are different kinds of sensors, including chemiresistive [4], field-effect transistor (FET) [5], and micro-electromechanical system (MEMS) [6], depending on how they detect things. Among these sensing mechanisms, chemiresistive sensors have been extensively studied. For a variety of useful applications, metal-oxide sensors (MOSs) are currently well commercialized. These include hand-held ethanol sensors for cases of driving while intoxicated, methane and hydrogen sensors for the protection of workers in industries and mines, and acetone and toluene gas sensors for the diagnosis of diabetes and lung cancer [7]. For these practical sensing applications up until now, SnO2, TiO2, ZnO, CuO, In2O3, CdO, and WO3 have received a lot of attention. These sensors have excellent sensitivity, but because of their high operating temperatures, they are more expensive to operate and maintain. Additionally, a modification to their surface shape impacts their sensitivity.
As a result, to resolve the aforementioned problems, CNMs like CB, CNHs, CNOs, ND, CQDs, CNTs, graphene, novel substitutes, have been investigated as sensing materials throughout the last two decades [8,9,10,11]. Compared to other commonly used materials, carbon nanomaterials provide a plethora of advantages. Due to their inherent electrical properties, which are sensitive to changes in the chemical environment, they stand out as the most important and optimistic choice for sensor manufacturing. Furthermore, they are suitable for high-efficiency chemical sensing because of characteristics including poor water solubility, good thermal and chemical consistency, low fluorescence and functionalization. More than 100 million unique compounds with various attributes can be created, thanks to their hybridization property, which enables the synthesis of several different chains with various lengths and electrical configuration [12]. Even while pristine CNMs have many benefits, they also have some significant disadvantages, including low selectivity, poor reproducibility, and irregularities in the functional groups on graphene derivatives or the number of layers [13, 14]. This has led to the exploration of nanocomposites of CNMs with metal and their oxides, organic materials, and polymers, and this novel class of CNMs hybrid sensing materials has showed exceptional performances without sacrificing their advantages. Additionally, CNMs have considerable flexibility [15, 16], making them suitable for use in the construction of wearable sensors.
In this chapter, I will discuss the most recent developments in the field of electrical gas sensors used for hazardous gases employing CNMs and their hybrid/composites materials. Several papers containing experimental and theoretical data will be examined and discussed. The main discoveries for CB, CNHs, CNOs, ND, CQDs, CNTs, graphene with particular focus on the composites/hybrids of CNT and graphene with metal oxides, polymers, metals, etc. that demonstrate sensing capabilities in numerous fields will be taken into account for the discussions. Finally, a prediction for the future will be discussed, along with its potential and any obstacles that stand out.
2 Mechanism
The conductivity change brought on by the transferring of charge between the target molecule and sensing layer is the fundamental process of chemical sensing. Due to their high surface-to-volume ratios, one and two dimensional nanostructures are especially suitable for this use. Therefore, even a modest charge shift at the surface can have a significant impact on how they conduct electricity. Gases can behave as holes or electron donors depending on their chemical structure. While p-type dopants like NO2, boost conductivity by enhancing hole conduction, n-type dopants, like NH3, deplete holes from the conduction band and cause a drop in material conductivity [17, 18].
Response time, recovery time, operating temperature, and sensitivity are four primary performance metrics used in the literature to assess sensors. Response time is defined as the time it takes for a sensor to attain 90% of its entire response, such as resistance, after being exposed to the target gas. Recovery time is defined as the time needed for a sensor to restore to 90% of its original baseline signal after removing the target gas. Sensitivity is defined as the ratio of a sensor's output signal change to a variation in the measured parameter. It measures how efficiently a sensor detects minor changes in the input signal. The operating temperature is the temperature range in which a sensor can work and produce accurate measurements.
A thin film's sensitivity or sensors response (R) is commonly computed using the formula:
Here, Rg is the film resistance when subjected to the target analyte and Ra is the film resistance solely when exposed to synthetic air. The sensing component, manufacturing procedure, and shape all play a significant role in how the sensor reacts when exposed to a particular gas.
3 Carbon Nanomaterials
Carbon nanostructures like CNTs and graphene are capable of sensing extremely low amounts of greenhouse and explosive gases. Therefore, using the gas sensitivity of graphene and CNTs to make highly sensitive, power-efficient gas sensors is of great interest to both industry and researchers. As a carbon nanomaterial for creating gas sensors, CNTs have recently attracted the greatest research attention. The supremacy of CNTs is currently being challenged by graphene, a carbon allotrope that has just recently been studied. However, nanotubes and graphene are not the only carbon nanomaterials used for sensing so for. Various other carbon nanomaterials such as CB, CNHs, CNOs, ND, and CQDs have also been studied. In this chapter, I will discuss the most recent developments in sensor technology based on the carbon nanomaterials i.e. CB, CNHs, CNOs, ND, CQDs, CNTs, graphene with particular focus on the composites/hybrids of CNT and graphene with metal oxides, polymers, metals, etc.
3.1 Carbon Black (CB) Based Gas Sensors
When gaseous or liquid hydrocarbons are burned insufficiently or thermally decompose under controlled conditions, colloidal particles of CB, which are almost entirely pure elemental carbon, are created. The CB in these sensors is spread inside the polymer and enhances the conductivity of the film. The polymer expands and ultimately changes in electrical conductivity and resistance when the required gas or vapour is available. The viscosity of the polymer/CB is appropriately adjusted using a suitable solvent before the polymer is patterned on the filter electrode's surface. The polymer is applied to the electrode surfaces using a variety of techniques, including spin coating and drops, before drying.
The notion of percolation is used to explain changes in a composite’s strength as a function of the percentage of CBs [20, 21]. If the proportion of CB is low, the composite becomes insulated due to the lack of connections between the conductive nanoparticles in the composite body. The electrical resistance of the polymer reduces exponentially with increasing CB content [22,23,24]. The connection is established using the penetration limit by raising the CB concentration and travelling to the transfer point. When the required vapour or gas is available, the polymer expands and changes in electrical conductivity/resistance; this change is used to detect the gas (Fig. 1).
Polymer/CB composite’s gas detecting mechanism. The polymer matrix contains CB that has been dispersed in it. The conduction path between the electrodes is shown by the white line in the image above. This path and direction alter when vapours are present. The steering wheel returns to its initial position when fumes are eliminated from the atmosphere [19]. Copyright (2013) Elsevier
To respond to various vapours, Lewis and coworkers employed arrays of components, the team accomplished this using several polymer/CB composites. These arrays emit electrical resistance signals, which are assessed using a common system [25, 26]. The objective is to find different organic solvent vapours that may be present. This approach can be used with different hardware and software platforms to create a small, convenient tool that is also affordable.
Figure 2 displays, for instance, the sensory reactions for the detection of benzene and methanol. The responses will be moderate when these harmful gases are present in high concentrations. Due to deterioration polymer matrix or the displacement of CB particles, the polymer/carbon composite sensors may take longer to respond to external stimuli and may exhibit reduced sensitivity and stability over time. A diffusion path is caused by particle displacement or matrix ageing [27, 28]. The recurrent swelling and shrinkage of the polymer matrix brought on by the repeated operation of the sensor results in this change in configuration.
Resistances of composite CB made of a poly(ethylene-co-vinyl acetate) and b poly(N-vinylpyrrolidone) after 15 exposures to benzene (1.1 ppt) and methanol (1.5 ppt). To show reproducibility and stability, exposures were spaced out between recovery times. Part per thousand (ppt) refers to the amount of airborne vapour in this context [29]. Copyright (1996) American Chemical Society
3.2 Carbon Nanohorns (CNHs) Based Gas Sensors
CNHs, a subclass of carbon nanomaterials with horn-like tips that mimic single-walled CNTs (SWCNTs), are depicted in Fig. 3. The main characteristic of CHNs is that they tend to form spherical clusters (dimensions: diameter; 2–5 nm, length; 40–50 nm) [30]. Flowers that resemble dahlias, buds, and seeds are the three different types of CNHs [31]. They are made by laser ablation of pure graphite at room temperature, which results in excellent yield and production rates, despite the fact that they have unique advantages like as increased surface area, improved electrical and thermal conductivity, and easiness in functionalization [32, 33]. CNHs are attractive prospects in a variety of applications [34] including biosensing, and gas sensors owing to their high surface area and many holes. The dielectrophoresis (DEF) technology was used by Suehiro et al. to create the CNHs gas sensor [35]. The performance of the CNHs sensor fabricated using DEP with respect to NO2 and NH3 gas was evaluated using impedance spectroscopy. Suehiro et al. demonstrated that CNHs can detect ppm-levels of NH3 and NO2 gases at ambient temperature [35]. Sano et al. examined the gas sensing properties of CNHs and presented a simple and affordable method for producing them. They established that the CNH’s gas sensor can detect NH3 and O3 at room temperature. They discovered that under identical conditions, gas sensors made up of CNHs were more sensitive than sensors based on SWCNTs. They linked this improvement with monolayer gas adsorption as well as the interactions between adsorbed gas molecules that affect the charge transfer through gas molecules to the sensor surface [36]. With the oxidized nanohorns acting as an active sensing layer, the use of CNHs for humidity sensing can be expanded. Sensing capability was evaluated in nitrogen and air with a humidity range of 10–90%. The sensitivity in air is two times greater than the sensitivity in humid nitrogen. The presence of carboxylic groups can assist to explain this since interactions involving water molecules (being electron donors) reduce the amount of holes and oxidized single-wall CNHs produce increased resistivity [37].
a Shows a schematic of CNHs, b a high-resolution transmission electron microscope (HRTEM) image of the CNHs, and c a HRTEM image of a single carbon nanohorn with a size bar of 2 nm [38]. Copyright (2005) American Chemical Society
3.3 Carbon Nano-Onions (CNOs) Based Gas Sensors
CNOs depicted in Fig. 4 are an allotrope of the fullerene-family of carbon nanomaterials, consisting of spherical, quasi-spherical, and polyhedral geometries with concentric graphitic shells [39]. The series C60@C240@C540@C960@C1500@C60n, where n denotes the shell number [40], can be used to define the many graphitic shell structures that make up CNOs. To create CNOs, a variety of synthesis techniques have been investigated, including thermal annealing of NDs, arc discharge, flame aided pyrolysis, chemical vapour deposition (CVD), and non-thermal plasma. The exceptional shell-shaped structure of CNOs may be the cause of their remarkable features, which include high specific surface area, incredible electrical conductivity, and good tribological behaviour [41]. The aforementioned characteristics of CNOs place them in the running for use as a material in a variety of applications, including gas sensors, supercapacitors, lubricants, electrochemical sensors, and optical limiting [39]. As far as I know, few investigations have been devoted to the CNOs’ gas sensing capabilities. Dhonge et al. investigated the behaviour of CNOs in sensing volatile organic molecules (VOCs) at ambient temperature. Figure 5 illustrates how they demonstrated a linear relationship between sensitivity and gas concentration between 34 and 148 ppm [40].
Shows CNOs [42]. Copyright (2017) Elsevier
Depicts, for a number of gases, the connection between sensitivity and gas concentration [43]. Copyright (2017) Springer Nature
3.4 Nanodiamond (ND) Based Gas Sensors
The zero-dimensional carbon allotrope known as (ND) is predominantly made up of carbon atoms arranged in short-range order tetrahedral sp3 bonds [44, 45]. There are three primary industrial production technologies that can be used to produce ND. The first approach involves mechanically grinding high-quality diamond microcrystals made from graphite under circumstances of high pressure and temperature (HTHP), the second one is made up of explosive detonation synthesis and the third one is Chemical Vapour Deposition (CVD). The ND manufactured under harsh circumstances is present with particles ranging in size from 10 to 25 nm. According to XRD estimates, the usual crystallize size of ND generated by the detonation process is 4–5 nm [46]. The most common detonations, HTHP and CVD, have recently been seen as inferior alternatives to atmospheric pressure microplasma (AMP) [47]. Due to their exceptional chemical, mechanical, and optical properties, NDs can be employed in a wide range of applications, including gas sensors. In terms of gas sensing applications, the synthesis of MWCNTs was carried out using nanocrystalline diamonds employing CVD procedure. In a nutshell, the materials that were synthesized have a quick response time for H2 gas detection. Furthermore, repeatability and selectivity were maintained throughout a two-month period. The increased response/recovery attitude may be attributed to increase in defect sites brought on by the presence of ND grains, which in turn encourages the creation of numerous hydrogen molecule binding sites [48].
3.5 Carbon Quantum Dots (CQDs) Based Gas Sensors
With a particle size of about 10 nm, the novel zero-dimensional carbon-based nanomaterial known as CQDs is easy to functionalize and exhibits strong fluorescence, high thermal stability, biocompatibility and an innocuous chemical structure [49]. CQDs upholds a considerable number of high criteria, including good photoluminance, simple preparation techniques, low cost, low degree of toxicity, and straightforward functionalization, to name a few. CQDs have recently been recognized as having applications in the sensing space with fine detection limits in the nano-, pico-, or even femto-molar range [50]. The standard hydrothermal technique is used to incorporate ZnO onto the matrix of CQDs to create a composite. Nitric oxide (NO) gas detection and monitoring are done using this compound. For a detection limit of 100 ppm, a recovery/ response time of 34 and 36 s, respectively, is captured. The silica aerogel functionalized CQDs are used to detect NO2 in addition. The demonstrated selectivity of NO2 gas among various gases, such as O2, CO2 and NH3, is confirmed in this work [51]. In keeping with this carbon dot gas sensing, H2S gas was detected utilising a specifically made Schottky apparatus using a composite of carbon dots reinforced with MgO nanoparticles. Both air and the gas being measured are used to evaluate the sensor’s I–V inclination. The 120 ppm gas concentration naturally results in an increased response that is 11 times greater than the MgO at external voltage of −0.7 V. The reason why the aforementioned gas responded more strongly than other gases is due to the apparent decrease in barrier height that occurred during the H2S gas exposure [51]. Graphene quantum dots produced utilising a straight forward solution manufacturing technique under ambient circumstances is used as an ammonia gas sensor. It's noteworthy to observe that two opposing current responses for the gas result from a flexible pH modulation from acidic to neutral [52].
3.6 Carbon Nanotubes (CNTs) Based Gas Sensors
The material for gas sensing applications that has gained the most research is the carbon nanotube. Gas molecules are more likely to bond to its surface due to its high aspect ratio, chemical, thermal, and mechanical stability, as well as its metallic and semiconductive qualities and functionalization capacities. In response to interactions with gas molecules deposited on them, CNTs experience a change in conductivity. They have the benefit of being able to detect the presence of gases at room temperature, as opposed to traditional metal-oxide gas sensors. The pure CNTs, on the other hand, have low selectivity, making it impossible for them to distinguish between various gases [53]. The sensing mechanism of pristine CNTs is also slightly troublesome since they frequently feature a combination of metallic and semiconducting tubes, in addition to the varied degrees of faults caused by the purification procedures. In light of this, alteration and functionalization have been suggested as a way to improve sensitivity and selectivity. CNT modification using different materials has been the subject of numerous investigations.
3.6.1 CNTs/Metals Gas Sensors
The effective detection of (Nitrogen Dioxide, Carbon monoxide, and Benzene) pollutants in CNTs embellished with gold nanoparticles is investigated using a mix of experimental and theoretical methods. In contrast to C6H6, where there was no discernible effect, gold nanoparticles show a direct impact on the detection of Nitrogen Dioxide and Carbon monoxide. By understanding the link between the Fermi level shift and the change in resistance following gas adsorption, this behaviour difference may be explained [54]. Additionally, rhodium nanoparticles were used to adorn CNTs added to these materials so they could function as sensors for the detection of Nitrogen Dioxide, Carbon monoxide, and Benzene. Because oxygenated vacancies serve as both anchoring sites for rhodium nanoparticles and active adsorption sites for gases, the presence of oxygen is crucial for enhancing gas responsiveness. It’s also conceivable for CNTs and Ru NPs to transmit charge in a more direct manner [55]. Sharafeldin et al. added copper, platinum, titanium, Ruthenium and Cu, Pt, Ti, Ru, and silver to the MWCNTs to investigate their gas sensing behaviour. The MWCNTs/Cu nanocomposites were discovered to have the maximum sensitivity, measuring 1.75% when subjected to 10 ppm H2S. Additionally, Sharafeldin et al. also showed that the MWCNTs/Pt responded more strongly, with a reaction of 1.96%, when exposed to 10 ppm NO2 [56]. At ambient temperature, various quantities of carbon monoxide and NO were subjected to the zigzag MWCNTs decorated with palladium and Platinum nanoparticles. In order to study this unique structure, first-principles computations were used. According to a thorough investigation, SWCNTs adorned with Pt are more sensitive to carbon monoxide while those adorned with palladium are extremely sensitive to NO [57]. An NO2 gas sensor was created using Au NPs with a regulated size and proportion over MWCNTs to detect low concentrations of NO2 as low as sub-ppm. This research came to conclusion that the sensing capacities are controlled by the amount of Au nanoparticles deposited, the heights reaction to H2S and the modest Au loading [58]. Ag/CNTs and ZnO/Ag/CNTs were deposited onto cellulose paper by Khan et al. using the simple and affordable spray method. Investigators reported that Ag/CNTs sensor responded more quickly and selectively to acetone than the Ag/ZnO/Ag sensor [59]. The CNTs with Ni NPs functionalized were created for the detection of SO2, H2S, and SO2F2. According to Gui et al., the Ni/CNTs sensor’s low detection limit (LOD) against SF6 was 1 ppm. H2S, SOF2, SO2, and SO2F2 were shown to increase the sensitivity to various gases in that sequence [60].
3.6.2 CNTs/Metal Oxides Gas Sensors
Different physical and chemical methods have been used to anchor a range of metal oxide semiconductors to CNTs [61]. In order to improve CNTs’ selectivity and sensitivity for use in gas sensing applications, the decorating process’ main objective is to do so. In-depth study has been conducted on both SWCNTs and MWCNTs. The SWCNTs are set up as gas sensors for various toxic chemicals, including NH3, NO, and NO2. The SWNT-Fe2O3 composite film exhibits a steady response, improved sensitivity for H2S, improved sensitivity for NO2, and improved sensitivity at room temperature in comparison to pure SWNT films. These deformable, flexible sensors have a tremendous potential for wearable monitoring [62]. Research is being done on the use of ZnO and SWCNTs together to detect ethanol gas. On a copper substrate, the nanostructured materials were prepared using a spray pyrolysis technique. At a 6% weight concentration of ZnO/SWCNT, the best device performance is visible. Chemisorption, which achieves the transfer of charges between the adsorbed gas species and the metal oxide surface, is what produces the gas sensor response [63]. The same SWCNTs covered ZnO produced using a wet chemical process is also used to construct gas sensing for nitrogen dioxide, with response and recovery times of 70 and 100 s, respectively. The ideal sensing conditions are attained at 150 °C and 1000 ppm nitrogen dioxide [64]. The electrochemical synthesis method is also used to evaluate gas sensing utilising ZnO/SWCNT hybrids. Performance is carried out at room temperature for a number of gases, including CO, CO2, NO2, H2S, O2, and NH3. By precisely altering the electrochemical variables, density, crystallinity, and eventual particle size, a correlation between the gas sensing behaviour and the ambient conditions is seen. In summary, compared to the non-functionalized MWCNTs, the functional ZnO/MWCNTs exhibit approximately 5% per ppm for the H2S gas.
A practical sol–gel approach was used to create SnO2 and TiO2 carbon nanotube mixtures for ethanol detection. Within a broad temperature range of ambient temperature to 250 ℃, the mixture exhibits good qualities such as thermal stability and enhanced sensitivity [65]. Vanadium oxide-filled MWCNTs were used to illustrate the utilization of physical attributes as well as gaseous tracking. The composite's methane gas detection response at room temperature approached 16 s due to an increase in state densities among Fermi energy levels. In CNTs-based sensors, the influence of atmospheric oxygen was stressed and highlighted [66]. Cháfer et al. investigated the possibility of employing an IrOx-MWCNT nanocomposite for simultaneous NO2 and NH3 detection. Cháfer et al. demonstrated that, in comparison to pure MWCNTs, IrOx-MWCNTs nanocomposite can detect NO2 and NH3 at various working temperatures exhibiting good reproducibility, stability, increased sensitivity down to 1 bbp, and lower noise levels [67]. ZnO that has been doped with MWCNTs is used for toluene based gas sensor. In a nutshell, a variety of ZnO/MWCNTs that were produced via the reflux method is presented. MWCNTs make it difficult to prepare agglomerations for ZnO nanostructures. The 3:1 ratio of ZnO to MWCNTs results in a 17% increase in the sensor response at 150 °C compared to pure ZnO, which has no response at the given temperature [68]. In another report, Sonker et al. reported efficient LPG gas sensor based on MWCNT Doped ZnO Nanocomposite Thin Film [69].
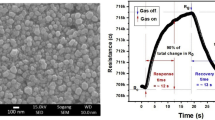
T. Guo et al. created the ammonia gas sensor that operates at ambient temperature. They employed a CNTs/Fe3O4 combination to discover a lower content of ammonia at ambient temperature. At ammonia concentrations of 20, 40, 60, and 80 ppm, they put the CNTs/Fe3O4 sensor to the test. The findings conclude that the sensor has a quick recovery time and responds selectively to ammonia. In addition to these benefits, as demonstrated in Fig. 6, the sensor also exhibits strong linearity, robust repeatability, and high stability [70]. ZnO nanostructures with MWCNT decorations are ready for hydrogen gas detection. Performance is increased by using Pt nanoparticles sputtered onto the composite surface. 78 s recovery times are attained for 0.05% of the targeted gas at room temperature, together with good reproducibility and stability. Furthermore, the MWCNTs/ZnO/Pt reaches 4% sensitivity, approximately twice that of the MWCNTs/ZnO [71]. The conventional chemical precipitation method was successfully applied to create the Al2O3/CeO2/MWCNTs nanostructure under the impact of an ultrasonic wave for CO2 gas. According to the data collected, the response and recovery times of the thermal conductivity sensor are 9 and 13 s, respectively, and are comparable to those of the majority of commercially available CO2 sensors [68]. The as-synthesized MWCNTs/ZnO using reflux technique at ~197 °C in ethylene glycol is employed for methanol gas detection. A wide range of temperatures between 100 and 300 ℃ were explored for the sensing behaviour, with the latter temperature yielding the best results. For the purpose of methane detection, Al2O3 doped with MWCNTs is also offered [72].
Shows the a repeatability, b stability, c responsiveness at various concentrations, and d linearity of the CNTs/Fe3O4 gas sensor [70]. Copyright (2018) MDPI
3.6.3 CNTs/Organic Materials Gas Sensors
Gas sensors for detecting volatile organic chemicals were made using CNTs and poly-ethylene glycol (VOCs). At room temperature, it was possible to achieve viable high response (110 s) and recovery (152 s) rates in different concentrations of acetone, isopropanol, isoprene and ethanol. These rates [70] characterize the sensor as a portable electronic-nose device [73]. As a sensitive material, poly (3,4-ethylenedioxythiophene) polystyrene sulfonate-multiwall CNTs can also be used to advance gas sensing. By integrating a sensing platform tailored to low power applications with the Internet of Things, this research intends to deliver a low-cost communicative sensor [74]. NO2 gas detection is performed by using single-walled nanotubes positioned on the flexible polytetrafluoroethylene (PTFE) filter substrate. A good stability of sensitivity was demonstrated when the substrates were bent repeatedly between 0.75 and 2 ppm concentrations. However, a substantial increase in sensitivity was found for concentrations of 3–5 ppm. The porousness of the substrates may have something to do with this. The sensitivity of the sensors can be doubled when compared to those made over a silicon substrate. Additionally, the electron-donor nature of water molecules causes a reduction in sensitivity at 10 and 30% humidity. These findings are helpful for flexible electronics and air quality monitoring [75].
Ammonia gas is detected using CNTs and polyaniline films. Sulfuric acid, camphor sulfonic acid and m-cresol were used for doping. Among which the sensing capability of camphor sulfonic acid was optimized and found equivalent to other responses. This is due to both the conservation of the initial volume of polyaniline and the evenly dispersed polarons induced by the concerned doping agent. This device works with improved sensitivity for ammonia gas detection, with a 4 ppm detection limit [76]. An acetone gas sensor made of a composite sheet of polyethylene glycol (PEG) and MWCNTs was demonstrated by Chiou et al. PEG/MWCNTs were more sensitive under mild temperature than they were in the lack of the thermal treatment, which is desirable for environmental applications, according to the results of the sensing performance tests [77]. W. Zhanget al. looked at how well the PANI/CNT composite worked for monitoring NO2 and NH3. To improve the sensing capabilities of the PANI/CNT composite, they developed a core–shell structure using n-type PANI and p-type MWCNTs. The low detection limits (LOD) for NO2 and NH3 were found to be 19.6 and 6.5 ppb, respectively, as depicted in Fig. 7 [75].
Illustrates the a response of the p-PANI/CNT sensor to NO2 gas, b a sensor's reaction to NO2 concentration fit curve, c responses to NH3 gas and d a fitting curve of the sensor’s response to NH3 concentration [78]. Copyright (2020) MDPI
3.7 Graphene Based Gas Sensors
One sheet of carbon atoms arranged in a hexagonal lattice is called graphene, and it has also been used to detect gases. With detection limits as low as ppb [14], graphene-based materials are good candidates for chemical sensing. However, additional precautions must be taken to prevent surface contamination brought on by the lithographic process [77]. This is a result of their inherently low noise structure, substantial specific surface area, and remarkable carrier mobility, which are all distinctive characteristics. Gas sensing performance of graphene can be considerably enhanced by the proper functionalization, according to theoretical and practical investigations. As in CNTs, dopants or defects also increase the adsorption energy in graphene which improves the sensitivity and selectivity. The interactions of four different forms of graphene (pristine, B- or N- doped, defective, and flaws) with minute gas molecules (CO, NO, NO2, and NH3) were investigated in order to investigate the potential of graphene as a gas sensor [79]. GO, reduced GO (rGO), and functionalized rGO conductometric devices have all been reported to have strong gas sensing capabilities [80,81,82]. Recently, graphene composites with other materials have shown tremendous sensing capabilities. The following section will discuss the composite/hybrid of graphene briefly.
3.7.1 Graphene/Metals Nanocomposite Gas Sensors
GO-metals have recently attracted a lot of attention due to their improved catalytic, electrical, and optical specifications [83]. Utilizing the Pt–Pd/rGO, hydrogen gas detection is carried out. It is established that the response is stable and repeatable. This could be explained by the crystal lattice expansion and carrier donation that occur during hydrogenation and dehydrogenation. By raising the hydrogen content or lowering the operating temperature, the responsiveness can be improved. No discernible changes in the sensor result are reflected in the flow rate variation. Higher response/recovery durations were attained when nitrogen was used as a gas carrier rather than air. This might be viewed as the reaction's oxygen contribution [84]. NO2 sensing is brought up by addressing the impact of electron beam exposure on Pd-fortified rGO composites. A range of irradiation dosages, from 0 to 500 kGy, were used; the latter level produced the best results. A reaction time of 345 s was measured at a NO2 concentration of 10 ppm, however the recovery time for the same concentration and dose is 816 s. This enhanced gas reaction is brought on by high energy defects and the abundance of oxygen functional groups [85].
The hydrogen gas is detected using a Pd/rGO hybrid. The hybrid was created with the use of microwave irradiation. From ambient temperature to 120 ℃, a wide temperature range was used to examine sensing performance. The 1% H2 sensing at 100 ℃ resulted in the greatest response of 14.5%. The increased interaction between hydrogen molecules and the sensor layer may explain this observation [86]. Reduced graphene oxide adorned with silver, gold, and platinum nanocomposites, which were made via a one-step chemical reduction technique, are also used to detect ammonia gas. Silver displayed the highest recovery, responsiveness, and sensitivity among these three nanoparticles [87]. In recent research, NO2 detection using graphene with an Au decorated porous structure was accomplished at a standard ambient temperature. When gas concentrations drop to 50 × 10–9, the sensor still responds within 30 s. The sensitivity was increased by au ornamentation to about 1.5 times that of clean graphene [88]. Ag-MoSe2/rGO ternary composites were used for hydrogen sulfide gas sensing. At room temperature, several values ranging from 0.1 ppm to 30 ppm were investigated. According to studies on how the potential barrier is regulated during electron transport and how the ternary composite structure works in concert, adding Ag to the compound appears to have an impact [89].
3.7.2 Graphene/Metal Oxide Nanocomposite Gas Sensors
For use in sensing applications, metal oxides such as ZnO, MnO2, WO3, MoO3, and CuO are extensively researched. On the one hand, these oxides have raised surface specific area and reasonable flexibility, but on the other hand, they do not have adequate electrical conductivity. By combining metal oxides with graphene and its derivatives, electrical conductivity can be naturally increased, which enhances sensor performance. There have been reports of graphene and metal oxide introduction [90]. The addition of metal oxides to graphene causes new physical and chemical properties to develop. Furthermore, it plays a crucial part in preventing the formation of aggregated graphene sheets. For the hydrothermal approach of methane detection and sensing, the nanocomposite of NiO/rGO was introduced. The sensing mechanism was proposed to be the Fermi energy band between NiO sheets and rGO nanoparticles. For concentrations between 100 and 500 ppm, long response times of roughly 6–18 s were observed [91]. In order to detect formaldehyde, Weiwei Guo et al. used a ZnO-rGO nanocomposite that they synthesized using a one-pot hydrothermal process and ZnO that was doped with Fe. The inclusion of Fe causes the ZnO hexagonal prism of the ZnO-rGO nanocomposite to shrink, while also increasing the ZnO’s surface area. Response-recovery times for a formaldehyde concentration of 12–5 ppm are maintained with a Fe doping of 5% [92]. ZnO treated with rGO was used for ultrasensitive monitoring of NO2 gas. The reaction at 100 ℃ is approaching a seven-fold improvement over pure ZnO. 5 ppm is the lowest detection limit that has been attained. The enhanced performance of the sensor is attributed to p-n heterojunctions between the ZnO and rGO [93].
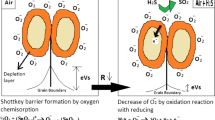
The hydrothermal method of production was used to obtain the rGO-TiO2 nanocomposite for ammonia sensing. In order to acquire the rGO-TiO2 nanocomposite with the intention of ammonia sensing, the hydrothermal method for preparation was introduced. At ambient temperature, the aforementioned nanocomposite showed improved selectivity and sensitivity for ammonia concentrations as low as 5 ppm [95]. But even at lower 100 ppm concentrations, the same nanocomposite can detect CO gas [96]. The rGO/ZnO nanocomposite was created by Vardan Galstyan et al. to detect H2, CH4 and NO2 gases. The resultant composite's conductivity is increased by the addition of rGO, which enhances the response to Nitrogen dioxide and Hydrogen gases as seen in Fig. 8. In contrast to pure ZnO, the rGO/ZnO nanocomposite reacts to NO2, gas preferentially at surprisingly low operating temperature [94].
Shows a schematic of how a gas sensor device is fabricated [94]. Copyright (2016) Royal Society of Chemistry
GO-WO3 nanocomposite films are used to demonstrate the detection of another gas (in this example, NO2). Combining the polyol method with metal–organic decomposition was used to carry out the synthesis. It was confirmed that there was immediate sensitivity within 0.5–5 ppm and excellent repeatability. Long-term stability for more than a month has been documented at room temperature [97]. The decoration of CuO with rGO was used for CO detection using the layer-by-layer method of self-assembly. The investigation covered a broad range of CO concentrations, from 0.25 to 1000 ppm. It was shown that the constructed heterojunction at the copper oxide–reduced graphene oxide interface produced excellent performance in terms of repeatability, sensitivity, and stability. SnO2-GO nanocomposite shown acknowledged sensitivity to detect NH3 with 10–50 ppm at ambient temperature [98]. The rGO-Fe3O4 nanocomposite is also used for carbon monoxide sensing; response-recovery periods of 32–35 s are obtained for a 5 ppm concentration, respectively [99]. Another method of carbon monoxide gas detection for NiO/graphene was provided using hydrothermal reflux technology [100]. For the purpose of monitoring H2S and SOF2, a hydrothermal process was used to generate SnO2-rGO nanocomposite. The ideal circumstances lead to the cleanest rGO sensors. For concentrations of 100 ppm H2S and 10 ppm SOF2, respectively, enhanced responses of 34.31 and 3.13% higher than those of a pristine rGO sensor at 125 °C were attained [101]. It was successful to create a rGO-SnO2-Au tri-structure system to identify formaldehyde. A remarkable increase in sensor responsiveness and selectivity was possible. This enhanced reaction could be attributed to the increased surface area, the catalytic action of the Au nanoparticles, and the ohmic contact synergistic interaction between SnO2 and rGO [102].
3.7.3 Graphene/Polymers Gas Sensors
With the use of rGO/polymer nanofibers, nitrogen dioxide was monitored and detected. A high sensitivity of 1.03 ppm and room temperature applicability were conceivably attained. Additionally, the electro-spun technique of synthesis provides a workable, environmentally friendly, and reliable path for preparation [103]. The detection limit of 150 ppm was also reached. The combination of rGO/conductive polymers was proposed as a potential tool for the Langmuir-Schaefer (LS) method to develop ammonia sensors. The four synthesized compounds showed the highest pyrrole-rGO-polyaniline sensitivity when pyrrole (Py) was utilised as the reducing agent. The detection limit was held at 0.2 ppm [104]. A graphene/ethyl cellulose nanocomposite was designed to provide a wearable gas sensor that is very sensitive and has a reduced strain response. At the minimum bending radius of 3.18 mm, this sensor exhibits a comparable resistance differential of 0.3% after 400 cycles of bending. For a 5 mm bending radius, 0.2% resistance change was attained. Monitoring was also done for detection limits, which ranged from 37 to 167 ppm. It was noted that ethanol, acetone, IPA, and hexane were detected [105].
3.7.4 Graphene/CNTs/Metal Oxide Nanocomposites Gas Sensors
Research is also being done on the nanocomposite of CNTs and derivatives of graphene that is used in gas sensing applications. These composite materials include NO2 gas sensors made of CNTs and rGO. These sensors have a flexible polyamide substrate and operate at ambient temperature. Both their great sensitivity and high bending ability have been noted for these sensors, with the former being attributed to the presence of CNT arrays while the second being credited to the remarkable flexibile nature of graphene sheets [106]. According to recent research by Morsy et al., the nanocomposite in combination with ZnO generated using the conventional precipitation method facilitates the detection of ammonia at ambient temperature. For the gas that was found and is currently under study, longer response and recovery periods were observed [107]. Vibha Srivastava et al. discovered that graphene/SnO2 synthesized using the sol–gel process has a greater intrinsic gas sensing response for NO2 than graphene/MWCNTs/SnO2 at ambient temperature. Reduced response times of less than one minute and a recovery period of almost five minutes are indicators of the composite. The increased reaction might be attributed to the surface’s complete exposure to the environment [108].
3.7.5 Graphene Foam and Three-Dimensional Graphene Gas Sensors
In addition, three-dimensional (3D) graphene foams are used for the detection of glucose employing the electrochemical sensing approach. The electrode scaffold was made of macroporous 3D graphene foam produced by chemical vapour deposition (CVD). A broad linear range (5–65 M) is used to reach the lowered detection limit of 1.5 M [109]. In a different report, a macro graphene foam-like material is used to detect gases. Here, there is a fusion of acceptable reliability and great sensitivity. Only a few ppm levels of NH3 and NO2 gases could be detected in the ambient air. Additionally, the proposed combination utilises less energy compared to Joule-heating, which combats molecules that have been chemisorbed off the surface of the foam, and produces an adequate level of mechanical strength and flexibility [110]. The ability of the three-dimensional reduced graphene oxide (3DRGO) decorated with ZnO nanoparticles to detect CO gas was investigated. The work by HaiHa et al. demonstrated that the sensor based on 3DRGO/ZnO has a rapid response and recovery, stability, good linearity and enhanced selectivity [111]. 3D graphene that was loaded with Co3O4 was used to detect VOCs. The GF/Co3O4 nanocomposite demonstrated great responsiveness and rapid response time at low Xylene concentrations [112].
4 Current Challenges and Outlook
Despite their high sensitivity, nano-carbon based sensors have a number of drawbacks, including limited repeatability, cross-sensitivity, irreversible recovery, non-uniform dispersion, defects, and low functional group stability, which necessitates further research and development before they can be commercialized. Different hazardous gases require gas sensors to have an extremely low detection limit. People might be warned about possible exposure to dangerous conditions beforehand using an ultralow detection limit as low as ppb. Future research should focus on developing gas sensors that are more sensitive, selective, and have lower detectivities for the intended gas analytes: (a) in order to improve gas sensing performance and achieve higher sensitivity, techniques like size control, doping, chemical modification, use of additional materials to modify functionality and defect generation and control have demonstrated their high efficacy. Through the use of these techniques, the interactions between analytes and sensing materials can be improved, resulting in a more sensitive reaction to the chemisorption or physisorption of molecular analytes; (b) in order to lower the detection limit of gas sensors, which is primarily determined by the sensitivity and resolution of the sensors, it is possible to increase surface areas, enhance material-analyte interactions, functionalize sensing materials, and employ analytical techniques; (c) for the selective detection of one or more analytes of interest, suitable host–guest hybrid material combinations can be used and created, improving the selectivity of gas sensors.
5 Conclusion
Traditional metal oxide semiconductor sensors offer several benefits and some limitations. The new class of sensors, which rely on nanoscale materials, are predicted to have some advantages over traditional sensors. As a result, carbon nanomaterials possess a number of outstanding mechanical, optical, electrochemical, and electrical features that make them suitable for use as sensors, either by themselves or in combination with other substances. This sensitivity to changes in their immediate chemical environment is one possible explanation for why these kinds of nanomaterials exhibit such sensitivity. This could be explained by the way that molecules that interact have an electrical structure. They are the ideal materials for sensing gases because of their sensitivity. In this book chapter, I covered the implications of carbon-based nanomaterials for gas sensing. I began with an introduction that features CB, CNHs and nano-onions before moving on to NDs and CQDs. Further I discussed about the sensing capabilities of CNTs and various CNTs combinations. This study outlines the enhanced detection limits and response/recovery timings for CNTs/metals, CNTs/metal oxides, and CNTs/organic materials in gas sensing performance and evolution. Finally, I discussed graphene-based gas sensor combinations including graphene/noble metals, graphene/metal oxide, graphene/polymers, and graphene/CNTs/metal oxide. I had covered a progression of the debate from 0D, 1D, 2D, and finally 3D foam graphene. Finally, the key lessons for selecting the best carbon-based materials along with the consequences for forthcoming challenges and outlook are discussed.
References
U. Kumar, H.-W. Hsieh, Y.-C. Liu, Z.-Y. Deng, K.-L. Chen, W.-M. Huang, C.-H. Wu, Revealing a highly sensitive sub-ppb-level NO2 gas-sensing capability of novel architecture 2D/0D MoS2/SnS heterostructures with DFT interpretation. ACS Appl. Mater. Interfaces 14, 32279–32288 (2022)
U. Kumar, B.C. Yadav, T. Haldar, C.K. Dixit, P.K. Yadawa, Synthesis of MWCNT/PPY nanocomposite using oxidation polymerization method and its employment in sensing such as CO2 and humidity. J. Taiwan Inst. Chem. Eng. 113, 419–427 (2020)
R.K. Sonker, B.C. Yadav, G.I. Dzhardimalieva, Preparation and properties of nanostructured PANI thin film and its application as low temperature NO2 sensor. J. Inorg. Organomet. Polym. Mater. 26, 1428–1433 (2016)
H. Li, J. Zhang, G. Li, F. Tan, R. Rui Liu, T.Z. Li, H. Jin, Q. Li, Triton assisted fabrication of uniform semiconducting single-walled carbon nanotube networks for highly sensitive gas sensors. Carbon 66, 369–376 (2014)
L. Sacco, S. Forel, I. Florea, C.-S. Cojocaru, Ultra-sensitive NO2 gas sensors based on single-wall carbon nanotube field effect transistors: monitoring from ppm to ppb level. Carbon 157, 631–639 (2020)
Z. Hou, J. Wu, W. Zhou, X. Wei, D. Xu, Y. Zhang, B. Cai,A MEMS-based ionization gas sensor using carbon nanotubes. IEEE Trans. Electron. Dev. 54(6), 1545–1548 (2007)
R. Malik, V.K. Tomer, Y.K. Mishra, L. Lin, Functional gas sensing nanomaterials: a panoramic view. Appl. Phys. Rev. 7(2), 021301 (2020)
S.W. Lee, W. Lee, Y. Hong, G. Lee, D.S. Yoon,Recent advances in carbon material-based NO2 gas sensors. Sens. Actuators B: Chem. 255, 1788–1804 (2018)
K. Xu, C. Fu, Z. Gao, F. Wei, Y. Ying, C. Xu, G. Fu, Nanomaterial-based gas sensors: a review. Instrum. Sci. Technol. 46(2), 115–145 (2018)
Y. Wang, J.T.W. Yeow, A review of carbon nanotubes-based gas sensors. J. Sens. 2009 (2009)
K. Toda, R. Furue, S. Hayami, Recent progress in applications of graphene oxide for gas sensing: a review. Analytica Chimica Acta 878, 43–53 (2015)
Q. Hu, E.K. Wujcik, A. Kelarakis, J. Cyriac, X. Gong, Carbon-based nanomaterials as novel nanosensors. J. Nanomater. 2017 (2017)
T. Han, A. Nag, S.C. Mukhopadhyay, Y. Xu, Carbon nanotubes and its gas-sensing applications: a review. Sens. Actuators A: Phys. 291, 107–143 (2019)
S. Demon, A.I. Kamisan, N. Abdullah, S.A.M. Noor, O.K. Khim, N.A.M. Kasim, M.Z.A. Yahya, N.A.A. Manaf, A.F.M. Azmi, N.A. Halim,Graphene-based materials in gas sensor applications: a review. Sens. Mater. 32(2), 759–777 (2020)
J. Wu, W. Zixuan, H. Ding, Y. Wei, W. Huang, X. Yang, Z. Li, L. Qiu, X. Wang, Flexible, 3D SnS2/reduced graphene oxide heterostructured NO2 sensor. Sens. Actuators, B Chem. 305, 127445 (2020)
E. Singh, M. Meyyappan, H.S. Nalwa, Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 9(40), 34544–34586 (2017)
O. Leenaerts, B. Partoens, F.M. Peeters, Adsorption of H2O, NH3, CO, NO2, and NO on graphene: a first-principles study. Phys. Rev. B. 77, 125416 (2008)
R.K. Sonker, B.C. Yadav, V. Gupta, M. Tomar, Fabrication and characterization of ZnO-TiO2-PANI (ZTP) micro/nanoballs for the detection of flammable and toxic gases. J. Hazard. Mater. 370, 126–137 (2019)
E. Llobet, Gas sensors using carbon nanomaterials: a review. Sens. Actuators, B Chem. 179, 32–45 (2013)
S. Mousavian, P. Faravar, Z. Zarei, R. Azimikia, M. Ghasemi Monjezi, E. Kianfar, Modeling and simulation absorption of CO2 using hollow fiber membranes (HFM) with mono-ethanol amine with computational fluid dynamics. J. Environ. Chem. Eng. 8, 103946 (2020)
U. Kumar, S. Sikarwar, R.K. Sonker, B.C. Yadav, Carbon nanotube: synthesis and application in solar cell. J. Inorganic Organomet. Polym. Mater. 26, 1231–1242 (2016)
R.K. Sonker, R. Shastri, B.C. Yadav, Theoretical and experimental investigation on structural stability, electronic and vibrational properties of polyaniline (PANI), in Proceedings of the Jangjeon Mathematical Society, vol. 22, no. 1 (2019), pp. 129–139
Y. Zilberman, U. Tisch, W. Pisula, X. Feng, K. Müllen, H. Haick, Spongelike Structures of hexa-peri-hexabenzocoronene derivatives enhance the sensitivity of chemiresistive carbon nanotubes to nonpolar volatile organic compounds of cancer. Langmuir 25, 5411–5416 (2009)
M. Ding, A. Star, Selecting fruits with carbon nanotube sensors. AngewandteChemie (International ed. in English) 51(31), 7637–7638 (2012)
R.K. Sonker, B.C. Yadav, S.R. Sabhajeet, Preparation of PANI doped TiO2 nanocomposite thin film and its relevance as room temperature liquefied petroleum gas sensor. J. Mater. Sci. Mater. Electron. 28, 14471–14475 (2017)
S.K. Asl, M. Namdar, Preparation of graphene/graphene oxide microsupercapacitor by using laser-scribed method. Chem. Methodol. 3, 183–193 (2019)
W. Kim, A. Javey, O. Vermesh, Q. Wang, Y. Li, H. Dai, Hysteresis caused by water molecules in carbon nanotube field-effect transistors. Nano Lett. 3, 193–198 (2003)
M. Muoth, T. Helbling, L. Durrer, S.-W. Lee, C. Roman, C. Hierold, Hysteresis-free operation of suspended carbon nanotube transistors. Nat. Nanotechnol. 5(8), 589–592 (2010)
M.C. Lonergan, E.J. Severin, B.J. Doleman, S.A. Beaber, R.H. Grubbs, N.S. Lewis, Array-based vapor sensing using chemically sensitive, carbon black−polymer resistors. Chem. Mater. 8, 2298–2312 (1996)
N. Comisso, L.E.A. Berlouis, J. Morrow, C. Pagura, Changes in hydrogen storage properties of carbon nano-horns submitted to thermal oxidation. Int. J. Hydrog. Energy 35, 9070–9081 (2010)
S. Zhu, G. Xu, Single-walled carbon nanohorns and their applications. Nanoscale 2(12), 2538–2549 (2010)
S. Zhu, J. Zhang, X. Zhao, H. Wang, G. Xu, J. You, Electrochemical behavior and voltammetric determination of L-tryptophan and L-tyrosine using a glassy carbon electrode modified with single-walled carbon nanohorns. Microchim. Acta. 181, 445–451 (2014)
M.R. Waikar, R.K. Sonker, S. Gupta, S.K. Chakarvarti, R.G. Sonkawade, Post-γ-irradiation effects on structural, optical and morphological properties of chemical vapour deposited MWCNTs. Mater. Sci. Semiconductor Process. 110, 104975 (2020)
Y. Liu, C.M. Brown, D.A. Neumann, D.B. Geohegan, A.A. Puretzky, C.M. Rouleau, H. Hui, D. Styers-Barnett, P.O. Krasnov, B.I. Yakobson, Metal-assisted hydrogen storage on Pt-decorated single-walled carbon nanohorns. Carbon 50(13), 4953–4964 (2012)
J. Suehiro, N. Sano, G. Zhou, H. Imakiire, K. Imasaka, M. Hara, Application of dielectrophoresis to fabrication of carbon nanohorn gas sensor. J. Electrostat. 64(6), 408–415 (2006)
N. Sano, M. Kinugasa, F. Otsuki, J. Suehiro, Gas sensor using single-wall carbon nanohorns. Adv. Powder Technol. 18(4), 455–466 (2007)
B.C. Serban, O. Buiu, N. Dumbravescu, C. Cobianu, V. Avramescu, M. Brezeanu, M. Bumbac, C.M. Nicolescu, Oxidized carbon Nanohorns as novel sensing layer for resistive humidity sensor. Acta Chimica Slovenica 67(2), 469–475 (2020)
K. Ajima, M. Yudasaka, T. Murakami, A. Maigné, K. Shiba, S. Iijima, Carbon nanohorns as anticancer drug carriers. Mol. Pharm. 2(6), 475–480 (2005)
O. Mykhailiv, H. Zubyk, M.E. Plonska-Brzezinska, Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorganica Chimica Acta 468, 49–66 (2017)
J.C. Zuaznabar-Gardona, A. Fragoso, Band structure, work function and interfacial diagrams of oxygen-functionalized carbon nano-onions. Synth. Met. 266, 116434 (2020)
T.H. Han, D. Mohapatra, N. Mahato, S. Parida, J.H. Shim, A.T.N. Nguyen, V.Q. Nguyen, M.H. Cho, J.-J. Shim, Effect of nitrogen doping on the catalytic activity of carbon nano-onions for the oxygen reduction reaction in microbial fuel cells. J. Ind. Eng. Chem. 81, 269–277 (2020)
A. Mahor, P.P. Singh, P. Bharadwaj, N. Sharma, S. Yadav, J.M. Rosenholm, K.K. Bansal, Carbon-based nanomaterials for delivery of biologicals and therapeutics: A cutting-edge technology. C 7(1), 19 (2021)
Y. Zhang, J. Zhao, Du. Tengfei, Z. Zhu, J. Zhang, Q. Liu, A gas sensor array for the simultaneous detection of multiple VOCs. Sci. Rep. 7(1), 1960 (2017)
Y. Zhang, K.Y. Rhee, D. Hui, S.-J. Park, A critical review of nanodiamond based nanocomposites: synthesis, properties and applications. Compos. B Eng. 143, 19–27 (2018)
D.H. Jariwala, D. Patel, S. Wairkar, Surface functionalization of nanodiamonds for biomedical applications. Mater. Sci. Eng. C 113, 110996 (2020)
Y. Gao, P. Yin, Determination of crystallite size of nanodiamond by Raman spectroscopy. Diam. Relat. Mater. 99, 107524 (2019)
S. Iqbal, M.S. Rafique, M. Zahid, S. Bashir, M.A. Ahmad, R. Ahmad, Impact of carrier gas flow rate on the synthesis of nanodiamonds via microplasma technique. Mater. Sci. Semicond. Process. 74, 31–41 (2018)
B.-R. Huang, D. Kathiravan, A. Saravanan, P.-H. Mai, Crystalline nanodiamond-induced formation of carbon nanotubes for stable hydrogen sensing. ACS Appl. Nano Mater. 4(3), 2840–2848 (2021)
L. Liu, Z. Mi, Z. Guo, J. Wang, F. Feng, A label-free fluorescent sensor based on carbon quantum dots with enhanced sensitive for the determination of myricetin in real samples. Microchem. J. 157, 104956 (2020)
M.J. Molaei, Principles, mechanisms, and application of carbon quantum dots in sensors: a review. Anal. Methods 12(10), 1266–1287 (2020)
A.G. El-Shamy, New nano-composite based on carbon dots (CDots) decorated magnesium oxide (MgO) nano-particles (CDots@MgO) sensor for high H2S gas sensitivity performance. Sens. Actuators B Chem. 329, 129154 (2021)
W. Chen, F. Li, P.C. Ooi, Y. Ye, T.W. Kim, T. Guo, Room temperature pH-dependent ammonia gas sensors using graphene quantum dots. Sens. Actuators B: Chem. 222, 763–768 (2016)
L. Camilli, M. Passacantando, Advances on sensors based on carbon nanotubes. Chemosensors 6(4), 62 (2018)
Z. Zanolli, R. Leghrib, A. Felten, J.-J. Pireaux, E. Llobet, J.-C. Charlier, Gas sensing with Au-decorated carbon nanotubes. ACS Nano 5(6), 4592–4599 (2011)
R. Leghrib, T. Dufour, F. Demoisson, N. Claessens, F. Reniers, E. Llobet, Gas sensing properties of multiwall carbon nanotubes decorated with rhodium nanoparticles. Sens. Actuators B: Chem. 160(1), 974–980 (2011)
I. Sharafeldin, S. Garcia-Rios, N. Ahmed, M. Alvarado, X. Vilanova, N.K. Allam, Metal-decorated carbon nanotubes-based sensor array for simultaneous detection of toxic gases. J. Environ. Chem. Eng. 9(1), 104534 (2021)
K. Li, W. Wang, D. Cao, Metal (Pd, Pt)-decorated carbon nanotubes for CO and NO sensing. Sens. Actuators B Chem. 159, 171–177 (2011)
E. Dilonardo, M. Penza, M. Alvisi, C. Di Franco, R. Rossi, F. Palmisano, L. Torsi, N. Cioffi,Electrophoretic deposition of Au NPs on MWCNT-based gas sensor for tailored gas detection with enhanced sensing properties. Sens. Actuators B: Chem. 223, 417–428 (2016)
Acetone sensing and modeling through functionalized Ag-catalysts and CNTs via low cost metal-organic framework-derived ZnO nanostructures
Y. Gui, X. Zhang, P. Lv, S. Wang, C. Tang, Q. Zhou, Ni-CNT chemical sensor for SF6 decomposition components detection: a combined experimental and theoretical study. Sensors 18(10), 3493 (2018)
R.K. Sonker, B.C. Yadav, Synthesis of ZNO/CNTS nanocomposite thin film and its sensing. Int. J. Appl. Bioeng. 10(1) (2016)
C. Hua, Y. Shang, Y. Wang, J. Xu, Y. Zhang, X. Li, A. Cao, A flexible gas sensor based on single-walled carbon nanotube-Fe2O3 composite film. Appl. Surf. Sci. 405, 405–411 (2017)
K. Kaviyarasu, G.T. Mola, S.O. Oseni, K. Kanimozhi, C.M. Magdalane, J. Kennedy, M. Maaza,ZnO doped single wall carbon nanotube as an active medium for gas sensor and solar absorber. J. Mater. Sci. Mater. Electron. 30, 147–158 (2019)
S. Barthwal, B. Singh, N.B. Singh, ZnO-SWCNT nanocomposite as NO2 gas sensor. Mater. Today: Proc. 5(7), 15439–15444 (2018)
N. Van Duy, N.V. Hieu, P.T. Huy, N.D. Chien, M. Thamilselvan, J. Yi, Mixed SnO2/TiO2 included with carbon nanotubes for gas-sensing application. Physica E: Low-Dimensional Syst. Nanostruct. 41(2), 258–263 (2008)
G. Chimowa, Z.P. Tshabalala, A.A. Akande, G. Bepete, B. Mwakikunga, S.S. Ray, E.M. Benecha,Improving methane gas sensing properties of multi-walled carbon nanotubes by vanadium oxide filling. Sens. Actuators B Chem. 247, 11–18 (2017)
J. Casanova-Cháfer, E. Navarrete, X. Noirfalise, P. Umek, C. Bittencourt, E. Llobet, Gas sensing with iridium oxide nanoparticle decorated carbon nanotubes. Sensors 19(1), 113 (2018)
N.L.W. Septiani, B. Yuliarto, H.K. Dipojono, Multiwalled carbon nanotubes–zinc oxide nanocomposites as low temperature toluene gas sensor. Appl. Phys. A 123, 1–9 (2017)
R.K. Sonker, M. Singh, U. Kumar, B.C. Yadav,MWCNT doped ZnO nanocomposite thin film as LPG sensing. J. Inorg. Organomet. Polym. Mater. 26, 1434–1440 (2016)
T. Guo, T. Zhou, Q. Tan, Q. Guo, F. Lu, J. Xiong,A room-temperature CNT/Fe3O4 based passive wireless gas sensor. Sensors 18(10), 3542 (2018)
S. Dhall, K. Sood, N. Jaggi, A hydrogen gas sensor using a Pt-sputtered MWCNTs/ZnO nanostructure. Meas. Sci. Technol. 25, 085103 (2014)
X.-L. Liu, B. Shen, H.-G. Zhang, Y.-Y. Sun, Q. Fang, Gas sensing properties of methane based on Al2O3-doped multi-walled carbon nanotubes. J. Nanoelectron. Optoelectron. 13(11), 1695–1700 (2018)
Z. Liu, T. Yang, Y. Dong, X. Wang, A room temperature VOCs gas sensor based on a layer by layer multi-walled carbon nanotubes/poly-ethylene glycol composite. Sensors 18, 3113 (2018)
P. Bahoumina, H. Hallil, J.-L. Lachaud, A. Abdelghani, K. Frigui, S. Bila, D. Baillargeat et al., Microwave flexible gas sensor based on polymer multi wall carbon nanotubes sensitive layer. Sens. Actuators B: Chem. 249, 708–714 (2017)
P.B. Agarwal, B. Alam, D.S. Sharma, S. Sharma, S. Mandal, A. Agarwal,Flexible NO2 gas sensor based on single-walled carbon nanotubes on polytetrafluoroethylene substrates. Flexible Printed Electron. 3(3), 035001 (2018)
M. Eising, C.E. Cava, R.V. Salvatierra, A.J. GorgattiZarbin, L.S. Roman,Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B: Chem. 245, 25–33 (2017)
J.-C. Chiou, C. C. Wu, T. M. Lin, Sensitivity enhancement of acetone gas sensor using polyethylene glycol/multi-walled carbon nanotubes composite sensing film with thermal treatment. Polymers 11, 423 (2019)
H. Kim, Y. Jang, G.W. Lee, S.Y. Yang, J. Jung, J. Oh, Tunable chemical grafting of three-dimensional poly (3, 4-ethylenedioxythiophene)/poly (4-styrenesulfonate)-multiwalled carbon nanotubes composite with faster charge-carrier transport for enhanced gas sensing performance. Sensors 20, 2470 (2020)
Y.-H. Zhang, Y.-B. Chen, K.-G. Zhou, C.-H. Liu, J. Zeng, H.-L. Zhang, Y. Peng, Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 20(18), 185504 (2009)
S. Prezioso, F. Perrozzi, L. Giancaterini, C. Cantalini, E. Treossi, V. Palermo, M. Nardone, S. Santucci, L. Ottaviano, Graphene oxide as a practical solution to high sensitivity gas sensing. J. Phys. Chem. C. 117, 10683–10690 (2013)
J. I. Paredes, S. Villar-Rodil, A. Martínez-Alonso, J.M.D. Tascon, Graphene oxide dispersions in organic solvents. Langmuir 24(19), 10560–10564 (2008)
M.-R. Yu, R.-J. Wu, G. Suyambrakasam, J. Joly, M. Chavali, Evaluation of graphene oxide material as formaldehyde gas sensor. Adv. Sci. Lett. 16(1), 53–57 (2012)
F. Liu, C. Wang, X. Sui, M.A. Riaz, M. Xu, L. Wei, Y. Chen, Synthesis of graphene materials by electrochemical exfoliation: recent progress and future potential. Carbon Energy 1(2), 173–199 (2019)
M. Choucair, P. Thordarson, J.A. Stride, Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 4(1), 30–33 (2009)
R.F. Davis, G. Kelner, M. Shur, J.W. Palmour, J.A. Edmond, Thin film deposition and microelectronic and optoelectronic device fabrication and characterization in monocrystalline alpha and beta silicon carbide. Proc. IEEE. 79, 677–701 (1991)
J. Kedzierski, P.-L. Hsu, P. Healey, P.W. Wyatt, C.L. Keast, M. Sprinkle, C. Berger, W.A. De Heer,Epitaxial graphene transistors on SiC substrates. IEEE Trans. Electron. Dev. 55(8), 2078–2085 (2008)
Y-M. Lin, C. Dimitrakopoulos, K.A. Jenkins, D.B. Farmer, H.-Y. Chiu, A. Grill, P. Avouris, 100-GHz transistors from wafer-scale epitaxial graphene. Science 327(5966), 662–662 (2010)
F. Schwierz, Graphene transistors. Nat. Nanotechnol. 5(7), 487–496 (2010)
N. Mishra, J. Boeckl, N. Motta, F. Iacopi, Graphene growth on silicon carbide: a review. Physica status solidi (a) 213(9), 2277–2289 (2016)
P. Tyagi, A. Sharma, M. Tomar, V. Gupta, A comparative study of RGO-SnO2 and MWCNT-SnO2 nanocomposites based SO2 gas sensors. Sens. Actuators, B Chem. 248, 980–986 (2017)
H.V. Roy, C. Kallinger, B. Marsen, K. Sattler, Manipulation of graphitic sheets using a tunneling microscope. J. Appl. Phys. 83, 4695–4699 (1998)
L. Ci, L. Song, D. Jariwala, A.L. ElÃas, W. Gao, M. Terrones, P.M. Ajayan, Graphene shape control by multistage cutting and transfer. Adv. Mater. 21, 4487–4491 (2009)
X. Liang, A.S.P. Chang, Y. Zhang, B.D. Harteneck, H. Choo, D.L. Olynick, S. Cabrini, Electrostatic force assisted exfoliation of prepatterned few-layer graphenes into device sites. Nano Lett. 9(1), 467–472 (2009)
V. Galstyan, E. Comini, I. Kholmanov, G. Faglia, G. Sberveglieri,Reduced graphene oxide/ZnO nanocomposite for application in chemical gas sensors. RSC Adv. 6(41), 34225–34232 (2016)
X. Liang, F. Zengli, S.Y. Chou, Graphene transistors fabricated via transfer-printing in device active-areas on large wafer. Nano Lett. 7(12), 3840–3844 (2007)
J.‐H. Chen, M. Ishigami, C. Jang, D.R. Hines, M.S. Fuhrer, E.D. Williams, Printed graphene circuits. Adv. Mater. 19(21), 3623–3627 (2007)
V. Huc, N. Bendiab, N. Rosman, T. Ebbesen, C. Delacour, V. Bouchiat, Large and flat graphene flakes produced by epoxy bonding and reverse exfoliation of highly oriented pyrolytic graphite. Nanotechnology 19, 455601 (2008)
M.K. Alam, M.M. Rahman, A. Elzwawy, S.R. Torati, M.S. Islam, M. Todo, A.M. Asiri, D. Kim, C.G. Kim, Highly sensitive and selective detection of Bis-phenol A based on hydroxyapatite decorated reduced graphene oxide nanocomposites. Electrochimica Acta 241, 353–361 (2017)
S. Park, K.-S. Lee, G. Bozoklu, W. Cai, S.B.T. Nguyen, R.S. Ruoff, Graphene oxide papers modified by divalent ions—enhancing mechanical properties via chemical cross-linking. ACS Nano 2(3), 572–578 (2008)
Y. Xu, H. Bai, L. Gewu, C. Li, G. Shi, Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 130(18), 5856–5857 (2008)
H. Chen, M.B. Müller, K.J. Gilmore, G.G. Wallace, D. Li, Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 20(18), 3557–3561 (2008)
M.D. Stoller, S. Park, Y. Zhu, J. An, R.S. Ruoff, Graphene-based ultracapacitors. Nano Lett. 8(10), 3498–3502 (2008)
S. Watcharotone, D.A. Dikin, S. Stankovich, R. Piner, I. Jung, G.H.B. Dommett, G. Evmenenko, S.-E. Wu, S.-F. Chen, C.-P. Liu, S.T. Nguyen, R.S. Ruoff, Graphene−silica composite thin films as transparent conductors. Nano Lett. 7, 1888–1892 (2007)
D.A. Dikin, S. Stankovich, E.J. Zimney, R.D. Piner, G.H.B. Dommett, G. Evmenenko, S.B.T. Nguyen, R.S. Ruoff, Preparation and characterization of graphene oxide paper. Nature 448(7152), 457–460 (2007)
S. Park, R.S. Ruoff, Chemical methods for the production of graphenes. Nat. Nanotechnol. 4(4), 217–224 (2009)
K.S. Kim, Y. Zhao, H. Jang, S.Y. Lee, J.M. Kim, K.S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi, B.H. Hong,Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457(7230), 706–710 (2009)
P.W. Sutter, J.-I. Flege, E.A. Sutter, Epitaxial graphene on ruthenium. Nat. Mater. 7(5), 406–411 (2008)
V. Srivastava, K. Jain, At room temperature graphene/SnO2 is better than MWCNT/SnO2 as NO2 gas sensor. Mater. Lett. 169, 28–32 (2016)
Q. Zhang, C. An, S. Fan, S. Shi, R. Zhang, J. Zhang, Q. Li, D. Zhang, X. Hu, J. Liu, Flexible gas sensor based on graphene/ethyl cellulose nanocomposite with ultra-low strain response for volatile organic compounds rapid detection. Nanotechnology 29, 285501 (2018)
V.S. Bhati, M. Hojamberdiev, M. Kumar, Enhanced sensing performance of ZnO nanostructures-based gas sensors: a review. Energy Rep. 6, 46–62 (2020)
N.H. Ha, D.D. Thinh, N.T. Huong, N.H. Phuong, P.D. Thach, H.S. Hong, Fast response of carbon monoxide gas sensors using a highly porous network of ZnO nanoparticles decorated on 3D reduced graphene oxide. Appl. Surf. Sci. 434, 1048–1054 (2018)
M. Morsy, A.I. Madbouly, Room temperature xylene sensor based on Co3O4/GF hybrid. Sens. Actuators A: Phys. 305, 111921 (2020)
Acknowledgements
I acknowledge the financial support from SERB for providing NPDF (File no. PDF/2021/001872).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tripathi, P. (2023). Carbon Based Functional Materials as Hazardous Gas Sensing. In: Sonker, R.K., Singh, K., Sonkawade, R. (eds) Advanced Functional Materials for Optical and Hazardous Sensing. Progress in Optical Science and Photonics, vol 27. Springer, Singapore. https://doi.org/10.1007/978-981-99-6014-9_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-6014-9_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6013-2
Online ISBN: 978-981-99-6014-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)